Ⅳ期胃癌转化治疗的实践
2016-12-14陈环球
周 斌,陈环球
作者单位: 210009 江苏 南京,江苏省肿瘤医院(南京医科大学附属肿瘤医院) 普外科
晚期胃癌转化治疗
Ⅳ期胃癌转化治疗的实践
周 斌,陈环球
作者单位: 210009 江苏 南京,江苏省肿瘤医院(南京医科大学附属肿瘤医院) 普外科
晚期胃癌的转化治疗是当下胃癌治疗的热点。转化治疗是指经转化化疗后,使原本不可切除或肿瘤学无法肉眼完整切除的晚期胃癌,争取获得R0切除。但目前,转化治疗的适应证未明确。综合文献报道的临床实践,根据胃癌生物学行为特点和高度异质性,Ⅳ期胃癌被分为:无肉眼种植转移(Ⅰ类:转移灶潜在可切除;Ⅱ类:转移灶大体可切除,手术可能获益小,不作为首选治疗);有肉眼种植转移(Ⅲ类:不可切除的腹腔种植转移病灶,转化手术后可能获益;Ⅳ类:不可切除转移灶,腹腔种植伴其他远处转移)。Ⅰ类病患的术前化疗归为新辅助化疗,转化治疗的适应人群应为Ⅱ类、部分Ⅲ类、极少数Ⅳ类Ⅳ期胃癌,Ⅰ类、Ⅱ类、少数Ⅲ类晚期胃癌有望通过围手术期化疗联合手术,获得相对较长的生存期。作者回顾Ⅳ期胃癌的治疗进展,并对Ⅳ期胃癌进行新的分级,试图在临床实践中厘清思路。
胃癌; Ⅳ期胃癌; 转化治疗; 新辅助化疗; 转化手术
随着早期诊断率提高和新药物的研发,胃癌治疗效果有所提高,但仍是全球第二位死因的恶性肿瘤[1]。日本胃癌诊疗规约的不断修订,规范了胃癌的标准治疗[2],已形成共识的有T1a期胃癌的内镜黏膜下剥离术(endoscopic submucosal dissection,ESD)、T1b期胃癌的腹腔镜下缩小手术、进展期可切除胃癌的D2清扫术等。对于转移或复发胃癌,治疗策略也在不断的调整。但Ⅳ期胃癌患者的中位生存时间始终只能徘徊在13~16个月[3-4],亟需我们重新审视Ⅳ期胃癌的治疗。
新的化疗方案和靶向药物(FOLFOX、FOLFIRI、XELOX、SOX联合bevacizumab, cetuximab、panitumab、regorafinib、ramcirumab等)使转移性结直肠癌的中位生存时间从6个月增加到30个月[5-6]。积极的对原发灶和转移病灶进行外科处理,在延长转移性结直肠癌生存期中作用显著[7]。转化治疗有效且转移灶达到R0切除的转移性结直肠癌患者,甚至有望获得临床治愈。反观晚期胃癌,使用化疗和靶向治疗后远未达到与转移性结直肠癌类似的临床结果。近年来有几项转化治疗方案的积极尝试给Ⅳ期胃癌的治疗带来一线曙光,但Ⅳ期胃癌转化治疗适应证、化疗药物的选择、手术时机的选择等关键问题仍困扰临床。本文通过回顾Ⅳ期胃癌的治疗进展,并对Ⅳ期胃癌进行新的分级,期待能为临床实践中的决策厘清思路。
1 当前的胃癌转化治疗实践
日本胃癌诊疗指南推荐:在HER 2(-)人群中S-1联合顺铂(CDDP)方案(SP)是标准一线治疗,卡培他滨联合顺铂方案(XP)、S-1联合多西他赛(docetaxel,DTX)为二线治疗;对HER 2(+)人群推荐XP联合曲妥珠单抗治疗[8]。此共识建立在多项开放临床试验的基础上(表1)。
JCOG9912[9]是日本首项对比5-FU vs S-1 vs伊利替康(CPT-11)联合顺铂的试验,结果未证实两药联合的优势,S-1可作为标准治疗。SPIRITS[10]研究对比S-1联合CDDP(顺铂60 mg/m2静注,1~8 d;S-1口服3周,停2周;每5周重复,直至肿瘤进展)vs单药S-1,结果前者中位生存时间(median survival time,MST)为13个月,S-1为11个月,S-1/CDDP优于单药S-1。另一项Ⅲ期研究TOP-002[11]证实了S-1联合CPT-11对照单药S-1治疗进展期胃癌的安全性和有效性,但两者无生存差异。
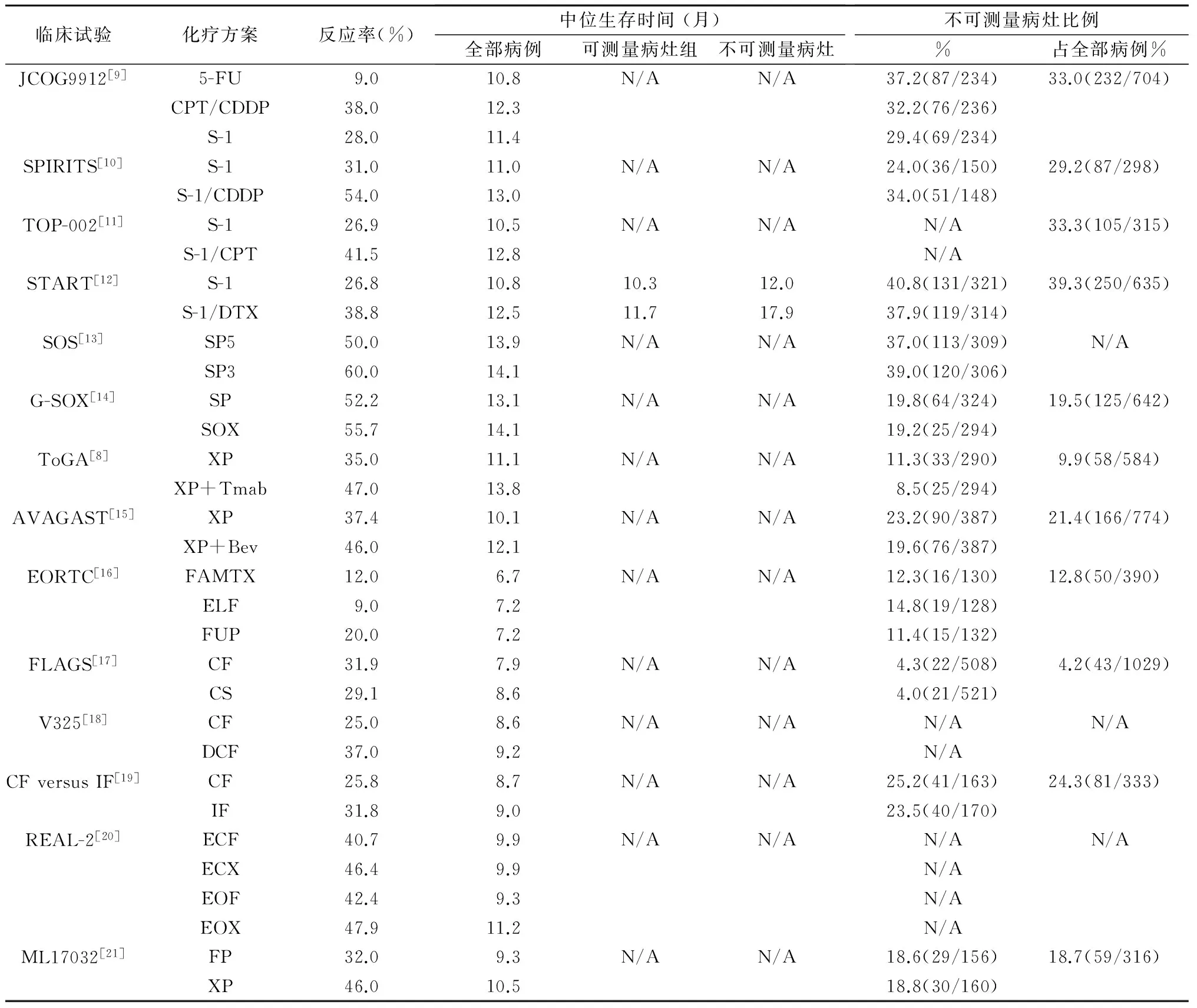
表1 多项临床试验:胃癌伴有不可测量转移灶的治疗
START[12]研究是一项由日、韩主导的多中心随机、对照、前瞻性Ⅲ期临床研究,目的是对比单药S-1和S-1联合DTX(DTX 40 mg/m2,d1;S-1 80 mg/m2/d,2周)治疗进展期胃癌的作用,结果S-1/DTX的MST为12.5个月,S-1为10.8个月,显示S-1/DTX优于单药S-1,化疗后病灶消失患者的MST,S-1/DTX组(17.9个月)也长于S-1组(12.0个月)。SOS[13]研究证实,改良的SP 3周方案对比5周方案具有同样的有效性。为减轻肾脏毒性,G-SOX试验[14]用奥沙利铂取代顺铂(SOX方案:奥沙利铂100 mg/m2,d1;S-1 80 mg/m2/d,口服2周后停1周,3周方案),证实奥沙利铂较顺铂具有同效低毒的优势,更适合在临床使用。
ToGA研究[8]证实,以卡培他滨或5-FU联合顺铂为对照组,曲妥珠单抗联合化疗组的MST为13.8个月,对比单独化疗组的11.1个月,P<0.01;联用曲妥珠单抗组进展时间(TTP)和无进展生存期(PFS)显著优于对照组,且毒性可控。AVAGAST研究和ToGA亚组分析显示XP方案后MST分别达到14.2和17.7个月[15,22]。因此,XP联合曲妥珠单抗作为HER-2阳性晚期胃癌的一线推荐方案,XP为HER-2阴性患者的二线推荐方案。
一项进行中的随机临床试验[23]证明DCS(Docetaxel/CDDP/S-1)方案优于S-1/CDDP方案,提示Ⅳ期胃癌的治疗效果较前有所改善。但如表1中所示,Ⅳ期胃癌的MST仍徘徊于3~17个月,多数的临床试验揭示有不可测量病灶(如腹膜转移、恶性胸腹水、残留微转移灶)患者对比有可测量病灶者,经治疗后有获得更佳生存的趋势。如START试验[12]证明,有不可测量病灶患者使用S-1/DTX方案后MST达17.9个月,单药S-1组为12.0个月,而有可测量病灶者两组MST无差异。类似的结果也见于SPIRITS研究[10]。
2 Ⅳ期胃癌的外科干预
胃空肠吻合、空肠造口、回肠或结肠造口术用于不可切除胃癌伴幽门梗阻和(或)腹腔广泛播散时。即使无法R0切除,如有肿瘤出血或胃肠梗阻,仍建议姑息切除胃部原发灶[24-27]。近年来,消化道内支架技术日趋成熟,应用逐渐增加,但有出血、支架移位等风险。肠内营养技术的发展提供新的姑息治疗方式,通过幽门后的营养管不仅可以进行营养支持,缓解化疗并发症,而且实现了口服氟尿嘧啶类药物的给药[28]。
REGATTA等[29]研究证实,Ⅳ期胃癌原发灶姑息切除可能无法获益。伴单处器官受累的Ⅳ期胃癌可考虑行手术切除,而多处肝转移、腹腔转移、包括16a1、16b2组淋巴结转移则不建议手术切除原发灶。
日本胃癌诊疗指南认为,Ⅳ期胃癌治疗应以化疗为主[2]。Ⅳ期胃癌的MST随着药物的进步而有所延长,但仍达不到让人满意的程度。如前所述,在有效的化疗后,继之予行胃癌原发灶和(或)转移灶的切除能改善患者的生存,其中肝转移和(或)淋巴结转移的患者治疗效果优于腹腔播散[30]。SATOH等[31]报道S-1/CDDP是一种有效的“引导”化疗方案,伴有远处转移或腹膜播散的晚期胃癌能从“引导化疗”有效后的手术中生存获益。
众所周知,晚期胃癌通常是多因素、多途径参与的过程,可能同时存在血行转移、远处淋巴转移及腹膜转移等,究竟哪一部分Ⅳ期胃癌患者能从转化治疗中获益?转化治疗的确切定义是什么?重新认识Ⅳ期胃癌的生物学行为,在转化治疗中有重要意义。
3 根据生物学行为划分的Ⅳ期胃癌新分类法
以往将Ⅳ期胃癌分为有或无腹膜种植转移两类。腹膜种植转移有着与经血行转移完全不同的生物学结局。腹腔种植的最终结局是引起恶性肠梗阻、癌性腹水和恶液质。而经血行途径引起的肝、肺等远处转移者,常死于器官衰竭。目前认为,广泛的腹腔内种植转移难以行完全的手术切除,而局限在某个或某些脏器的远处转移有切除的可能。
根据生物学行为的不同,可将Ⅳ期胃癌新分为四类,如图1所示。

图1 Ⅳ期胃癌新分类和转化治疗策略
其中,没有肉眼种植转移的Ⅳ期胃癌可分为:Ⅰ类,转移灶潜在可切除;Ⅱ类,转移灶大体可切除[32]。有肉眼种植转移的Ⅳ期胃癌可分为:Ⅲ类,不可切除转移灶(但有姑息治疗必要);Ⅳ类:不可切除转移灶。Ⅲ类患者的确认有待于剖腹探查或腹腔镜下探查分期,常规手段往往无法确诊。几乎所有伴腹腔种植转移的胃癌都是不可治愈的,即使治疗前分期认为或可达到病灶的R0切除。不同的肿瘤进展程度、速度和对治疗的敏感度导致Ⅳ期胃癌生存期存在差异。Ⅰ、Ⅱ类患者生存期相对较长,Ⅲ类次之,而Ⅳ类患者只能寄希望以姑息处理。
此分类可理解为“不需引导化疗可手术”和“需引导化疗后可手术”两类。转化治疗的目的是追求化疗后的R0切除[33]。转化治疗的对象主要以Ⅱ类Ⅳ期胃癌为主,包括某些Ⅲ类患者,而Ⅳ类患者几乎无转化治疗的机会。
3.1 Ⅰ类Ⅳ期胃癌(转移灶潜在可切除) 包括单发肝转移合并细胞学阳性,或腹主动脉16a2和(或)16b1组淋巴结转移,可行外科手术切除。
KODERA等[34]报道,细胞学阳性但无肉眼种植灶的Ⅳ期胃癌患者,手术后5年生存期超过20 %。生存显著改善的原因可能是术后口服S-1。化疗后获得R0切除的P0CY1(无腹腔种植,腹腔游离癌细胞阳性)期患者的预后尚不清楚。这些患者能否归为Ⅰ类,尚需要更多的临床证据验证。单发肝转移预后显著优于多发肝转移者,因此强烈推荐同时切除胃癌原发灶和肝脏单发灶[35-38]。
JCOG多项研究[39-43]证明:S-1/CDDP治疗后有效的16a2、16b1组淋巴结转移患者,手术切除后其生存期明显延长。REGATTA试验未包括这类患者,因为从外科角度评估,不管有无新辅助化疗,原发病灶和远处转移淋巴结都可切除。新辅助治疗是指针对可切除胃癌的术前冲击化疗。新辅助化疗联合手术,最终病理可证实达到的缓解程度有完全缓解(CR)、部分缓解(PR)以及稳定(SD),新辅助化疗后若出现新发病灶(进展)则应选择继续姑息化疗。Ⅰ类Ⅳ期胃癌患者可选择新辅助化疗+手术或手术+手术后辅助化疗的治疗路径。
3.2 Ⅱ类Ⅳ期胃癌(转移灶大体可切除) 是指肿瘤学或操作上难以实现切除,手术非治疗的首选方法。包括肝转移灶≥2个;转移灶大小>5 cm;肿瘤靠近肝静脉或门静脉;16a1, 16b2组淋巴结转移或更远的如纵隔、锁骨上或腋窝淋巴结转移。这些患者应该首选转化治疗,因转化治疗可能获得原发病灶和远处转移灶的完全缓解,从而获得原发病灶的切除,使生存延长。如治疗后仅剩孤立转移灶,则可一并切除。Ⅱ类Ⅳ期胃癌人群庞大,转化化疗是其标准治疗策略,医生通过转化性化疗可了解肿瘤对化疗药物的敏感性,治疗后是否进展,以及判断有无手术机会。
3.3 Ⅲ类Ⅳ期胃癌(不可切除转移灶,但有姑息治疗必要) 包括剖腹或腹腔镜探查发现已有腹腔种植转移。化疗能使较大的肿块或腹膜种植灶缩小,但无法达到消除微转移的程度,即使化疗后肿瘤明显退缩[44-46]。当化疗效果明显且腹腔镜探查分期为:CY0和P0者,胃原发癌和(或)转移灶应争取手术切除。这类手术被称为“细胞减灭手术”或“减瘤手术”,因为即使认为切除完整,大部分病例还是会在腹腔内复发。这种“减瘤手术”部分与“转化手术”重叠,患者获益程度还有待进一步的临床观察验证。有单发或多发的肉眼可见的腹腔转移灶,且局限于大小网膜者也被归为此类。这些病灶虽外科可切除,但肿瘤学上是无法根治性清除的。
3.4 Ⅳ类Ⅳ期胃癌(不可切除转移灶) 大部分存在肉眼可见的腹膜播散灶和其他器官转移,被认为是不可切除或不许切除的。极为罕见的病例能获得异常好的化疗后缓解,肿瘤可达到R0切除,但绝大多数患者只能行持续的姑息化疗。也有一些合并出血、胃排出道梗阻的患者需行姑息切除或短路手术。
4 结语
在Ⅳ期胃癌的治疗过程中,手术是转化治疗的重要治疗环节。在前期化疗有效的基础上,手术可延长患者生存期。越来越多的临床证据证明了这一点,但是晚期胃癌转化治疗的定义和手术适应证的选择尚存争议。前述的研究已证实,有部分转移灶可切除的生存期显著优于转移灶不可切除的患者。但也有研究发现,伴腹膜转移的患者在引导化疗有效后行原发灶切除(减瘤手术),其生存期仍是有限的。基于此,转化治疗的意义值得进一步探究。
综合以往Ⅳ期胃癌的临床实践,结合治疗中遇到的困惑,从胃癌生物学及胃癌异质性角度提出的新的分类法,为手术的选择提供了更细化的选择依据。Ⅰ类Ⅳ期胃癌,即无论有无术前化疗转移灶可切除,除非化疗中有新发转移,否则应不被归为转化治疗的范畴。
转化治疗是指化疗联合手术的治疗策略,可定义为:原本局部晚期无法切除或肿瘤学无法根治的胃癌,经化疗后获得R0切除的治疗模式。这类手术称为转化手术或辅助手术。术后应尽早继续化疗,直至肿瘤耐药或不可控的治疗副反应等情况发生。转化手术是以化疗为基石的。挽救手术是指肿瘤侵犯临近组织经化疗或放疗后,对残留灶或局部复发灶追加的切除术。两者主要区别是,挽救手术针对局部晚期肿瘤,而转化治疗兼顾原发灶和转移灶。
上世纪80年代,原发灶联合转移灶切除被认为是减瘤手术,但患者生存率极低,因为当时化疗的有效率仅为20%~30%[18]。日本的多项临床研究认为,以S-1为基础的化疗联合手术使部分患者的病理达到完全缓解。REGATTA试验认为,Ⅱ、Ⅲ类Ⅳ期胃癌在姑息手术后再接受化疗,生存获益极小,应在切除病灶前行化疗[29]。原因可能是:手术前化疗耐受性较好;手术后各类细胞因子活化,刺激肿瘤增殖[47-48]。
综上,转化治疗适合人群是Ⅱ类、部分Ⅲ、Ⅳ类Ⅳ期胃癌患者。在有效的化疗后达到R0切除:原发肿瘤和区域淋巴结完整切除,而转移灶如腹腔种植、肝转移、远隔淋巴结转移等消失。转化治疗还有很多问题缺乏定论,如手术时机如何选择,哪种治疗方案最佳及R0手术后是否继续化疗等。
YOSHIKAWA等[49]认为,胃癌在新辅助化疗两个周期后手术较为合理。参考胃肠道间质瘤的治疗,Ⅳ期胃癌手术最佳时机是化疗后肿瘤缓解最明显时,而不是等到肿瘤进展或复发时。因此,经4~6周期有效的治疗达到CR或PR,可能是转化手术介入的时间点。当然,手术后化疗应该继续,直至化疗耐药、肿瘤进展或出现严重的副反应使化疗终止[50]。
转化治疗是目前Ⅳ期胃癌治疗的方向,迫切需要有更多的前瞻性、随机对照试验去提供证据、指导治疗。
[1] GLOBOCAN 2012 database GLOBOCAN database. http:∥www-dep.jarc.fr/globocan/globocan.html.
[2] SANO T, AIKO T.New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points[J].Gastric Cancer,2011,14(2):97-100.doi: 10.1007/s10120-011-0040-6.
[3] SHEN L, SHAN Y S, HU H M,et al. Management of gastric cancer in Asia: resource-stratified guidelines[J]. Lancet Oncol,2013,14(12):e535-e547.
[4] LORDICK F, SIEWERT J.Recent advances in multimodal treatment for gastric cancer: a review[J].Gastric Cancer,2005,8(2):78-85.
[5] TAIEB J, TABERNERO J, MINI E,et al.Oxaliplatin, fluorouracil, and leucovorin with or without cetuximab in patients with resected stage III colon cancer (PETACC-8): an open-label, randomised phase 3 trial[J].Lancet Oncol,2014,15(8):862-873.
[6] SCHWARTZBERG L S,RIVERA F,KARTHAUS M,et al.PEAK:a randomized, multicenter phase Ⅱ study of panitumumab plus modified fluorouracil,leucovorin,and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer[J]. J Clin Oncol,2014,32(21):2240-2247.
[7] ADAM R, ALOIA T, LVI F,et al.Hepatic resection after rescue cetuximab treatment for colorectal liver metastases previously refractory to conventional systemic therapy[J]. J Clin Oncol,2007,25(29):4593-4602.
[8] BANG Y,VAN CUTSEM E,FEYEREISLOVA A,et al.Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial[J]. Lancet,2010,376(9742):687-697.
[9] TAKASHIMA A,BOKU N,KATO K,et al.Survival prolongation after treatment failure of first-line chemotherapy in patients with advanced gastric cancer: combined analysis of the Japan Clinical Oncology group trials JCOG9205 and JCOG9912[J].Gastric Cancer,2014,17(3):522-528.
[10] KOIZUMI W,NARAHARA H,HARA T,et al.S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase Ⅲ trial[J].Lancet Oncol,2008,9(3):215-221.
[11] NARAHARA H,IISHI H,IMAMURA H,et al.Randomized phase Ⅲ study comparing the efficacy and safety of irinotecan plus S-1 with S-1 alone as first-line treatment for advanced gastric cancer (study GC0301/TOP-002)[J].Gastric Cancer,2011,14(1):72-80.
[12] KOIZUMI W,KIM Y H,FUJII M,et al.Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START)[J]. J Cancer Res Clin Oncol,2014,140(2):319-328.
[13] RYU M H,BABA E,LEE K H,et al. Phase Ⅲ trial of a 3-weekly versus 5-weekly schedule of S-1 plus cisplatin (SP) combination chemotherapy for first-line treatment of advanced gastric cancer (AGC): SOS study[C]. J Clin Oncol,2013,31:LBA4024.
[14] YAMADA Y,HIGUCHI K,NISHIKAWA K,et al. Phase Ⅲ study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer[J].Ann Oncol,2014,26(1):141-148.
[15] OHTSU A,SHAH M A,VAN CUTSEM E,et al.Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer:a randomized, double-blind,placebo-controlled phase Ⅲ study[J].J Clin Oncol,2011,29(30):3968-3976.
[16] VANHOEFER U, ROUGIER P, WILKE H,et al. Final results of a randomized phase Ⅲ trial of sequential high-dose methotrexate,fluorouracil,and doxorubicin versus etoposide, leucovorin,and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer:A trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group[J]. J Clin Oncol,2000,18(14):2648-2657.
[17] AJANI J A,BUYSE M,LICHINITSER M,et al.Combination of cisplatin/S-1 in the treatment of patients with advanced gastric or gastroesophageal adenocar-cinoma:Results of noninferiority and safety analyses compared with cisplatin/5-fluorouracil in the First-Line Advanced Gastric Cancer Study[J]. Eur J Cancer,2013,49(17):3616-3624.
[18] VAN CUTSEM E, MOISEYENKO V M, TJULANDIN S,et al.Phase Ⅲ study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group[J]. J Clin Oncol,2006,24(31):4991-4997.
[19] DANK M,ZALUSKI J,BARONE C,et al.Randomized phase Ⅲ study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction[J]. Ann Oncol,2008,19(8):1450-1457.
[20] CUNNINGHAM D, STARLING N, RAO S,et al.Capecitabine and oxaliplatin for advanced esophagogastric cancer[J]. N Engl J Med,2008,358(1):36-46.
[21] KANG Y K,KANG W K,SHIN D B,et al.Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer:a randomised phase Ⅲ noninferiority trial[J].Ann Oncol,2009,20(4):666-673.
[22] YAMAGUCHI K,SAWAKI A,DOI T,et al.Efficacy and safety of capecitabine plus cisplatin in Japanese patients with advanced or metastatic gastric cancer:subset analyses of the AVAGAST study and the ToGA study[J]. Gastric Cancer,2013,6(2):175-182.
[23] KATAYAMA H,ITO S,SANO T,et al.A phase Ⅱ study of systemic chemotherapy with docetaxel, cisplatin, and S-1 (DCS) followed by surgery in gastric cancer patients with extensive lymph node metastasis: Japan Clinical Oncology Group study JCOG1002[J]. Jpn J Clin Oncol,2012,42(6):556-559.
[24] PROSERPIO I, RAUSEI S, BARZAGHI S,et al.Multimodal treatment of gastric cancer[J].World J Gastrointest Surg,2014,6(4):55-58.
[25] SUN J,SONG Y,WANG Z,et al.Clinical significance of palliative gastrectomy on the survival of patients with incurable advanced gastric cancer: a systematic review and meta-analysis[J]. BMC Cancer,2013,13:577.
[26] DITTMAR Y,RAUCHFUSS F,GOETZ M,et al.Non-curative gastric resection for patients with stage 4 gastric cancer—a single center experience and current review of literature[J]. Langenbecks Arch Surg,2012,397(5):745-753.
[27] MAHAR A, COBURN N, KARANICOLAS P,et al. Effective palliation and quality of life outcomes in studies of surgery for advanced, non-curative gastric cancer: a systematic review[J]. Gastric Cancer,2012,15(Suppl 1):S138-s145.
[28] LEE H O,LEE J J.Nutritional intervention using nutrition care process in a malnourished patient with chemotherapy side effects[J].Clin Nutr Res,2015,4(1):63-67.
[29] FUJITANI K,YANG H K,KUROKAWA Y,et al.Randomized controlled trial comparing gastrectomy plus chemotherapy with chemotherapy alone in advanced gastric cancer with a single non-curable factor: Japan Clinical Oncology Group Study JCOG 0705 and Korea Gastric Cancer Association Study KGCA01[J]. Jpn J Clin Oncol,2008,38(7):504-506.
[30] YOSHIDA K,YAMAGUCHI K,OKUMURA N,et al.The roles of surgical oncologists in the new era—minimally invasive surgery for early gastric cancer and adjuvant surgery for metastatic gastric cancer[J].Pathobiology,2011,78(6):343-352.
[31] SATOH S, OKABE H, TERAMUKAI S,et al. Phase II trial of combined treatment consisting of preop-erative S-1 plus cisplatin followed by gastrectomy and postop-erative S-1 for stage IV gastric cancer[J]. Gastric Cancer,2012,15(1):61-69.
[32] VAN CUTSEM E,CERVANTES A,NORDLINGER B,et al.Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up[J].Ann Oncol,2014,25(Suppl 3):iii1-iii9.
[33] FOLPRECHT G,GRUENBERGER T,BECHSTEIN W,et al.Survival of patients with initially unresectable colorectal liver metastases treated with FOLFOX/cetuximab or FOLFIRI/cetuximab in a multidisciplinary concept (CELIM study)[J].Ann Oncol,2014,25(5):1018-1025.
[34] KODERA Y, ITO S, MOCHIZUKI Y,et al. Long-term follow up of patients who were positive for peritoneal lavage cytology: final report from the CCOG0301 study[J].Gastric Cancer,2012,15(3):335-337.
[35] TAKEMURA N,SAIURA A,KOGA R,et al.Long-term outcomes after surgical resection for gastric cancer liver metastasis: an analysis of 64 macroscopically com-plete resections[J].Langenbecks Arch Surg,2012,397(6):951-957.
[36] KODERA Y,FUJITANI K,FUKUSHIMA N,et al.Surgical resection of hepatic metastasis from gastric cancer: a review and new recommendation in the Japanese gastric cancer treatment guidelines[J].Gastric Cancer,2014,17(2):206-212.
[37] QIU J L,DENG M G,LI W,et al.Hepatic resection for synchronous hepatic metastasis from gastric cancer[J]. Eur J Surg Oncol,2013,39(7):694-700.
[38] OKI E,TOKUNAGA S,EMI Y,et al.Surgical treatment of liver metastasis of gastric cancer:a retrospective multicenter cohort study (KSCC1302)[J]. Gastric Cancer,2015.[Epub 2015 Aug 11].
[39] MATSUMOTO T,SASAKO M,MIZUSAWA J,et al.HER2 expression in locally advanced gastric cancer with extensive lymph node (bulky N2 or paraaortic) metastasis (JCOG1005-A trial)[J].Gastric Cancer,2015,18(3):467-475.
[40] KODERA Y,KOBAYASHI D,TANAKA C,et al.Gastric adenocarcinoma with para-aortic lymph node metastasis:a borderline resectable cancer?[J].Surg Today,2015,45(9):1082-1090.
[41] TSUBURAYA A,MIZUSAWA J,TANAKA Y,et al.Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis[J].Br J Surg,2014,101(6):653-660.
[42] IWASAKI Y,SASAKO M,YAMAMOTO S,et al.Phase Ⅱ study of preoperative chemotherapy with S-1 and cisplatin followed by gastrectomy for clinically resectable type 4 and large type 3 gastric cancers (JCOG0210)[J].J Surg Oncol,2013,107(7):741-745.
[43] INOUE T,YACHIDA S,USUKI H,et al.Pilot feasibility study of neoadjuvant chemoradiotherapy with S-1 in patients with locally advanced gastric cancer featur-ing adjacent tissue invasion or JGCA bulky N2 lymph node metastases[J].Ann Surg Oncol,2012,19(9):2937-2945.
[44] YAMAGUCHI H,KITAYAMA J,ISHIGAMI H,et al.A phase 2 trial of intravenous and intraperitoneal paclitaxel combined with S-1 for treatment of gastric cancer with macroscopic peritoneal metastasis[J].Cancer,2013,119(18):3354-3358..
[45] COCCOLINI F,COTTE E,GLEHEN O,et al.Intraperitoneal chemotherapy in advanced gastric cancer.Meta-analysis of randomized trials[J].Eur J Surg Oncol,2014,40(1):12-26.
[46] MEZHIR J J,SHAH M A,JACKS L M,et al.Positive peritoneal cytology in patients with gastric cancer: natural history and outcome of 291 patients[J].Ann Surg Oncol,2010,17(12):3173-3180.
[47] WADA Y,YOSHIDA K,HIHARA J,et al.Sivelestat,a specific neutrophil elastase inhibitor,suppresses the growth of gastric carcinoma cells by preventing the release of transforming growth factor-alpha[J]. Cancer Sci,2006,97(10):1037-1043.
[48] WADA Y,YOSHIDA K,TSUTANI Y,et al.Neutrophil elastase induces cell proliferation and migration by the release of TGF-alpha, PDGF and VEGF in esophageal cell lines[J].Oncol Rep,2007,17(1):161-167.
[49] YOSHIKAWA T,Tanabe K,Nishikawa K,et al.Induction of a pathological complete response by four courses of neoadjuvant chemotherapy for gastric cancer:early results of the randomized phase Ⅱ COMPASS trial[J].Ann Surg Oncol,2014,21(1):213-219.
[50] BAUER S,RUTKOWSKI P,HOHENBERGER P,et al.Long-term follow-up of patients with GIST undergoing metastasectomy in the era of imatinib—analysis of prognostic factors (EORTC-STBSG collaborative study)[J]. Eur J Surg Oncol,2014,40(4):412-419.
周 斌,男,医学硕士,主治医师,研究方向:胃肿瘤的临床与基础研究,E-mail: zbjszl@126.com
陈环球,男,主任医师,硕士生导师,擅长胃癌的外科和综合治疗,E-mail: drchenhuanqiu@sina.com
10.3969/j.issn.1674-4136.2016.04.002
1674-4136(2016)04-0217-06
2016-01-05] [本文编辑:李筱蕾]
