金黄色葡萄球菌经静脉和腹腔诱导小鼠血流感染的炎症反应和病理变化
2016-12-13周树生胡仕静
吴 丹 周树生 胡仕静 刘 宝
(安徽医科大学附属省立医院重症医学科,合肥230001)
金黄色葡萄球菌经静脉和腹腔诱导小鼠血流感染的炎症反应和病理变化
吴 丹 周树生 胡仕静 刘 宝①
(安徽医科大学附属省立医院重症医学科,合肥230001)
目的:建立简单可靠的金黄色葡萄球菌血流感染小鼠模型,探讨血流感染小鼠的炎症反应和病理变化。方法:C57BL/6小鼠分别通过静脉或腹腔注射金黄色葡萄球菌标准菌株以诱导血流感染;观察各组小鼠生存率、体重变化及脓毒症评分(MSS);进行血样本及组织匀浆培养以确定细菌负荷;ELISA法检测血清或组织匀浆中的CRP、PCT及细胞因子(IL-1β、IL-6和TNF-α)水平;同时观察各组小鼠肝、肺、肾脏的病理改变及病理炎症评分。结果:4.5×108CFU/ml金黄色葡萄球菌血流感染小鼠的生存率约为70%。感染后24 h,小鼠体重明显减轻,MSS明显升高,且静脉组高于腹腔组。WBC于感染后3 h显著升高;感染后48 h血清CRP、PCT水平达到峰值,分别为静脉组:60.80±5.63、6.796±1.16,腹腔组:40.58±7.54、2.740±0.36;血清及组织匀浆(肝、肺、肾)中IL-1β、IL-6 和TNF-α水平均有不同程度的升高,且静脉组高于腹腔组。感染12 h后开始出现肝、肺、肾组织不同程度的病理炎症改变,且病理炎症评分随之显著升高。结论:成功建立金黄色葡萄球菌小鼠血流感染模型,比较静脉及腹腔两种途径的炎症反应强度及病理改变,有助于进一步探讨血流感染的早期诊断及临床治疗预后。
金黄色葡萄球菌;血流感染;炎症;细胞因子;组织病理学
各种细菌、病毒、真菌及其他微生物充斥着环境,并在我们免疫力低下时造成不同程度的感染。当今世界,严重感染已经成为威胁人类健康的致死性疾病之一,尤其是在重症监护病房(ICUs)有着更高的发病率[1]。最近的报道认为革兰氏阳性菌金黄色葡萄球菌引起的重症感染有着越来越高的发生率,其导致了肺部感染、消化道感染、泌尿道感染、皮肤软组织感染,甚至血流感染(BSIs)[2]。当血流感染发展为多器官衰竭的脓毒症或脓毒性休克时,其成为ICU感染死亡的重要原因。一些研究认为脓毒症死亡率为20%~30%[2],尽管临床上对其有着广泛的研究以及治疗方案的发展,脓毒症及相关死亡的发生率仍在上升[3],尤其是金黄色葡萄球菌导致的BSIs呈显著增加[4]。随着金黄色葡萄球菌诱导的BSIs的增加,更好的理解金黄色葡萄球菌感染的发病机理,对发展临床有用的治疗方法和改善预后显得尤为重要和有价值。目前,已有几种脓毒症动物模型,它们都试图模拟脓毒症病人典型的病理生理改变[5]。其中一些是输入外源性内毒素或者活菌以造成毒血症或菌血症,另一些包括盲肠结扎和穿孔法(CLP)及其他的腹部脓毒症[6]。在我们的研究中,我们建立了一种简便但有价值的模型,即通过给C57BL/6小鼠尾静脉或腹腔注射金黄色葡萄球菌的方法,比较两者炎症应答和病理变化,以助于理解金黄色葡萄球菌诱导的BSIs发病机制。
1 材料与方法
1.1 材料
1.1.1 菌株来源 S.aureus 标准菌株ATCC29213 由安徽医科大学附属省立医院临床微生物实验室惠赠。
1.1.2 实验动物 SPF级C57BL/6 雄性小鼠(20~25 g,6~8周) ,购自安徽省实验动物中心[SCXK(皖)2011-002)]。动物饲养于ASBL2动物室,实验前适应环境一周。
1.1.3 仪器和材料 血细胞分析仪(Coulter LH 750,美国Beckman Coulter公司),小鼠CRP、PCT ELISA试剂盒(美国LifeDiagnostics公司),小鼠IL-1β、 IL-6、TNF-α ELISA试剂盒(深圳欣博盛生物科技有限公司)。
1.2 方法
1.2.1 细菌培养 菌株于LB培养液中37℃摇菌过夜,然后以1∶100(v/v) 稀释于新鲜LB中继续摇菌4~6 h。菌液离心(3 000 r/min,5 min)后重悬于无菌PBS离心,反复三次,麦氏比浊法确定菌液浓度。
1.2.2 确定最适感染浓度 每组小鼠分别尾静脉和腹腔注射等剂量的不同浓度(4.5×109、4.5×108、4.5×107、4.5×106、4.5×105和4.5×104CFU/ml)的细菌菌液,每组5只,注射量为0.1 ml/10 g,连续观察7天,每日观察小鼠发病和死亡情况。按Karber方法计算LD50作为最适感染浓度。
1.2.3 感染实验 选择上述LD50的浓度感染小鼠,小鼠随机分为1、3、6、12、24、48、72、96、120、168 h实验组(静脉组和腹腔组) 及PBS对照组,每组5只,共150只,注射量0.1 ml/10 g,记录体重、症状、死亡情况,每组小鼠麻醉后收集血液标本检测WBC、CRP、PCT、IL-1β、 IL-6、TNF-α及细菌菌落计数,收集肝脏、肺脏、肾脏进行组织匀浆的IL-1β、 IL-6、TNF-α检测,以及组织的HE染色。小鼠脓毒症评分(MSS)用于血流感染后的临床症状评分,病理炎症评分(PIS)用于HE染色后的组织急性炎症评分。MSS评分包括七项单独的标准,即外表、意识状态、活动力、对刺激的反应、眼睛分泌物、呼吸频率、呼吸质量,每一项分别有0~4分,最终评分包括四项的总分[7]。PIS评分主要用于评估组织HE切片中炎症细胞(主要是中性粒细胞和巨噬细胞)的数量,分为4个等级:0=没有或者极少数的细胞,1=少量松散的细胞,2=血管周围多数的细胞,3=血管周围满布细胞,最终评分包括各个组织的评分总和[8]。
2 结果
2.1 最适感染浓度的确定 利用比浊仪计数在1.5左右时确定细菌计数为4.5×108CFU/ml细菌数。用不同浓度的菌液感染小鼠,观察周期7d,静脉注射4.5×109CFU/ml组的小鼠全部死亡,4.5×108CFU/ml和4.5×107CFU/ml组的生存率分别为 70% 和90%,其余各组全部生存;腹腔注射4.5×109CFU/ml组的小鼠全部死亡,4.5×108CFU/ml组的生存率为80%,其余各组全部生存,见图1。
2.2 小鼠感染后的症状表现 静脉组和腹腔组感染的小鼠体重均在48h出现明显的下降,并随时间的推移逐渐减轻,但静脉组更为显著,而PBS对照组小鼠体重逐渐增加。静脉组小鼠在感染后3h即出现竖毛,活动减少等表现,并随时间的延长逐渐出现萎靡、对刺激反应迟钝、眼睛有脓性分泌物、呼吸急促或微弱,甚至死亡等,而腹腔组仅在数天以后出现轻微的上述症状,PBS对照组无上述症状,静脉组小鼠MSS评分自3h开始较腹腔组有显著意义的增加,见表1。
2.3WBC、CRP和PCT水平的变化 静脉各组WBC在3h后显著升高,而腹腔各组在12h后明显升高;感染小鼠的CRP在12h后明显升高,持续至96h,PCT在12h后明显升高,持续至120h,且静脉各组在同一时间段高于腹腔各组,见表2。

图1 不同浓度金黄色葡萄球菌菌液感染小鼠后7天生存曲线Fig.1 Percent survival of mice with different concentrati-ons S.aureus within 7 daysNote: 1.Mice were infected with 4.5×109 CFU/ml,4.5×108 CFU/ml,4.5×107 CFU/ml,4.5×106 CFU/ml,4.5×105 CFU/ml and 4.5×104 CFU/ml S.aureus respectively (n=10/group).A.IV infected;B.IP infected.
2.4 血标本和组织匀浆中细菌计数 静脉组和腹腔组在感染1h~168h各个时间点的血标本及组织匀浆(肝、肺、肾)经培养后均见有细菌生长,且随着感染时间的延长,各组细菌计数逐渐增加,达到感染高峰(96h~120h)后又呈下降趋势,且静脉感染各组细菌计数明显高于腹腔感染各组,见表3。
2.5 各组小鼠血浆及组织匀浆中IL-1β、IL-6、TNF-α水平 静脉感染各组小鼠IL-1β、IL-6、TNF-α水平较腹腔各组的血浆及组织匀浆升高,几乎所有细胞因子的峰值都出现在6~24h,然而肾脏匀浆中IL-1β峰值在48~72h,肝脏和肾脏匀浆中IL-6及肾脏匀浆中TNF-α水平在7d的观察时间内持续地升高,见表4~7。
表1 各组小鼠的平均体重和脓毒症症状评分(MSS)
Tab.1 Body weight and MSS of mice(MSS)

TimenWeight(g)(x±s)IVIPMSS(x±s)IVIPPIS(x±s)IVIP0h1020.60±1.3520.70±1.340.00±0.000.00±0.000.04±0.250.03±0.121h1020.52±1.6320.88±1.510.25±0.080.17±0.081.60±1.140.40±0.553h1020.53±1.7020.73±1.401.07±0.122)0.75±0.084.80±1.303)1.20±0.846h1020.43±1.5520.80±1.451.69±0.102)1.06±0.044.60±1.343.40±1.1412h1020.43±1.1921.08±1.772.24±0.282)2.17±0.087.20±0.843)6.00±0.7124h1020.30±1.0921.23±1.927.73±0.122)5.56±0.107.60±1.346.20±0.8348h1019.88±1.051)20.96±1.888.99±0.672)7.53±0.098.40±0.896.80±1.4372h1019.00±0.921)20.45±1.579.73±0.272)7.57±0.156.40±0.553)4.60±0.5796h1018.07±1.101)20.20±0.9413.51±2.152)8.72±0.136.20±0.453)3.80±0.84120h1017.10±1.451)19.70±1.5616.15±0.122)9.08±0.084.60±0.563.00±1.87168h1015.00±0.711)19.60±1.8220.04±0.122)9.15±0.222.69±0.571.80±0.84
Note:1)P<0.01,2)P<0.001,3)P<0.05.

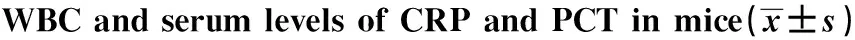

TimenWBC(×109L-1)IVIPCRPlevels(μg/ml)IVIPPCTlevels(ng/ml)IVIP0h101.81±1.041.21±0.353.20±0.623.22±0.610.036±0.020.038±0.021h101.70±1.911.71±1.143.28±0.533.04±0.650.048±0.040.036±0.023h104.44±0.701)1.20±0.653.32±0.533.12±0.660.049±0.040.038±0.026h105.68±0.721)2.74±0.976.00±0.794.50±0.790.202±0.060.098±0.0112h107.09±0.361)3.89±0.5922.90±5.662)13.40±3.402.000±0.382)0.347±0.0824h107.34±0.401)4.55±0.4938.40±6.512)23.94±4.613.962±0.342)1.246±0.2848h109.13±0.451)5.20±0.7260.80±5.632)40.58±7.546.796±1.162)2.740±0.3672h1010.44±0.851)7.26±0.4930.96±5.872)21.50±8.284.684±0.662)2.280±0.3996h1013.71±1.291)8.46±1.3015.00±4.062)11.56±3.312.496±0.562)1.344±0.35120h1011.75±1.678.85±1.024.66±0.684.30±0.920.806±0.972)0.276±1.13168h1010.68±1.479.32±1.193.52±0.403.57±0.700.064±0.010.185±0.36
Note:1)P<0.01,2)P<0.001.


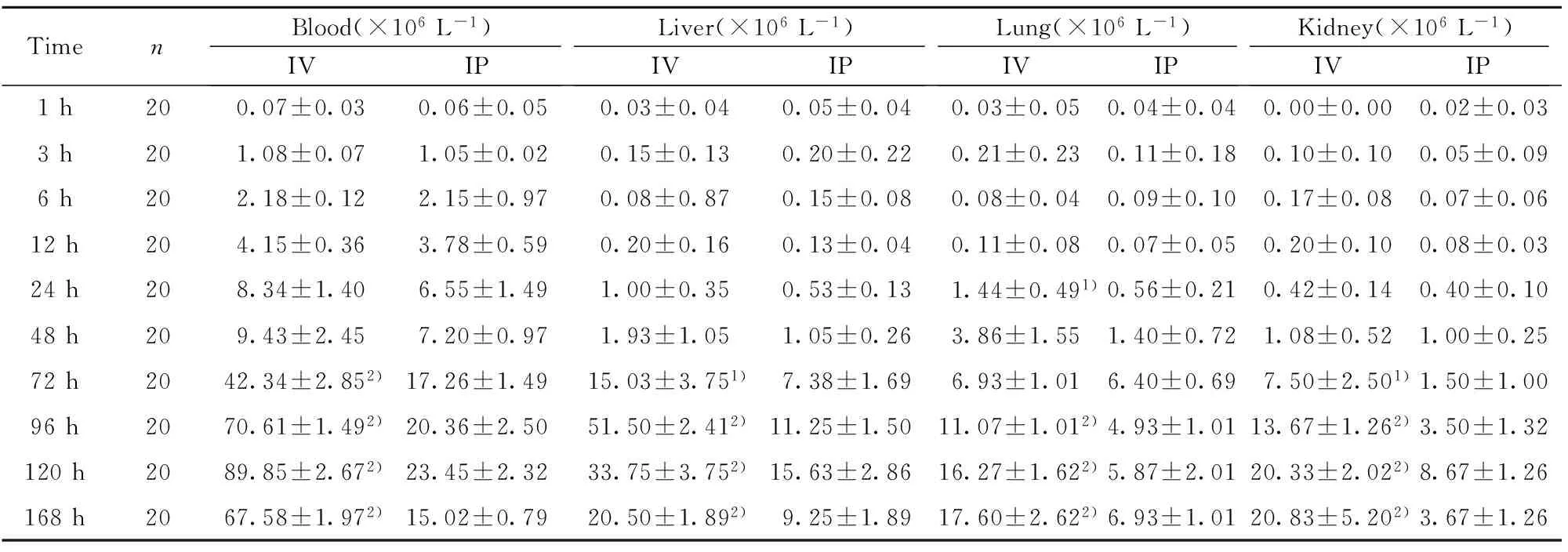
TimenBlood(×106L-1)IVIPLiver(×106L-1)IVIPLung(×106L-1)IVIPKidney(×106L-1)IVIP1h200.07±0.030.06±0.050.03±0.040.05±0.040.03±0.050.04±0.040.00±0.000.02±0.033h201.08±0.071.05±0.020.15±0.130.20±0.220.21±0.230.11±0.180.10±0.100.05±0.096h202.18±0.122.15±0.970.08±0.870.15±0.080.08±0.040.09±0.100.17±0.080.07±0.0612h204.15±0.363.78±0.590.20±0.160.13±0.040.11±0.080.07±0.050.20±0.100.08±0.0324h208.34±1.406.55±1.491.00±0.350.53±0.131.44±0.491)0.56±0.210.42±0.140.40±0.1048h209.43±2.457.20±0.971.93±1.051.05±0.263.86±1.551.40±0.721.08±0.521.00±0.2572h2042.34±2.852)17.26±1.4915.03±3.751)7.38±1.696.93±1.016.40±0.697.50±2.501)1.50±1.0096h2070.61±1.492)20.36±2.5051.50±2.412)11.25±1.5011.07±1.012)4.93±1.0113.67±1.262)3.50±1.32120h2089.85±2.672)23.45±2.3233.75±3.752)15.63±2.8616.27±1.622)5.87±2.0120.33±2.022)8.67±1.26168h2067.58±1.972)15.02±0.7920.50±1.892)9.25±1.8917.60±2.622)6.93±1.0120.83±5.202)3.67±1.26
Note:1)P<0.01,2)P<0.001.

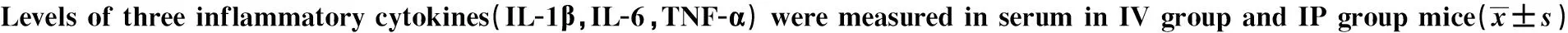

TimenIL-1β(pg/ml)IVIPIL-6(pg/ml)IVIPTNF-α(pg/ml)IVIP0h108.72±1.018.72±0.9487.78±6.4088.99±7.3319.59±2.0719.91±2.261h1024.66±2.902)19.48±1.681102.30±85.781014.10±82.2126.34±2.3024.97±1.223h1077.00±5.291)66.60±5.942676.03±95.602577.85±55.04104.02±4.492)90.20±3.706h10170.78±4.632)150.00±7.913254.03±50.291)3117.30±59.12229.08±7.422)193.60±6.1112h10187.30±5.701)177.60±5.593127.63±96.851)2988.03±39.62173.20±6.461)160.00±7.2124h1050.80±4.6649.60±6.54509.62±26.661)458.13±25.8344.20±5.1237.00±6.0848h109.30±1.798.90±1.1988.80±7.9886.01±9.6222.80±3.9620.80±3.1972h109.27±0.788.73±0.9293.52±6.3189.74±7.4623.60±4.5119.40±2.3096h108.86±0.808.53±0.9493.60±4.7790.80±4.5522.80±3.5620.01±1.87120h109.29±0.619.00±0.7488.18±11.7490.60±9.5620.23±2.5520.40±2.79168h108.76±1.078.98±0.8690.88±8.0290.40±7.9620.25±1.6919.19±1.29
Note:1)P<0.05,2)P<0.01.



TimenIL-1β(pg/ml)IVIPIL-6(pg/ml)IVIPTNF-α(pg/ml)IVIP0h105.00±1.113.00±2.102.00±0.001.00±002.00±2.071.50±2.081h101801.01±247.281561.53±281.2715.00±1.4115.23±5.0034.80±9.9426.01±7.753h102120.03±168.062370.13±448.2347.61±13.461)27.81±5.63517.82±58.171)440.18±46.866h102578.03±139.672)2141.30±113.49219.26±22.542)158.02±10.371341.13±45.952)974.26±59.2612h102942.08±264.232)2086.03±128.37500.20±72.112)344.03±48.27712.27±52.592)568.09±44.2124h102696.63±124.212)1957.13±105.45770.20±84.262)544.00±48.27182.83±14.292)139.02±16.7348h101808.13±123.301900.09±145.40918.02±58.052)688.04±52.6434.61±19.8923.76±13.7572h101475.83±205.541296.03±213.071250.03±180.282)842.00±51.1911.01±5.165.00±1.1896h10728.16±53.39377.22±49.731720.30±135.092)1057.83±95.254.00±4.444.05±3.32120h10685.43±74.492)353.87±45.452110.43±167.302)1231.63±149.313.60±1.044.45±0.79168h10256.33±56.69193.35±47.272290.03±159.692)1240.03±216.282.23±1.291.19±1.89
Note:1)P<0.05,2)P<0.01.



TimenIL-1β(pg/ml)IVIPIL-6(pg/ml)IVIPTNF-α(pg/ml)IVIP0h107.12±3.879.16±1.4262.60±7.8364.33±7.7934.01±1.5834.33±1.371h10462.92±88.692)227.24±49.9379.60±8.5673.61±9.50106.20±9.5464.01±11.933h101269.53±62.891)595.98±98.36440.00±78.18340.02±80.39420.02±75.361)177.72±56.116h101646.63±69.152)1005.52±92.28990.02±151.662)664.00±119.291100.01±171.562)550.02±76.7812h10756.68±82.91671.66±20.981768.03±97.152)1050.23±112.141870.03±158.852)1126.03±135.5724h10663.09±75.492)219.60±61.241448.03±69.762)860.02±108.391200.00±158.11970.04±92.3548h10205.50±39.902)42.60±15.43238.00±63.07157.00±65.53310.02±67.33208.02±62.5772h1048.80±34.9525.60±28.2766.21±12.1361.00±6.52130.02±40.6767.21±12.9196h105.00±8.111.90±6.0763.60±4.7257.81±5.4957.21±10.3238.80±6.76120h103.29±0.652.00±3.9055.40±11.2854.01±8.2241.20±2.5934.01±2.45168h105.76±1.094.98±1.8652.21±7.6953.41±8.8233.10±4.8433.80±3.56
Note:1)P<0.05,2)P<0.01.



TimenIL-1β(pg/ml)IVIPIL-6(pg/ml)IVIPTNF-α(pg/ml)IVIP0h1015.00±4.1214.72±3.472.34±4.402.67±3.331.95±3.072.06±1.261h1020.39±9.3812.91±7.927.76±6.344.10±2.2120.00±6.9810.01±4.143h1042.59±14.5625.88±18.7131.01±9.8110.11±8.4529.00±7.8120.09±9.036h1061.49±24.6741.54±12.8145.40±26.0421.00±9.4942.80±9.7434.09±9.8112h10246.81±39.99226.82±54.75112.02±21.962)65.01±16.9651.80±5.811)34.80±11.9424h10714.06±54.63707.83±64.62536.00±61.072)259.05±37.8163.61±6.111)44.81±15.2148h101976.73±213.121854.23±194.391260.03±119.372)666.02±88.2097.60±5.592)59.80±9.1872h101701.63±53.491)915.43±52.631340.09±118.362)750.67±79.06242.02±31.942)133.20±31.5496h10410.78±55.931)321.03±87.961)1810.09±96.182)1140.30±155.72421.62±36.372)257.02±35.99120h10224.45±46.13219.90±29.922130.30±233.452)1446.63±164.56608.02±49.692)340.45±38.08168h1065.68±13.994.11±15.781830.03±177.491506.03±224.00757.80±67.852)521.62±41.75
Note:1)P<0.05,2)P<0.01.

图2 静脉组和腹腔组小鼠三种器官的大体病理变化Fig.2 Gross pathological changes of three organs of IV and IP group miceNote: Lungs,liver and kidneys were observed at 48 h to 168 h post-infection in IV and IP infected mice.Organs of PBS control group were also observed.In lungs and liver,arrow indicates hemorrhage on the surface of tissues.In kidneys,arrows indicate abscesses on the surface.

图3 静脉组、腹腔组及PBS对照组小鼠器官的HE病理变化(×400)Fig.3 Representative photomicrographs of HE-stained organs from BSIs or PBS control mice(×400)Note: Sections from the lungs,liver and kidneys of infected mice at 12 h-120 h post-infection and PBS control mice were examined respectively.Arrow indicates neutrophil infiltration;asterisk indicates tissue necrosis.
2.6 小鼠脏器组织病理学观察结果
2.6.1 大体观察 在肺组织和肝组织中,感染后48~168 h静脉组和腹腔组均表现为肺脏和肝脏的出血,尤其在72~96 h,且静脉组更为明显;在肾组织中,感染后48~168 h静脉组和腹腔组均表现为肾脏充血肿大,且在72~120 h静脉组还表现为多发肾脓肿,见图2。
2.6.2 HE染色观察 金黄色葡萄球菌感染后12 h镜下开始观察到各组织的炎症变化,静脉组的肺、肝、肾组织在感染后12~120 h表现为炎症细胞浸润及不同程度组织的坏死,而腹腔组仅表现为不同程度炎症细胞浸润,未见明显组织坏死,见图3。
2.6.3 病理炎症评分结果 感染组各时间段均表现为评分的显著提高,且静脉组与腹腔组比较有统计学意义,见表1。
3 讨论
近年来,金黄色葡萄球菌诱导的血流感染逐年上升,已严重威胁人类健康[9]。一些涉及脓毒症机制的研究已经为我们提供了改善患者预后的方向,然而,对于其潜在的病理生理机制仍理解甚少。建立简单可靠的动物模型是进一步研究的基础,CLP法被认为是目前研究脓毒血症最常见的动物模型[6],然而CLP法通过盲肠结扎和穿孔造成腹腔感染播散而形成全身血流感染,其主要是革兰氏阴性杆菌或混合细菌的感染,而对于研究特定细菌的血流感染则存在局限性,我们的研究建立了简单易行的尾静脉或腹腔注射金黄色葡萄球菌,并成功摸索最适感染浓度,造成稳定的血流感染模型,该动物模型可进一步推广,研究其他特定细菌或者多种已知细菌混合感染后的炎症应答及病理变化。
CRP及PCT被认为是炎症及感染的生物标志之一。我们的实验结果表示血流感染组较对照组CPR及PCT水平显著升高,并分别开始于6 h及3 h,两者分别达高峰于24~48 h及12~24 h,这些与国内外相关研究相符[10-12],且静脉感染组明显高于腹腔感染组。
先前的实验已经证明,脓毒症时患者体内细胞因子(IL-1β、IL-6和TNF-α)水平显著提高。我们的实验表明血流感染小鼠血清及组织匀浆中(肝、肺、肾)上述三种细胞因子均显著提高,且静脉感染组高于腹腔感染组,而且还发现除了肝、肾组织匀浆中IL-6及肾组织匀浆中TNF-α水平在观察周期7 d内保持持续上升外,其余血清及组织匀浆中的细胞因子基本于感染后12~24 h达到高峰,这一结果与国外相关文献报道一致[10,13]。这可能是由于血清及各种组织中分泌的不同细胞因子的特性或者观察时间限制引起的,所以进一步有关细胞因子的机制作用有待研究。
我们观察到血流感染的小鼠组织细胞显著的病理学改变,如炎症细胞(中性粒细胞及巨噬细胞)浸润以及组织坏死等,这与Elif Cadirci等[8]的研究结果相似,而另一些研究认为脓毒症小鼠肾脏的病理改变较肺脏及肝脏明显[14,15],这可能与不同脓毒症模型的特性有关。病理炎症评分已被用于评估急性炎症感染程度,我们的结果与已有的研究结果一致,即血流感染组的评分远高于未感染组[16],且静脉组高于腹腔组。
感染后小鼠血液及组织匀浆中培养出金黄色葡萄球菌能够确定小鼠发生血流感染及播散至组织器官。我们的实验发现经腹腔感染的小鼠血液及组织中的细菌计数明显少于经静脉感染组,且各组血液培养出的细菌计数少于组织器官,这可能是由于血液中的补体系统及溶酶体能够直接吞噬一部分细菌,而已扩散至组织器官的细菌可以不断繁殖[17]。
体重的减轻被认为是脓毒症小鼠营养不良的表现之一。我们的实验结果与国外的研究一致,在血流感染后24 h发现小鼠体重显著下降或者不升,直至死亡或到达观察周期,而对照组小鼠的体重持续增长。我们还发现在感染后的24~48 h,体重的下降与细胞因子的上升相一致。这可能有以下解释:IL-1β有直接抑制食物摄取的作用,也有间接调节IL-1R的作用[18];TNF-α同样受IL-1R表达的影响,参与体重调节[19];此外,IL-6诱导发热,被认为是调节体重增长的重要因子[20]。
我们通过静脉及腹腔注射两种途径建立了金黄色葡萄球菌血流感染的小鼠模型,并比较两组小鼠的炎症反应及病理学改变,有助于进一步探明血流感染的病理生理学机制,并指导临床诊断治疗及预后。
[1] Vincent JL,Rello J,Marshall J,etal.EPIC II Group of Investigators.International study of the prevalence and outcomes of infection in intensive care units[J].JAMA,2009,302(21):2323-2329 .
[2] Garrouste-Orgeas M,Timsit JF,Tafflet M,etal.Excess risk of death from intensive care unit acquired nosocomial bloodstream infections:a reappraisal[J].Clin Infect Dis,2006,42(8):1118-1126.
[3] Martin GS,Mannino DM,Eaton S,etal.The epidemiology of sepsis in the United States from 1979 through 2000[J].N Engl J Med,2003,348(16):1546-1554.
[4] Koupetori M,Retsas T,Antonakos N,etal.Bloodstream infections and sepsis in Greece:over-time change of epidemiology and impact of de-escalation on final outcome[J].BMC Infect Dis,2014,14:272-281.
[5] Rittirsch D,Hoesel LM,Ward PA.The disconnect between animal models of sepsis and human sepsis[J].J Leukoc Biol,2007,81(1):137-143.
[6] Dyson A,Singer M.Animal models of sepsis:Why does preclinical efficacy fail to translate to the clinical setting?[J].Crit Care Med,2009,37(1 Suppl):S30-S37.
[7] Shrum B,Anantha RV,Xu SX,etal.A robust scoring system to evaluate sepsis severity in an animal model[J].BMC Res Notes,2014,7:233-243.
[8] Cadirci E,Altunkaynak BZ,Halici Z,etal.α-Lipoc acid as a potential target for the treatment of lung injury caused by cecal ligation and punctureyindced sepsis model in rats[J].Shock,2010,33(5):479-484.
[9] Lowy FD.Staphylococcus aureus infections[J].N Engl J Med,1998,339(8):520-532.
[10] van den Berg S,Laman JD,Boon L,etal.Distinctive cytokines as biomarkers predicting fatal outcome of severe Staphylococcus aureus bacteremia in mice[J].PLoS One,2013,8(3):e59107.
[11] Riedel S,Melendez JH,An AT,etal.Procalcitonin as a marker for the detection of bacteremia and sepsis in the emergency department[J].Am J Clin Pathol,2011,135(2):182-189.
[12] 谢尹晶,段晋燕,张洪瑞,等.四种常用细菌感染指标的动物实验研究[J].中华微生物学和免疫学杂志,2014,34(1):29-33.
[13] Ashare A,Powers LS,Butler NS,etal.Anti-inflammatory response is associated with mortality and severity of infection in sepsis[J].Am J Physiol Lung Cell Mol Physiol,2005,288(4):633-640.
[14] Doi K,Leelahavanichkul A,Hu X,etal.Pre-existing renal disease promotes sepsis-induced acute kidney injury and worsens outcome[J].Kidney Int,2008,74(8):1017-1025.
[15] Fisher BJ,Kraskauskas D,Martin EJ,etal.Attenuation of sepsisinduced organ injury in mice by vitamin C[J].JPEN J Parenter Enteral Nutr,2014,38(7):825-839.
[16] Stearns-Kurosawa DJ,Osuchowski MF,Valentine C,Kurosawa S,etal.The pathogenesis of sepsis[J].Ann Rev Pathol Mechanisms Dis,2011,6:19-48.
[17] Leendert A,Trouwa,Mohamed R,etal.Role of complement in innate immunity and host defense[J].Immunology Letters,2011,138(1):35-37.
[18] McCarthy DO,Kluger MJ,Vander AJ.Suppression of food intake during infection:is interleukin-1 involved?[J].Am J Clin Nutr,1985,42(6):1179-1182.
[19] Cerami A,Ikeda Y,Le Trang N,etal.Weight loss associated with an endotoxin-induced mediator from peritoneal macrophages:the role of cachectin (tumor necrosis factor)[J].Immunol Lett,1985,11(3-4):173-177.
[20] Wallenius V,Wallenius K,Ahrén B,etal.Interleukin-6-deficient mice develop mature-onset obesity[J].Nat Med,2002,8(1):75-79.
[收稿2015-07-10 修回2015-07-23]
(编辑 倪 鹏)
Mechanism of inflammatory responses and histopathological changes in Staphylococcus aureus induced bloodstream infections in mice
WU Dan,ZHOU Shu-Sheng,HU Shi-Jing,LIU Bao.Department of Critical Care Medicine,Affiliated Provincial Hospital of Anhui Medical University,Hefei 230001,China
Objective:To establish mice models of Staphylococcus aureus bloodstream infections so as to investigate the inflammatory responses and histopathological changes in bloodstream infections(BSIs) mice.Methods: C57BL/6 mice were inoculated with S.aureus intravenously or intraperitoneally to induce BSIs.Survival rate,weight loss and murine sepsis scores(MSS) were observed.Blood samples and tissue homogenates were plated on agar to determine bacterial burden.Inflammatory proteins(CRP,PCT) and cytokines(IL-1β,IL-6 and TNF-α) were determined by ELISA kits.Histopathologic changes were also assessed by pathological inflammation scores(PIS),macroscopic and microscopic examination.Results: About 70% survival rate was observed in 4.5×108CFU/ml S.aureus induced BSIs mice.Body weight decreased and sepsis scores increased significantly since 24 h post-infection in BSIs mice,and more prominent in IV group.The counts of WBC began to significantly increase at 3 h post-infection,while CRP and PCT levels peaked at 48 hours in IV and IP groups (60.80±5.63 vs 40.58±7.54 for CRP;6.796±1.16 vs 2.740±0.36 for PCT).Moreover,the levels of IL-1β,IL-6 and TNF-α in serum and tissue homogenates(liver,lungs,and kidneys) were significantly elevated in BSIs mice.Pathological changes in tissues(liver,lungs and kidneys) and higher pathological inflammation scores(PIS) were also observed in BSIs mice.Conclusion: Our study represents an effective approach for S.aureus BSIs model to mimic human sepsis.Our results demonstrated that inflammation protein(PCT,CRP) and cytokines(IL-6,IL-1β and TNF-α) play an important role in the inflammatory response and histopathological changes during BSIs caused by S.aureus.
Staphylococcus aureus;Bloodstream infections;Inflammation;Cytokines;Histopathology
10.3969/j.issn.1000-484X.2016.04.024
吴 丹(1988年-),女,硕士,主要从事细菌感染与免疫方面研究。
R378.1
A
1000-484X(2016)04-0556-07
①通讯作者,E-mail:DrLiubao@outlook.com。
指导教师:周树生(1973年-),男,博士,硕士生导师,主要从事重症感染细菌耐药方面研究。
