BABA诱导烟草抵御镉胁迫初步研究
2016-11-23刘松郭家明何宽信尤本武肖先仪陈学平
刘松,郭家明,何宽信,尤本武,肖先仪,陈学平
1中国科学技术大学烟草与健康研究中心,安徽省合肥市徽州大道1129号 230051;2 江西省烟草公司,江西省南昌市洪城路298号 330025
BABA诱导烟草抵御镉胁迫初步研究
刘松1,郭家明1,何宽信2,尤本武1,肖先仪2,陈学平1
1中国科学技术大学烟草与健康研究中心,安徽省合肥市徽州大道1129号 230051;2 江西省烟草公司,江西省南昌市洪城路298号 330025
通过施加外源BABA来减轻镉胁迫对烟草的毒害,并从抗氧化系统、离子含量及相关基因表达来探讨其作用机理。结果表明:0.2 mmol/L BABA和0.5 mmol/L BABA能使镉胁迫下的烟苗根长、鲜重及叶绿素含量显著增加;外源BABA处理不仅能显著提高镉胁迫下烟苗脯氨酸、谷胱甘肽(GSH)、还原型抗坏血酸(AsA)、可溶性糖和总酚含量,还能增强超氧化物歧化酶(SOD)、抗坏血酸还原酶(APX)、过氧化物酶(POD)和过氧化氢酶(CAT)活性,降低过氧化氢(H2O2)和丙二醛(MDA)含量,可以促进烟苗根部镉的积累及降低地上部镉的含量。实时定量PCR结果表明:BABA参与NtIRT1、NtNramp5、NtPCS1、NtGSH1、NtHMA4、NtPCR1、NtPDR5b基因表达调控。综合来看, BABA缓解烟草镉胁迫可能是激发植物体抗氧化系统、降低地上部镉的积累及调节相关基因表达的综合结果。
β-氨基丁酸;烟草;镉胁迫
镉是毒性较强的一种重金属元素。据估计,我国有高达13,000 hm2耕地被镉污染[1-2]。研究人员发现土壤中高浓度镉抑制小白菜的生长,影响氮素代谢,阻碍对铜,钙,铁,镁的吸收;镉胁迫主要抑制植物的光合作用和呼吸作用,降低氮素代谢、水分和矿质元素吸收[3]。镉还可诱导植物遭受氧化胁迫,产生大量活性氧(ROS),扰乱细胞膜的组成和功能[4-6]。镉胁迫对烟草的影响也有大量报道。马新明等人[7]发现烟草吸收过多的镉会使烟碱含量降低,糖碱比、氮碱比趋于不协调,导致烟叶品质降低。郭江波等[8]证实镉污染会抑制烟草叶绿素合成及氧化酶活性,进而对烟草细胞造成伤害。
β-氨基丁酸(BABA)是一种非蛋白氨基酸,能够调节植物对多种胁迫的抵御能力。研究表明,BABA可以诱导水稻抗线虫[9]、葡萄抗霜霉病[10]、烟草抗烟草花叶病毒(TMV)[11]。BABA还可以诱导拟南芥抗镉[12]、烟草抗高铜[13]和高盐[14]。有关BABA缓解镉胁迫对烟草毒害的研究未见报道。烟草是我国重要的经济作物,也是镉易富集植物(富集系数可达5~10)[15],且吸收的镉易分配积累于叶片中。本研究以云烟87为试验材料,研究不同浓度BABA对镉胁迫下烟草抗氧化物质含量、抗氧化酶活性及镉吸收转运及解毒基因的表达量,测定了地上部和根部镉含量,以期为烟草镉污染控制提供一定的理论依据。
1 材料与方法
1.1 材料
烟草品种“云烟87”由安徽农业科学院提供,BABA(纯度为94 %)市购。
1.2 生长条件和处理
水培条件:将消毒后的烟草种子均匀撒在由霍格兰营养液润湿的珍珠岩里,选取生长一致烟草幼苗移入已加入霍格兰营养液的12孔黑色育苗盆中,轻质泡沫固定,温度(28 ± 2)℃,16 h光照/d培养至3、4叶真叶期。再从中选取一致烟草幼苗进行胁迫实验,每盆10株烟草幼苗,每组设置三盆重复。BABA预处理3 d后,将镉胁迫组和预处理组烟草幼苗移入含有100 μmol/L CdCl2的1/2霍格兰营养液中处理4 d,每隔2 d更换一次处理液,营养液pH值保持在6.5,具体操作如表1所示。镉胁迫4 d后分别使用直尺和万分之一分析天平测量烟草幼苗根长和鲜重(滤纸吸干表面水分,迅速放入铝盒);同时取烟草幼苗倒数第2片叶测定生理指标、提取RNA;实验设置3次重复。

表1 水培条件Tab.1 Hydroponic condition
1.3 生理指标的测定
取新鲜叶片约0.2 g,在预先冷冻的研钵中加入2 mL 50 mmol/L pH=7.0的缓冲液(含有1 %PVP,0.1 %EDTA-2Na),迅速研磨至匀浆,再加入3 mL缓冲液,16000 rad/s高速冷冻离心机4 ℃离心20 min,上清液即为待测酶液。
采用丙酮提取法测定叶绿素含量[16];采用酸性茚三酮法测定脯氨酸含量[17];采用硫代巴比妥酸比色法测定丙二醛含量[18];AsA和可溶性糖的测定参照邹奇的方法[19];采用福林酚法测定总酚的含量[20];GSH和H2O2的测定分别参照南京建成生物试剂公司GSH、H2O2试剂盒。
APX活力的测定参照Nakano和Asada的方法[21];愈创木酚法测定过氧化物酶(POD)活性[22];氮蓝四唑(NBT)光化还原法测定超氧化物歧化酶(SOD)活性[23];CAT活力的测定参照南京建成生物试剂公司CAT试剂盒。
1.4 镉和铁元素含量测定
镉胁迫处理4 d后烟草幼苗使用20 mmol/L EDTA-2Na溶液浸泡20 min,再用去离子水冲洗3遍,滤纸吸干水分后置于105 ℃的烘箱杀青15 min,再70 ℃烘干至恒重。根和叶分别研磨成粉,采用Optima 7300DV等离子体原子发射光谱仪测定镉和铁含量。
1.5 荧光定量PCR
叶片总RNA提取参照北京天根公司植物总RNA提取试剂盒说明书,RNA反转录参照TaKaRa反转录试剂盒说明书,荧光定量PCR测定参照北京天根公司SuperReal PreMix Plus(SYBR Green)试剂盒说明书,使用LightCycler96采用两步法PCR反应程序进行反应。内参为Ntubc2,目的基因为NtIRT1、NtNramp5、NtPCS1、NtGSH1、NtHMA4、NtPCR1、NtPDR5b,目的基因引物序列参考表2。

表2 基因引物序列Tab.2 Gene sequence of primers
1.6 数据分析
实验数据表示为平均值 ± 标准偏差,数据分析采用单因素方差分析,置信区间P<0.05,统计分析软件使用origin 9.1。
2 结果与分析
2.1 BABA对镉胁迫下烟草幼苗生长的影响
与对照组相比,镉胁迫下烟草幼苗的生长明显受到抑制,叶部出现萎黄(图1A),根长缩短(图1B),地上部和根部鲜重减小(图1C),叶绿素含量降低(图1D)。而经BABA预处理的烟草幼苗的根长、鲜重、叶绿素含量均显著增加。其中,施加0.2 mmol/L BABA处理组烟苗比镉胁迫组根长、地上部鲜重和根部鲜重分别增加了19.82 %、18.67 %和82.93 %;施加0.5 mmol/L BABA处理组烟苗比镉胁迫组的根长、地上部鲜重和根部鲜重分别增加了16.22 %、25.36 %和53.14 %。由图1D可知,镉胁迫下烟草幼苗叶绿素a、b均出现下降。0.2 mmol/L、0.5 mmol/L BABA预处理组烟草幼苗较镉胁迫组叶绿素a含量分别提高了27.40 %和34.53 %;叶绿素b含量前者提高10.07 %,后者虽有增加,但差异不显著。

图1 镉胁迫下BABA对烟草幼苗长势(A)、根长(B)、鲜重(C)及叶绿素(D)的影响Fig.1 Effects of BABA-pretreatment on seedlings' growth (A), root length (B), fresh weight(C) and chlorophyll content (D) under Cd stress
2.2 BABA对镉胁迫下烟草幼苗MDA和H2O2含量的影响
由图2A可知,镉胁迫组烟草幼苗中MDA、H2O2含量较对照组增加了2.2倍、1.2倍。经0.2 mmol/L、0.5 mmol/LBABA预处理的烟草幼苗MDA含量较镉胁迫组分别降低了30.56 %、32.37 %。而其H2O2含量较镉胁迫照组分别降低了26.20 %、39.18 %(图2B)。该结果表明,BABA预处理可以缓解镉胁迫引起的氧化胁迫对烟草幼苗的损害。

图2 BABA对镉胁迫下烟苗MDA和H2O2含量的影响Fig.2 Effects of BABA on MDA and H2O2 contents in the tobacco seedlings under Cd stress
2.3 BABA对镉胁迫下烟草幼苗抗氧化物质及抗氧化酶的影响
由图3A可知, 0.2 mmol/L、0.5 mmol/L BABA预处理组GSH含量较镉胁迫组分别提高了60.39 %、94.64 %。AsA可以直接清除单线态氧(1O2)和O2-和·OH[24]。由图3B可知,镉胁迫下烟草幼苗AsA含量增加。0.2 mmol/L、0.5 mmol/L BABA预处理组AsA含量较镉胁迫组分别提高了16.81 %、8.15 %。
由图3C可知,镉胁迫下烟草幼苗总酚含量大量积累。0.2 mmol/L、0.5 mmol/LBABA预处理的烟草幼苗总酚含量较镉胁迫组含量分别提高了9.76 %、10.78 %。证实BABA可能诱导总酚的积累来抵御镉胁迫引起的氧化损伤。
由图3D可知,0.2 mmol/L、0.5 mmol/L BABA预处理的烟草幼苗可溶性糖含量较镉胁迫组分别提高了1.77 %、8.62 %,具有显著性差异。由图3E可知,脯氨酸含量较镉胁迫组分别提高了281.58 %、225.55 %。结果表明,镉胁迫下BABA能够诱导烟草幼苗可溶性糖和脯氨酸的积累,调节渗透平衡,保护细胞结构和功能的完整性。

图3 BABA对镉胁迫下烟苗GSH(A), AsA(B),总酚(C),可溶性糖(D)和脯氨酸(E)含量的影响Fig.3 Effects of BABA-pretreatment on GSH (A), AsA (B), total phenolic(C), soluble sugar (D) and proline (E) content in the tobacco seedlings under Cd stress
由图4A可知,在镉胁迫下,镉胁迫组烟草幼苗体内的SOD活性较对照组降低了16.46 %,而经0.2 mmol/L、0.5 mmol/L BABA预处理的烟草幼苗体内的SOD活性较镉胁迫组分别提高了41.37 %、44.86 %。由图4D可知,镉胁迫下烟草幼苗体内的POD活性显著增加。而经0.2 mmol/L、0.5 mmol/L BABA预处理的烟草幼苗体内POD活性较镉胁迫组分别提高了65.09 %、27.82 %。由图4B可知,经0.2 mmol/L、0.5 mmol/L BABA预处理的烟草幼苗体内CAT活性较镉胁迫组分别提高了100.89 %、32.64 %。由图4C可知,经0.2 mmol/L、0.5 mmol/L BABA预处理的烟草幼苗体内APX活性较镉胁迫组分别提高了38.76 %、60.77 %。
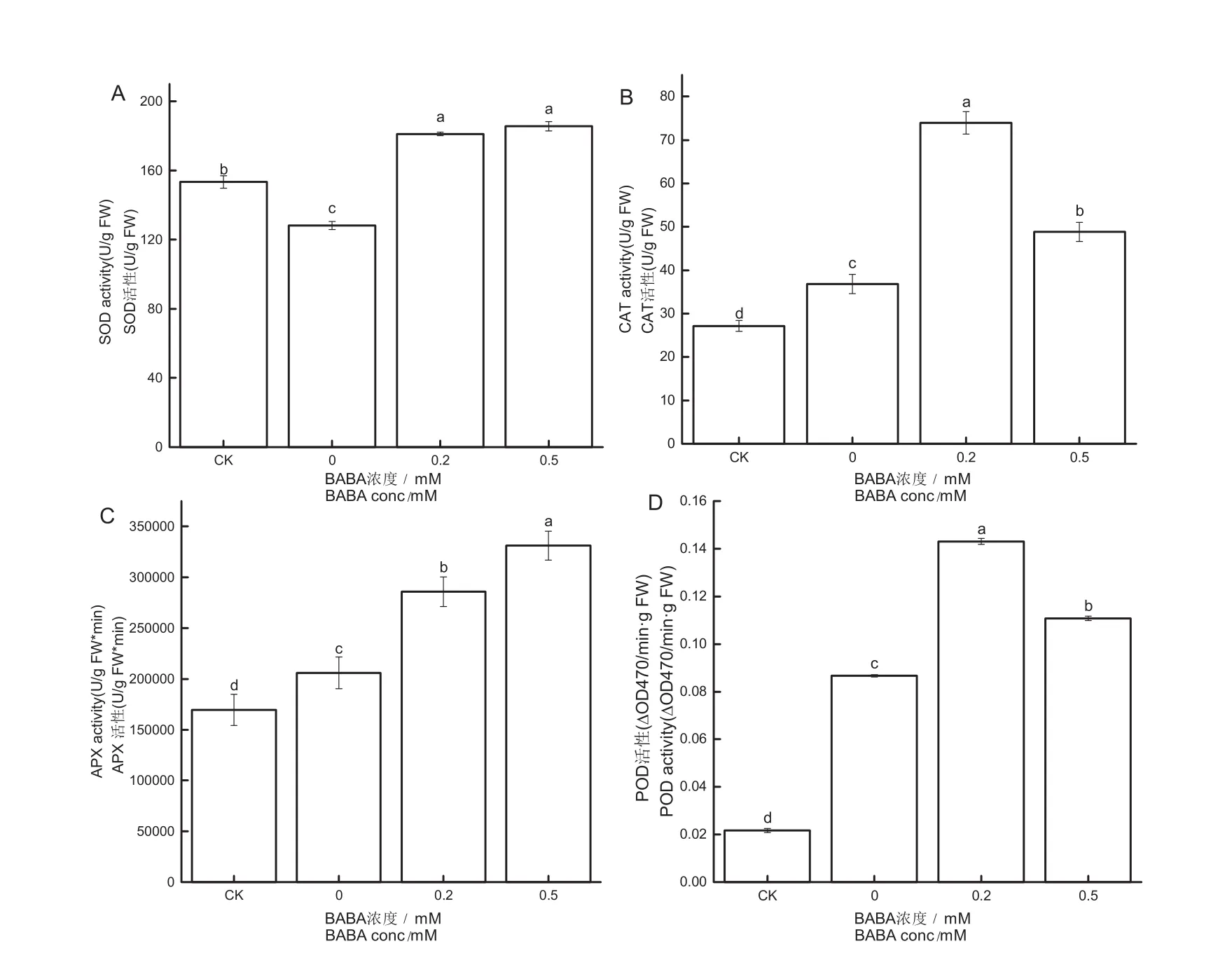
图4 BABA对镉胁迫烟草幼苗SOD(A),CAT(B),APX(C),POD(D)活力的影响Fig.4 Effects of BABA-pretreatment on SOD (A), CAT (B), APX(C), POD (D) activity in the tobacco seedlings under Cd stress
2.4 BABA对镉胁迫下镉吸收转运及解毒基因表达的影响
由图5A可知,0.2 mmol/L、0.5 mmol/L BABA预处理组NtIRT1基因表达量较镉胁迫组分别提高了36.4倍、61.74倍。NtIRT1基因大量上调表明BABA预处理不仅促进烟草幼苗对铁的吸收,同时也促进其对镉的积累。由图5B可知,0.2 mmol/L、0.5 mmol/L BABA处理组NtNramp5基因表达量较镉胁迫组分别提高了6.68倍、4.0倍。这表明BABA可能通过NtNramp5上调来调节镉的转运,造成地上部镉含量较根部低。
由图5C可知,BABA预处理组NtPCS1较镉胁迫组均出现下调,分别降低了23.69 %和22.72 %。而由图5D可知,经0.2 mmol/L、0.5 mmol/L BABA预处理,烟草幼苗NtGSH1基因较镉胁迫组分别提高了0.78倍和9.73倍。
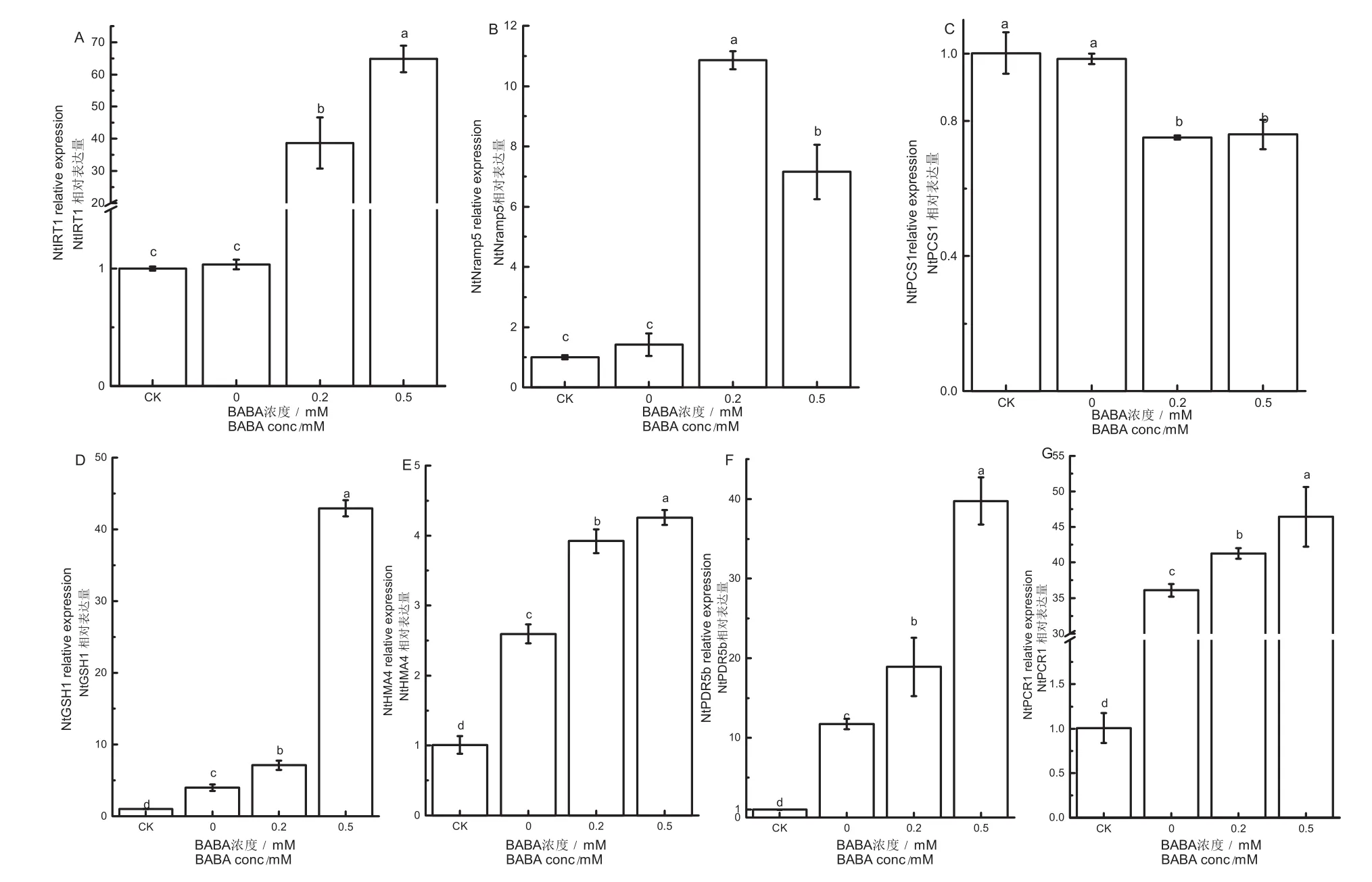
图5 BABA对镉胁迫下烟苗NtIRT1(A),NtNramp5(B),NtPCS1(C),NtGSH1(D),NtHMA4(E),NtPDR5b(F),NtPCR1(G)基因相对表达量的影响Fig.5 Effects of BABA on gene expression of NtIRT1(A), NtNramp5(B), NtPCS1(C), NtGSH1(D), NtHMA4(E), NtPDR5b(F), NtPCR1(G)in the tobacco seedlings under Cd stress
由图5E可知,0.2 mmol/L、0.5 mmol/L BABA处理组NtHMA4基因表达量较镉胁迫组分别提高了0.51倍、0.64倍。其中,拟南芥中AtPDR8经同源比对后在烟草中为NtPDR5b。由图6F可知,0.2 mmol/L、0.5 mmol/L BABA处理组NtPCR1基因表达量较对照组分别提高了0.14倍、0.29倍;由图5G可知,0.2 mmol/L、0.5 mmol/L BABA处理组NtPDR5b基因表达量较对照组分别提高了0.61倍、2.39倍。
2.5 BABA对镉胁迫下烟苗镉和铁含量的影响

图6 BABA对镉胁迫下烟苗镉和铁含量的影响Fig.6 Effects of BABA-pretreatment on Cd and Fe contents in the tobacco seedlings under Cd stress
由图6可知,对照组烟草幼苗地上部未检测出镉。镉胁迫下,烟草幼苗根部镉含量高于地上部镉含量。研究发现,0.2 mmol/L、0.5 mmol/L BABA预处理组烟草幼苗总镉含量较镉胁迫组分别提高了28.71 %、7.85 %;其中,根部镉含量分别提高了47.64 %、20.83 %,而地上部镉含量分别降低了17.14 %、23.60 %。同时,研究还发现,0.2 mmol/L、0.5 mmol/L BABA预处理的烟草幼苗总Fe含量分别提高了16.92 %、20.97 %。以上结果表明,BABA增强了根部对镉的积累,降低地上部镉的含量,促进了Fe的吸收。
3 讨论
镉胁迫下,烟草幼苗H2O2大量累积,脂质过氧化加剧,植株生长缓慢。SOD、POD、CAT和APX是四种重要的抗氧化酶[25]。其中,SOD是唯一可以清除的抗氧化酶[26],其能将转化为 H2O2,再由POD、CAT、APX将过量H2O2转化为H2O。BABA预处理组烟草幼苗抗氧化酶SOD、POD、CAT和APX的活性增强,GSH、AsA和总酚等抗氧化物质含量增加,可溶性糖、脯氨酸等渗透调剂物质含量增加,说明烟草幼苗通过增强抗氧化酶活性、抗氧化物质含量和提高渗透调节物质含量来缓解镉胁迫引起的膜脂过氧化,降低MDA和H2O2含量,从而较好的维持细胞膜的稳定性,提高对镉的抗性。
另一方面,镉胁迫下,植物体相关基因的表达对镉离子吸收转运及解毒起到重要作用。GSH和植物螯合肽(PC)由于其巯基部分对金属有强亲和力,可充当金属螯合剂,提高植物对重金属的抗性并解除重金属对植物的毒害[26]。镉胁迫下,植物螯合肽合成酶(PCS1)可以GSH为底物催化PC合成。本文中,NtGSH1上调且GSH含量显著增加,但NtPCS1出现下调。Sangman Lee等人[27]指出AtPCS1过表达会导致转基因植物对镉的超敏性,添加GSH后超敏性消失。NtHMA4、NtPCR1、NtPDR5b上调将镉排出细胞体,以降低植物体镉的含量。有研究指出烟草中AtHMA4异位表达上调会抑制根部镉向地上部迁移[28],NtNramp5上调也限制镉向地上部转运,这可能是BABA预处理组地上部镉含量较少的原因。图6中BABA预处理组根部镉含量增加,这可能与细胞壁中半纤维素含量增加有关[29],具体原因仍待进一步研究。
4 结论
研究发现,外源施加0.2 mmol/L和0.5 mmol/L BABA能有效改善烟草幼苗不良生长状况。BABA诱导烟草幼苗抵御镉胁迫,一方面是通过增加烟草体内脯氨酸、可溶性糖、总酚、叶绿素、GSH含量,另一方面是增强烟草幼苗抗氧化酶SOD、POD、CAT、APX活性,有效降低过量产生的H2O2。促进根部镉的积累,降低地上部镉的含量以减轻毒害。此外,BABA还参与调节烟草幼苗金属吸收转运(NtIRT1、NtNramp5、NtHMA4、NtPDR5b、NtPCR1)及金属解毒(NtGSH1、NtPCS1)基因的表达。BABA缓解烟草幼苗镉胁迫可能是激发植物体内抗氧化系统、抑制地上部镉的累积和调节金属吸收转运及解毒基因表达的综合结果。
[1] Huamain C, Chunrong Z, Cong T, et al. Heavy Metal Pollution in Soils in China: Status and Countermeasures [J]. Ambio, 1999, 28(2): 130-134.
[2] Zhao Zhongqiu, Yong Guanzhu,Yun Longcai. Transport and transformation of cadmium in soil-plant systems and the influence factors [J]. Ecol Environ, 2005, 14(2): 282-286.
[3] Sanita di Toppi L, Gabbrielli R. Response to cadmium in higher plants[J]. Environmental and Experimental Botany, 1999, 41(2): 105-130.
[4] Azevedo R A, Gratão P L, Monteiro C C, et al. What is new in the research on cadmium-induced stress in plants? [J]. Food and Energy Security, 2012, 1(2): 133-140.
[5] Cuypers A, Karen S, Jos R, et al. The cellular redox state as a modulator in cadmium and copper responses in Arabidopsis thaliana seedlings [J]. Journal of plant physiology, 2011, 168(4): 309-316.
[6] Gallego S M, Pena L B, Barcia R A, et al. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms [J].Environmental and Experimental Botany, 2012, 83:33-46.
[7] 马新明,李春明,袁祖丽,等. 镉和铅污染对烤烟根区土壤微生物及烟叶品质的影响 [J]. 应用生态学报, 2015, 16(11): 2182-2186.Ma Xinming, Li Chunming, Yuan Zuli, et al. Impacts of Cd and Pb pollution on soil microbes in tobacco root zone and on tobacco leaf quality][J]. Chinese Journal of Applied Ecology, 2005, 16(11): 2182-2186. (in Chinese)
[8] 郭江波,唐炳,王建英,等. 镉胁迫对烟草生理特性的影响 [J].浙江农业学报, 2013, 25(6): 1279-1283.Guo Jiangbo,Tang Bing,Wang Jianying,et al. Influence of cadmium stress on physiological indexes in tobacco[J].Acta Agriculture Zhejiangensis,2013,25(6): 1279-1283. (in Chinese)
[9] Ji H, Kyndt T, He W, et al. β-aminobutyric acid-induced resistance against root-knot nematodes in rice is based on increased basal defence[J]. Mol Plant-Microbe Interact, 2015, 28(5): 519-533.
[10] Hamiduzzaman M M, Jakab G, Barnavon L, et al. β-Aminobutyric Acid-Induced Resistance Against Downy Mildew in Grapevine Acts Through the Potentiation of Callose Formation and Jasmonic Acid Signaling [J]. Mol Plant-Microbe Interact, 2005, 18(8): 819-829.
[11] Siegrist J, Orober M, Buchenauer H. Aminobutyric acid-mediated enhancement of resistance in tobacco to tobacco mosaic virus depends on the accumulation of salicylic acid [J]. Physiological and Molecular Plant Pathology, 2000, 56(3): 95-106.
[12] Cao S Q, Ren G, Jiang L, et al. The role of beta-aminobutyric acid in enhancing cadmium tolerance in Arabidopsis thaliana [J]. Russian Journal of Plant Physiology, 2009, 56(4): 575-579.
[13] 朱奎正,彭耀东,陈祝,等. β-氨基丁酸对铜胁迫下烟草生长的影响 [J]. 烟草科技, 2015, 48(4): 7-12.Zhu Kuizheng,Peng Yaodong,Chen Zhu,et al. Effects of β-aminobutyric acid on tobacco growth under copper stress[J].Tobacco Science & Technology,2015,48(4):7 -12 . (in Chinese)
[14] 张清莉,刘再强,钟玉德,等. BABA 诱导烟草抵御高盐胁迫的初步研究 [J]. 中国烟草学报, 2015, 21(3): 72-81.Zhang Qingli,Liu Zaiqiang,Zhong Yude, et al. A preliminary study on BABA-induced resistance to high salt stress in tobacco [J] Acta Tabacaria Sinica, 2015, 21(3):72-81. (in Chinese)
[15] 杨欣,陈江华,张艳玲. 烟草对镉的吸收及控制措施研究综述 [J].中国烟草科学, 2010, 31(2): 70-75.Yang Xin, Chen Jianghua, Zhang Yanling. A review on cadmium uptake of tobacco and its control [J]. Chinese tobacco science, 2010,31(2): 70-75. (in Chinese)
[16] 朱广廉. 植物生理学.植物生理学实验 [M]. 北京: 北京大学出版社, 1990.Zhu Guanglian. Plant physiology [M]. BeiJing: Peking University Press,1990. (in Chinese)
[17] Michael P I, Krishnaswamy M. The effect of zinc stress combined with high irradiance stress on membrane damage and antioxidative response in bean seedlings [J]. Environmental and experimental botany, 2011,74:171-177.
[18] Stewart R R C, Bewley J D. Lipid peroxidation associated with accelerated aging of soybean axes [J]. Plant physiology, 1980, 65(2):245-248.
[19] 邹奇. 植物生理学实验指导 [M]. 北京:中国农业出版社. 2000.Zou Qi. Plant physiology experimental guidance [M].BeiJing; China Agriculture Press.2000. (in Chinese)
[20] Tawaha K, Alali F Q, Gharaibeh M, et al. Antioxidant activity and total phenolic content of selected Jordanian plant species [J]. Food Chemistry, 2007, 104(4): 1372-1378.
[21] Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbatespecific peroxidase in spinach chloroplasts [J]. Plant and cell physiology, 1981, 22(5): 867-880.
[22] Ramakrishna B, Rao S S R. 24-Epibrassinolide alleviated zinc-induced oxidative stress in radish (Raphanus sativus L.) seedlings by enhancing antioxidative system [J]. Plant Growth Regulation, 2012, 68(2): 249-259.
[23] Bewley J D. Physiological aspects of desiccation tolerance-a retrospect[J]. International Journal of Plant Sciences, 1995, 393-403.
[24] Ahmad P, Sarwat M, Sharma S. Reactive oxygen species, antioxidants and signaling in plants[J]. Journal of Plant Biology, 2008, 51(3): 167-173.
[25] Dubey R S. Metal toxicity, oxidative stress and antioxidative defense system in plants [J]. Reactive oxygen species and antioxidants in higher plants, 2010:177-233.
[26] Lin Y F, Aarts M G M. The molecular mechanism of zinc and cadmium stress response in plants [J]. Cellular and molecular life sciences ,2012, 69(19): 3187-3206.
[27] Lee S, Moon J S, Ko T S, et al. Overexpression of Arabidopsis phytochelatin synthase paradoxically leads to hypersensitivity to cadmium stress [J]. Plant physiology, 2003, 131(2): 656-663.
[28] Siemianowski O, Barabasz A, Kendziorek M, et al. AtHMA4 expression in tobacco reduces Cd accumulation due to the induction of the apoplastic barrier [J]. Journal of Experimental Botany, 2014, 65(4):1125-1139.
[29] Zhu Xiaofang, Wang Zhiwei, Dong Fang, et al. Exogenous auxin alleviates cadmium toxicity in Arabidopsis thaliana by stimulating synthesis of hemicellulose 1 and increasing the cadmium fixation capacity of root cell walls [J]. Journal of hazardous materials, 2013,263 :398-403.
A preliminary study on BABA-induced resistance to cadmium stress of tobacco
LIU Song1, GUO Jiaming1, HE Kuanxin2, YOU Benwu1, XIAO Xianyi2,CHEN Xueping1
1 Tobacco and Health Research Center, University of Science and Technology of China, Hefei 230051, China;2 Jiangxi Provincial Tobacco Company, Nanchang 330025, China
β–aminobutyric acid (BABA) is a potent inducer of resistance, which can be used against a wide range of biotic and abiotic stresses in plants. The present study highlighted the protective role of BABA in alleviating cadmium (Cd) stress of tobacco and attempted to explore the mechanism through measuring anti-oxidative molecular content, anti-oxidative enzyme activities, concentration of Cd and Fe and also related gene expression. Results indicated that both 0.2 mmol/L BABA and 0.5 mmol/L BABA could effectively improve plant length, fresh weight and chlorophyll content than Cd treatment alone. Pretreatment of BABA not only increased accumulation of proline,GSH, AsA, soluble sugar and total phenolic, but also markedly stimulated activities of superoxide dismutases (SOD), ascorbate peroxidases(APX), peroxidase (POD) and catalases (CAT) compared with Cd treatment alone, thereby enhancing anti-oxidative capacity in leaves under Cd stress. BABA also partly counteracted Cd toxicity by reducing H2O2and MDA contents of Cd -exposed seedlings. Moreover,BABA significantly increased Cd content in roots, but reduced Cd content in shoots. In addition, related gene expression indicated that BABA took part in the regulation of the transcript levels of NtIRT1, NtNramp5, NtGSH1, NtPCS1, NtHMA4, NtPDR5b, and NtPCR1. It was concluded that BABA pretreatment helped plants to combat Cd stress by activating antioxidant system, restricting Cd content in shoot as well as modulating related gene expression to protect cells from Cd induced oxidative stress damages.
β-aminobutyric acid; tobacco; Cd stress
刘松,郭家明,何宽信,等. BABA诱导烟草抵御镉胁迫初步研究[J]. 中国烟草学报,2016,22(3)
江西省烟草公司“提高烟草抗逆性的新型调节物质研制及应用”(赣2011.01.001号)和“江西省烟区烟叶品质定位及其区域划分研究”(赣2011.01.002号)项目经费资助。
刘 松(1990—),硕士,主要研究方向:植物抗逆性研究, Email:uliusong@ mail. ustc. edu. cn
陈学平(1956—),博士,教授,主要研究方向:植物生物技术及遗传改良, Email: chenxp08@ ustc. edu. cn
2015-09-24
:LIU Song, GUO Jiaming, HE Kuanxin, et al. A preliminary study on BABA-induced resistance to cadmium stress of tobacco[J].Acta Tabacaria Sinica, 2016,22(3)
