环氧化酶基因多态性及交互作用与脑梗死患者阿司匹林抵抗相关性
2016-10-14易兴阳周强范真林静刘平成文
易兴阳 周强 范真 林静 刘平 成文
环氧化酶基因多态性及交互作用与脑梗死患者阿司匹林抵抗相关性
易兴阳周强范真林静刘平成文
目的探讨脑梗死患者阿司匹林抵抗(AR)发生率,环氧化酶(COX)基因多态性及其间交互作用与AR相关性。方法收集2009-08—2011-08在温州医科大学第三附属医院和德阳市人民医院住院就诊的634例急性脑梗死患者,于入院当天开始服用阿司匹林,7~10 d后检测血小板聚集率,筛选出AR者及阿司匹林敏感(AS)者。采用质谱法对患者COX-1和COX-2 共4个基因位点多态性进行检测。采用广义多因子降维法(GMDR)分析多基因位点交互作用。多因素Logistic回归分析AR发生的独立危险因素。结果634例脑梗死患者发生AR者129例(20.35%),半抵抗(ASR)者28例(4.42%),AS者477例(75.23%)。AR组和AS组间COX-1和COX-2各基因位点基因型分布差异无统计学意义(P>0.05);GMDR分析显示,COX-1和COX-2基因存在交互作用,最优模型为rs3842787和rs20417两个基因位点的联合作用模型,交叉检验一致性为10/10,符号检验P=0.0116。糖尿病(OR=2.16, 95%CI:1.25~4.67,P<0.01)、rs3842787和rs20417高风险交互(OR=2.51, 95%CI:1.38~5.96,P<0.01)为发生AR的独立危险因素。结论中国脑梗死患者AR发生率高,rs3842787和rs20417联合交互作用可能增加了AR风险,对基因与基因间的交互作用分析有助于深入研究AR的机制。
阿司匹林抵抗;脑梗死;环氧化酶;基因多态性;广义多因子降维法
阿司匹林是脑梗死急性期治疗和二级预防主要药物之一,能明显降低心脑血管事件和死亡风险[1-2],得到国内外指南的广泛推荐[3-4]。但部分坚持服用阿司匹林的患者,其血小板聚集程度仍不能得到很好的抑制,不能避免缺血事件的发生,称为阿司匹林抵抗(aspirin resistance,AR)[5]。既往研究表明,脑梗死患者AR发生率高,AR与随访期脑梗死复发和其他血管事件相关[6-7]。AR发生机制复杂,目前尚未完全阐明。基因机制,特别是环氧化酶(cyclooxygenase,COX)基因多态性与AR相关性是目前研究的热点[7-8],但研究结果存在分歧[7,9-11]。AR基因机制复杂,多个基因位点参与,目前有关基因-基因交互作用与AR相关性的研究较少。本研究探讨了COX-1、COX-2基因4个位点多态性及其交互作用与脑梗死患者AR的相关性,旨在更好地指导脑梗死进行二级预防。
1 对象和方法
1.1研究对象连续纳入2009-08—2011-08首次发病72 h内入住作者医院的脑梗死患者,全部病例均经头MRI扫描证实,病因学分型[12]为动脉粥样硬化血栓形成型(atherothrombosis,AT)及小动脉病变型(small artery disease,SAD)患者。排除标准:(1)对阿司匹林过敏者;(2)病因不明型、其他少见病因型脑梗死以及心源性脑栓塞;(3)接受溶栓治疗或近1周内有使用除阿司匹林外其他抗血小板药、低分子肝素、华法林等药物;(4)有家族或个人出血疾病史;(5)血小板计数> 450×109/L 或<100×109/L;(6)骨髓增生异常综合征及其他血液系统疾病;(7)近期行较大外科手术或有严重外伤者;(8)伴严重心、肝、肾疾病者;(9)阿司匹林联用其他抗血小板药物者;(10)既往有心肌梗死和脑卒中病史者。所有研究对象知情同意并签署知情同意书。符合上述标准患者共634例,其中女302例,男332例,年龄45~85岁,平均(69.62±10.45)岁。
1.2方法
1.2.1资料收集:所有入组患者接受基于指南的治疗[3-4],入院当天即服用阿司匹林200 mg,每晚顿服,2周后改用100 mg,每晚顿服维持治疗。记录患者一般情况和传统危险因素(包括既往病史、个人史、吸烟、酗酒情况,有无高血压、糖尿病、冠心病、脑卒中史等)。入院后次日抽空腹静脉血检查血常规、凝血常规、生化常规、血脂全套等,并留置静脉血2 mL,用乙二胺四乙酸二钠盐抗凝处理,-80℃保存备检基因多态性。
1.2.2血小板聚集率测定和AR判断:采用光学比浊法检测血小板聚集率,具体步骤按参考文献[6-7]操作。服用阿司匹林后第7~10天采空腹肘静脉血6 mL,注入含3.8%(质量浓度)枸橼酸钠660 μL试管中,将新鲜血标本以223.6g离心10 min,提取富含血小板血浆,剩余血液再以894.4g离心10 min,制备乏血小板血浆,用乏血小板血浆做空白对照,进行不同诱导剂〔二磷酸腺苷(ADP)10.0 μmol/ L和花生四烯酸(AA)0.5 mg / mL〕血小板聚集实验,记录最大血小板聚集率。采用Gum等[13]和作者既往研究[6-7]所采用的标准进行AR判断:服用阿司匹林后第7~10天,在10 μmol/L ADP浓度下血小板最大聚集率≥70%;在0.5 mg/mL AA浓度下血小板最大聚集率≥20%。同时符合上述2项标准者称为AR,符合其中1项标准者为阿司匹林半反应 (aspirin semiresponde,ASR),反之为阿司匹林敏感(aspirin sensitivity,AS)。
1.2.3COX-1基因和COX-2基因多态性的检测:(1)研究位点的选择:参考国内外相关研究位点,并登录NCBI数据库(http://www.ncbi.nlm.nih.gov/SNP)最新登记的各基因人类SNP中的COX基因,设置最小等位基因频率≥0.05,获得标签SNP,即为本研究基因位点。据此本研究检测COX-1 rs1236913、rs3842787和COX-2 rs689466、rs20417共4个位点基因型。(2)检测方法:上述留置乙二胺四乙酸二钠抗凝血2 mL,采用低渗溶血、酚氯仿法抽提DNA。通过PCR扩增第1个靶序列,然后加入特异性延伸产物与扩增的片段SNPs连接。PCR引物和单碱基延伸引物均使用Assay Designer软件包设计,引物序列见表1。采用基质辅助激光解析电离飞行时间质谱法(上海生物工程有限公司)对基因位点进行基因分型,具体试验过程参见参考文献[14]。
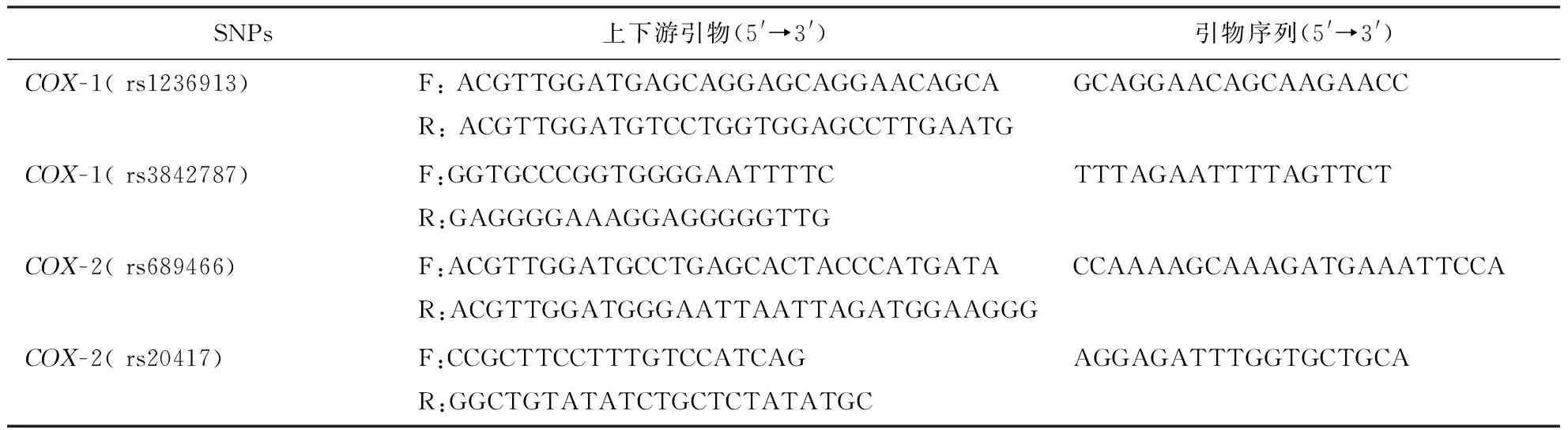
表1 脑梗死患者COX-1基因和COX-2基因多态性PCR检测的引物序列表
注:COX:环氧化酶,表3、4同;SNPs:单核苷酸多态性
1.3 统计学处理采用SPSS16.0统计软件包进行统计分析,采用χ2检验分析基因型频率的哈迪-温伯格平衡偏倚;计量资料用均数±标准差表示,组间比较采用t检验;计数资料用率表示,组间比较采用χ2检验;采用Logistic回归分析AR发生的危险因素;采用广义多因子降维法(generalized multifactor dimensionality reduction,GMDR)Beta 0.7版软件 (www.healthsystem.virginia.edu/internet/addiction-genomics/Software)分析多基因位点交互作用[14]。以P<0.05为差异有统计学意义。
2 结果
2.1AR发生率634例脑梗死患者发生AR 129例(20.35%),ASR 28例(4.42%),AS者477例(75.23%)。因ASR者少且有研究认为ASR与AR有共同危险因素和预后[6],故将AR与ASR合并为AR组,共157例(24.76%)。AR组女性和糖尿病患者多于AS组(P<0.05,P<0.01),低密度脂蛋白胆固醇水平高于AS组(P<0.01),而年龄、体重指数等其他因素比较差异无统计学意义(P>0.05)。具体结果见表2。
2.2COX-1和COX-2基因多态性与AR的相关性单基因分析发现,COX-1和COX-2各基因位点基因型在AR组和AS组比较差异无统计学意义(表3)。
2.3COX-1和COX-2基因交互作用与AR相关性GMDR分析显示,COX-1基因和COX-2基因存在交互作用,最优模型为rs3842787和rs20417两个基因位点的联合作用模型,交叉检验一致性为10/10,符号检验P=0.0116(表4)。将rs3842787和rs20417两个基因位点共9种基因型组合进行分析发现,与AR发生风险高的组合为rs3842787CT+ rs20417CC和rs3842787CT+ rs20417GC。具体结果见表4。

表2 AS组与AR组脑梗死患者入院时一般情况和危险因素比较
注:AS:阿司匹林敏感,AR:阿司匹林抵抗,表3同;NIHSS:美国国立卫生研究院卒中量表;AT:动脉粥样硬化血栓形成型;SAD:小动脉病变型

表3 脑梗死AS和AR组基因型分布比较〔n(%)〕

表4 脑梗死患者COX基因-基因交互作用与阿司匹林抵抗的GMDR分析
注:1~4分别代表rs3842787、rs20417、rs2269231、rs1236913、rs689466

表5 影响脑梗死患者AR的多因素Logistic回归分析
注:a表示rs3842787和rs20417高风险交互
2.4AR危险因素分析将rs3842787和rs20417两个基因位点共9种基因型组合设为交互变量,分为高风险(赋值1)和低风险(赋值0)。将单因素分析有统计学意义的因素,包括女性、糖尿病、高低密度脂蛋白、高空腹血糖、交互变量作为自变量,AR作为因变量进行多因素Logistic回归分析,结果显示,糖尿病以及rs3842787和rs20417高风险交互为AR发生独立危险因素(表5)。
3 讨论
国内外研究表明,AR发生率为5%~65%[15],高加索和亚裔人群AR发生率高,而欧美人群发生率低[16],采用不同评估方法所得AR发生率也存在差异性,光学比浊法所得AR发生率为10.3%~51.7%,血小板功能分析仪-100(PFA-100)分析所得AR发生率为59.5%,尿血栓素B2评估法为22.9%[17]。本组634例脑梗死患者发生AR者129例(20.35%),ASR者28例(4.42%),糖尿病、高低密度脂蛋白与AR相关。这与国内外其他学者研究结果一致[5,8-9]。高低密度脂蛋白、高血糖能促使血小板和单核细胞活化,增加体内血小板和单核细胞间的黏附,可导致AR的发生[18]。因此,积极控制高脂血症、高血糖等危险因素,将有助于降低AR的发生率。
阿司匹林通过不可逆地乙酰化COX活性部位的529位丝氨酸,抑制血栓素A2合成,发挥抗血小板聚集作用。因此,COX基因多态性与AR相关性是近来研究的热点。COX基因在中国人群突变率低[7],在高加索人群中突变率高,直接影响高加索人群对服用阿司匹林的效果[19]。Fan等[9]和Sharma等[10]研究表明,COX基因多态性与AR相关。然而对日本人群和中国人群研究没有发现COX多态性与AR有关联[7,11]。本研究单基因分析发现COX-1和COX-2 基因4个位点基因型在AR组和AS组分布差异无统计学意义,但GMDR分析显示COX-1基因和COX-2基因存在交互作用,最优模型为rs3842787和rs20417两个基因位点的联合作用模型,这2个基因位点高风险交互为AR发生独立危险因素,提示单个基因位点可能对AR影响小甚至检测不到,但多个弱势位点的联合作用可能为AR的主要诱因。rs3842787和rs20417两个基因位点交互作用,可能归因于这两个基因位点均参与了花生四烯酸COX通路的代谢[20]。花生四烯酸在COX作用下生成血栓素A2,血栓素A2具有强烈血管收缩和促血小板聚集作用[21]。rs3842787和rs20417两个基因位点交互作用可能影响阿司匹林对COX的抑制作用,从而影响阿司匹林抗血小板聚集效果。
综上所述,中国人群AR发生率高,单基因分析COX-1基因和COX-2基因与AR发生无相关性,但GMDR分析表明,COX-1基因和COX-2基因高风险交互作用为AR发生的独立危险因素。AR基因机制复杂,研究基因-基因交互作用与AR相关性可能是今后研究重点和发展趋势。
[1]CAST (Chinese Acute Stroke Trial) Collaborative Group. CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke[J]. Lancet, 1997, 349 (9066):1641-1649.
[2]International Stroke Trial Collaborative Group. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke[J]. Lancet, 1997, 349 (9065):1569-1581.
[3]Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association[J]. Stroke, 2014, 45(7):2160-2236.
[4]中华医学会神经病学分会脑血管病学组.中国急性缺血性脑卒中诊治指南2014[J].中华神经科杂志, 2015, 48(4):246-257.
[5]Dretzke J, Riley RD, Lordkipanidzé M, et al. The prognostic utility of tests of platelet function for the detection of aspirin resistance in patients with established cardiovascular or cerebrovascular disease: a systematic review and economic evaluation[J]. Health Technol Assess, 2015,19(37):1-366.
[6]Yi XY, Zhou Q, Lin J, et al.Aspirin resistance in Chinese stroke patients increased the rate of recurrent stroke and other vascular events[J]. Int J Stroke, 2013,8(7):535-539.
[7]Yi XY, Zhou Q, Lin J, et al. Platelet response to aspirin in Chinese stroke patients is independent of genetic polymorphisms of COX-1 C50T and COX-2 G765C[J]. J Atheroscler Thromb, 2013, 20(1):65-72.
[8]Xu ZH, Jiao JR, Yang R, et al. Aspirin resistance: clinical significance and genetic polymorphism[J]. J Int Med Res, 2012, 40(1):282-292.
[9]Fan L, Cao J, Liu L, et al. Frequency, risk factors, prognosis, and genetic polymorphism of the cyclooxygenase-1 gene for aspirin resistance in elderly Chinese patients with cardiovascular disease[J]. Gerontology, 2013, 59(2):122-131.
[10]Sharma V, Kaul S, Al-Hazzani A, et al. Association of COX-2 rs20417 with aspirin resistance [J]. J Thromb Thrombolysis, 2013, 35(1):95-99.
[11]Takahashi S1, Ushida M, Komine R, et al. Platelet responsiveness to in vitro aspirin is independent of COX-1 and COX-2 protein levels and polymorphisms[J]. Thromb Res, 2008,121(4):509-517.
[12]Han SW,Kim SH,Lee JY,et al. A new subtype classification of ischemic stroke based on treatment and etiologic mechanism[J].Eur Neurol, 2007, 57(2):96-102.
[13]Gum PA, Kottke MK, Welsh PA, et al. A prospective, blinded determination of the natural history of aspirin resistance among stable patients with cardiovascular disease[J]. J Am Col Cardiol, 2003,41(6):961-965.
[14]Yi X, Zhang B, Wang C, et al. CYP2C8 rs17110453 and EPHX2 rs751141 two-locus interaction increases susceptibility to ischemic stroke[J].Gene, 2015, 565(1):85-89.
[15]Snoep JD, Hovens MM, Eikenboom JC, et al. Association of laboratory-defined aspirin resistance with a higher risk of recurrent cardiovascular events:a systematic review and meta-analysis[J]. Arch Intern Med, 2007,167(15):1593-1599.
[16]Feher G, Feher A, Pusch G, et al. Clinical importance of aspirin and clopidogrel resistance[J]. World J Cardiol, 2010,2(7):171-186.
[17]Lordkipanidzé M, Pharand C, Schampaert E, et al. A comparison of six major platelet function tests to determine the prevalence of aspirin resistance in patients with stable coronary artery disease[J]. Eur Heart J,2007,28(14):1702-1708.
[18]Andreas S, Daniela F, Martin E,et al. Rosuvastatin reduces platelet activation in heart failure role of NO bioavailability[J]. Arterioscler Thromb Vasc Biol, 2005,25(5):1071-1077.
[19]Halushka MK, Walker LP, Halushka PV. Genetic variation in cyclooxygenase1: effects on response to aspirin[J]. Clin Pharmacol Ther, 2003, 73(1): 122-130.
[20]Yi XY, Zhou Q, Lin J, Chi LF, Chi WZ. The interaction between ALOX5AP-SG13S114A/T and COX-2-765G/C increases susceptibility to cerebral infarction in a Chinese population[J].Genet Mol Res, 2013,12(2):1660-1669.
[21]Rink C, Khanna S. Significance of brain tissue oxygenation and the arachidonic acid cascade in stroke[J]. Antioxid Redox Signal, 2011,14(10):1889-1903.
(本文编辑:时秋宽)
The correlation between cyclooxygenase genetic polymorphisms and their interactions with aspirin resistance
YIXingyang*,ZHOUQiang,FANZhen,LINJing,LIUPing,CHENGWen.
*DepartmentofNeurology,People’sHospitalofDeyangCity,DeyangSichuan618000,China
Corresponding author:YI Xingyang,Email:yixingyang64@126.com
ObjectiveTo investigate the prevalence of aspirin resistance (AR) in patients with cerebral infarction, and the correlation between cyclooxygenase(COX) genetic polymorphisms and their interaction with AR. MethodsWe prospectively enrolled 634 patients with cerebral infarction in the Third Affiliated Hospital of Wenzhou Medical University and People’s Hospital of Deyang City from Aug 2009 to Aug 2011. Aspirin was administrated to every patient from the first day of admission. Platelet aggregation testing was performed after 7-10 days of aspirin administration to screen the patients with AR or aspirin sensitive (AS). COX-1(rs1236913,rs3842787) and COX-2(rs689466, rs20417) genetic polymorphisms were measured by using mass spectrometry. Gene-gene interactions were analyzed by using generalized multifactor dimensionality reduction (GMDR) analysis. Logistic regression was performed to find independent risk factors of AR. ResultsAmong 634 patients,AR was detected in 129 patients (20.35%), aspirin semi-resistance (ASR) was detected in 28 patients (4.42%), and AS was detected in 477 patients (75.23%). There were no significant differences in the genotype distributions of the 4 genetic polymorphisms between the AR group and AS group by using single-locus analytical approach. However, the GMDR analysis showed a significant gene-gene interaction among rs3842787 and rs20417, and scored 10 for Cross-Validation Consistency and 9 for Sign Test (P= 0.0116). Diabetes mellitus (OR=2.16, 95%CI: 1.25-4.67,P<0.01), and the gene-gene interaction among rs3842787 and rs20417 (OR=2.51, 95%CI: 1.38-5.96,P<0.01) were independent risk factors of AR. ConclusionsThe incidence of AR is high in Chinese patients with cerebral infarction. The gene-gene interaction among rs3842787 and rs20417 may confer higher risk for AR. The combinatorial analysis used in this study may be helpful to elucidate mechanisms for AR.
aspirin resistance; cerebral infarction; cyclooxygenase; genetic polymorphisms; generalized multifactor dimensionality reduction
10.3969/j.issn.1006-2963.2016.05.010
成都中医药科大学校基金资助项目(YYZX1510)
618000 德阳市人民医院神经内科(易兴阳、范真、刘平、成文);325200 温州医科大学附属第三医院神经内科(周强、林静)
易兴阳,Email:yixingyang64@126.com
R379.41文献识别码:A
1006-2963(2016)05-0351-06
2016-03-18)
