铀酰-Salophen受体对α,β-不饱和羰基化合物及手性客体的分子识别
2016-09-13高莎兰文波林英武廖力夫聂长明
高莎 兰文波 林英武 廖力夫 聂长明
(南华大学化学化工学院,湖南衡阳421001)
铀酰-Salophen受体对α,β-不饱和羰基化合物及手性客体的分子识别
高莎兰文波林英武廖力夫聂长明*
(南华大学化学化工学院,湖南衡阳421001)
基于密度泛函理论(DFT)的计算方法,研究了不对称铀酰-sa lophen受体对α,β-不饱和羰基化合物客体及手性小分子的分子识别。理论计算结果表明:配合物中受体的U原子与客体的O3原子配位,且受体与客体之间结合能随受体上芳环取代基的增大而增大;R2,R3-系列配合物中U―O3键的稳定性比R1-系列的更强;配位后的α,β-不饱和羰基化合物中C=C与C=O之间的共轭效应减弱。而且,通过圆二色谱(CD)及结合能计算表明:芘基铀酰-sa lophen(受体3)对(R)-1-(2-萘基)乙胺的分子识别选择性优于(S)-1-(2-萘基)乙胺。因而,这些研究结果为不对称铀酰-salophens具有分子识别能力提供了新的信息。
密度泛函理论;铀酰-salophen;α,β-不饱和羰基化合物;分子识别
Besides,Dalla Cort etal.8used nonsymmetrically substituted uranyl-salophensas receptors and found that the receptors could producemolecular recognition9-12for aldehydes and/or ketones. With the inherent chirality of the nonsymmetrically substituted uranyl-salophens,these complexes could thus have potential applications.Inaddition,molecular recognitionsof chiralhosts for chiralguestswere studied bymany authors.For example,Liu et al.13studied themolecular recognition behavior of the chiralsalenmetalhosts towardsguestmolecules,and found that the circular dichroism(CD)spectrum method could beused to characterize the strength of thehost-guest interaction.According to Zhou etal.14the absolute configurations of chiralmolecules could be obtained by the CD spectrum calculations,which were consistentw ith the X-ray single-crystalstructure.
Despite theseadvances,themoleculargeometriesof1-3 uranylsalophen receptors and the coordination mode betw een these receptors and guests have not been reported in theory15,16.A lso, there are no attem pts to study themolecular recognitions of the asymmetrical uranyl-salophens for chiral molecules by CD spectrum calculations.Meanwhile,it is well-known that theoretical investigation can provide theoretical guidance for experiments.Therefore,in thisstudywe selected three typical receptors and corresponding compounds as samples,as shown in Fig.1,and theoretically studied theabovemeaningfulproblems.
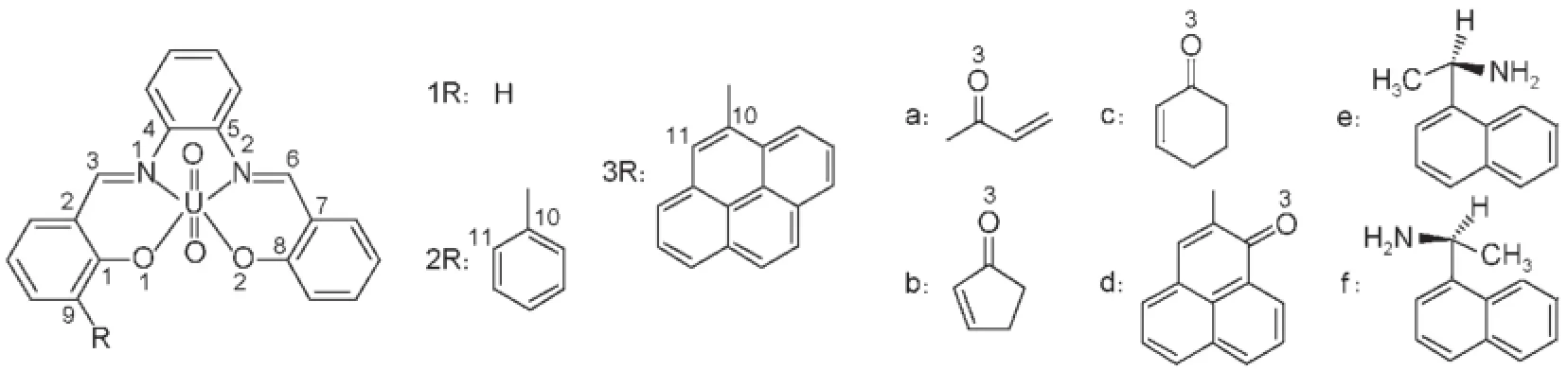
Fig.1 Molecu lar structural form ulasand the num bers ofm ajor atom s

Fig.2 Geometriesof the three optim ized uranyl-salophen recep tors
2 Computational details
All the calculations were performed using the Gaussian 09 software17.A ll geometrieswere optim ized using B3LYP18,19of density functional theory(DFT)method20,21.Relativistic effective core potential(RECP)22basis setwas taken into account for U atom.Forotheratoms(C,H,O,N),the 6-311G(d,p)basisset23was used.It had been studied24-26that this level of theoretical method isappropriate for lanthanide and actinide complexes to get better results of geometriesand energetics.Then theWiberg bond order indices(WBIs)27of the coordination complexeswere calculated using naturalbond orbital(NBO)analysis28,29.Based on the method of the basis setsuperposition error(BSSE)correction,the binding energies ofα,β-unsaturated carbonyl compounds w ith uranyl-salophen receptorswere also calculated.In addition,the ultraviolet-visible(UV-Vis)spectrum was calculated using B3LYP method of the time-dependentDFT(TD-DFT)30,and CD31analysis was carried outw ith theMultiw fn32program.
3 Results and discussion
3.1Geom etricalstruc tures o f recep tors
At the B3LYP/6-311G(d,p)/RECP level of theory,the geometrical structuresand the harmonic vibrational frequencies of the threeuranyl-salophen receptorswere calculated,respectively.The results showed that the normalmodes of vibrations were not imaginary frequencies in these three uranyl-salophen receptors, suggesting that the geometrical configurations of the three receptorswere situated at them inimum pointof potential energy surface.The optimized geometrical structures and a partof the structural parametersof the three receptorswere shown in Fig.2 and listed in Table 1.Firstly,the calculated C2―C1―O1―U dihedral angles/C7―C8―O2―U dihedral angles in the three receptorswere 35.81°/-35.83°,38.26°/-36.56°,41.14°/-36.30°, respectively.Thesedata indicated thatthe twowingsof theuranylsalophenswere sim ilar to thoseof birds and theuranyl-salpohen moleculesw ere distorted in the plane.The average bond lengthsof the U―N,U―O,and U=O in the receptorswere consistent with theexperimental resultsvery well33.The carbon-carbon bond lengths of thebenzene rings in the three kindsof receptorswere 0.1365-0.1435nm.With the enlargementof thearomaticwalls, the N 1―U―N 2 bond-anglesbecame smaller,the O1―U―O2 bond-anglesbecame larger,butall the N1―U―O1 and N2―U―O2 bond-angles gotsmaller.For the uranyl-salophen receptors2 and 3,the dihedral angles between the benzene ring of salicylaldehyde and its unilateral aromatic group were-47.56°,-113.26°,respectively.The N1―O1―O2―N2dihedralangelof receptor1wasalmostclose to zero,showing thatN1,O1,O2,N2 were situated at the same plane;but after introduction of the aromaticwalls,the N1―O1―O2―N2dihedral angelsof receptors 2 and 3 equaled-0.23°and-0.55°,respectively.TheO1―O2―N2―U and O2―O1―N1―U dihedralangelsof three receptors did notequal zero,indicating thatN1,O1,O2,N2,and U were not situated at the same planes.Indeed,the salophens in coordination complexes adopted twisted plane structures1.Note that the geometriesof the threeuranyl-salophensdisplayed the C1symmetry.

Table1 A par t of the structuralparam eters of three uranyl-salophen receptors

Fig.3 Geometriesof the optim ized com plexes
3.2Coordinationm ode o f urany l-salophens w ithα,βunsatu rated carbonyl com pounds
3.2.1Geometricalstructures o f coordination com plexes
At the same level of theory,the geometrical structures of a seriesof coordination complexeswereexplored.The coordination complexes(R1-series)1,2,3,4were formed by the receptor 1 coordinated with theα,β-unsaturated carbonyl compoundsa,b,c, d.In the same way,the complexes(R2-series)5,6,7,8 were produced by the receptor 2 coordinated with a,b,c,d,and the complexes(R3-series)9,10,11,12 w ere formed by the receptor 3 coordinated w ith a,b,c,d,respectively.Some of optimalgeometric configurations of receptors coordinated withα,β-unsaturated carbonyl compound guestswere shown in Fig.3.Itcould be seen that theguestswere located insideof the cleftof receptors in the coordination complexes.The planes of theunilateralaromatic wallswere almost parallel to the planes ofα,β-unsaturated carbonyl compounds in R2-seriesand R3-series complexes.Parts of themolecular geometry parameters of comp lexesw ere listed in Table 2.The simulation results show ed that theC2―C1―O1―U dihedralangelsand C7―C8―O2―U dihedralangelsof all the coordination complexeswere from 38°to 43°.The N―U bond lengths and O―U bond lengths in complexeswere longer thanthose in corresponding receptors,and the N1―U―N2 bondangles became smaller,whereas the O1―U―O2 bond-angles became larger,w hich suggests that after receptors coordinated withα,β-unsaturated carbonyl compounds,the coordination interactionsbetween N1,O1 atomsof receptors and U atom were weakened.

Table2 A partof the structuralparametersof the com plexes
On the other hand,for the sameα,β-unsaturated carbonyl compound guest,w ith the expanding of the unilateral aromatic walls in complexes,the O3―U bond lengthswere shortened, indicating that the coordination of O3 to the U atom weremore stable,which was consistentw ith the literature34.The dihedral angles in the coordination complexeswere obviously changed com pared to the corresponding receptors,especially for the C1―C9―C10―C11 dihedral angles in complexes thatwere smaller than those in corresponding receptors.In all the R2-series,R3-series comp lexes,the unilateral aromatic groups could provide stronger van derWaals interactionsand largerπ-πstacking to the guests.In addition,the unilateral aromatic walls of the uranylsalophen receptors 2,3 were served as steric blocking groups, which played an important role in the reactions with the asymmetric uranyl-salophen receptorsacting asasymmetric catalyst. 3.2.2 IR spec trum
The calculated IR spectra of the three receptors and corresponding complexes3,7,11were shown in Fig.4and Fig.5,respectively.The calculatedmajor IR absorptions of the receptors 1,2,3 and the corresponding complexes 3,7,11 were listed in Table 3.The results showed the stretching frequencies of C=N and C―O of the com plexeswere close to those of corresponding receptors.The complexes had absorption peaks in 923-929 cm-1but thecorresponding receptorsappeared in 938-940cm-1,which w as assigned to the asymmetric stretching vibration absorption peak of U=O in UO22+.The reasonmay be thatafter the U atom of the receptors coordinated by O3 atom ofguests,the U=O bond weakened,resulting in red-shiftsof vibration absorption for U=O.Themain absorption peaks of the comp lexesmoved towards the low wavelength compared to the corresponding receptors,such that the stretching frequency of C=O in cyclohexenone shifted from 1762 to 1693 cm-1and that of C=C moved from 1674to ~1665cm-1.These observations indicated that the conjugation effect betw een C=O and C=C inα,β-unsaturated carbonyl compoundswasweakened after the carbonyloxygens coordinated by U atom of the receptors.Experimental studies also demonstrated that the urany l-salophen receptors show ed good catalytic activity for the conjugateaddition of cyclohexenonewith other molecules7.Wealso calculated theother two complexesand found that,for the other two guests,the rules of the stretching frequencieswere sim ilar to theabove complexes.

Fig.4IR spectra of receptors1,2,3
3.2.3Mo lecularorbitals
The MOs(molecule orbitals)of severalmolecules were calculated,and Fig.6show ed Kohn-Sham representations of HOMO (the highest occupied molecular orbital),LUMO(the lowestunoccupied molecular orbital).In Fig.6,each a small parts of molecular orbitalswere com posed by the positive and negative aspectsand they were parallelwith each other.From Fig.6(left), the HOMO of uranyl was the 5f5z3-3zr2orbital,which was the hybridized orbital of the uranium combined w ith the other four atoms(O1,O2,N1,N2).But it was obviously changed when cyclohexenonewas coordinated by receptor3,asshown in Fig.6(right).It could prove that there existed bonding interaction between the carbonyl oxygen of cyclohexenone and the uranium atom.A partof occupiedmolecularorbitalsof the complex 11was shown in Fig.7.The occupiedmolecular orbitalof theunilateral aromatic wallwas almost paralleled to the occupiedmolecular orbitalof cyclohexenone.Moreover,the positive aspectof theπ bonding orbital of the unilateralaromatic wallwas faced to the negativeaspectof thebonding orbitalof the cyclohexenone.The distance between the two planes was 0.375nm.Therefore,unilateral aromatic wall in the complexes displayed certain steric hindrance effecton the conjugate addition of cyclohexenonew ith othermolecules,whichm ightbe themain reason for the chiral addition ofα,β-unsaturated carbonyl compoundsw ith reagents catalyzed by unilateralaromatic substituted uranyl-salophen.

Fig.5IR spectra for com p lexes 3,7,11

Tab le 3 Major IR data of the three receptorscoordinated w ith cyclohexenone

Fig.7 A partofoccupiedmolecular orbitalsof com plex 11
3.2.4Electronic structu re
Asobtained by Mulliken population calculations,a part of the netatomic chargesof three kindsof receptorsand three complexes 4,8,12were listed in Table4.The complexeswere formed by the three receptors coordinatingwith perinaphthenone.Electric dipole moment(p),polarizability(α),and the totalenergy(E)werealso listed in Table 4.The unitof E iseV and p is C∙m.The positive charges of U atom and negative chargesof O1 atom in the three complexesweremore than thoseof the corresponding receptors. The positive chargesof C3 atom and negative chargeof N1 atom of the three complexeswere less than ones of the corresponding receptors.The negative charges of O3 atom s of the three complexeswere-0.464e,-0.501e,-0.504e,respectively,whereas in the perinaphthenone itwas-0.362e.The electric dipolemomentsand the polarizabilitiesof three complexeswere larger than those of corresponding receptors.These changesmaymainly be attributed to the coordination between O3 and U atom.TheO3 atom offers the lone pairelectron to theU atom tomakeelectronic structure changed.

Fig.6HOMOsof recep tor 3 and com plex 11
3.2.5Bond orderand binding energy
In order to better understand themetal-ligand bonding and to explain the coordination effect between the uranyl-salophen receptorsand theα,β-unsaturated carbonyl compounds,theWiberg bond order indices(WBIs)of the coordination complexeswere calculated using NBO analysis,and based on themethod of the basis set superposition error(BSSE)correction,the binding energiesofα,β-unsaturated carbonyl com pound guestsw ith uranylsalophen receptorswere also calculated.The resultsshowed thetrendsof theWBIsvaluesof the U―O3 bonds for the complexes in Fig.8.Itcould be seen that the U―O3WBIswere found to be 0.350-0.450in all the studied com plexes,thus the bonding between themetalU atomsand ligandsO atomshasa certain degree of covalent character.In addition,the U―O3 WBIs in the asymmetrically substituted uranyl-salophen complexesare higher than those in the symmetrically uranyl-salophen complexes,which indicated that,for the sameα,β-unsaturated carbonyl com pound, the U―O3 bonds of all the R2-series,R3-series complexeshave more covalentcharacters than thoseof R1-series complexes.The probable reasonsmay be that theunilateral substituted aromatic groups could have the van derWaals interaction andπ-πstacking effectson theα,β-unsaturated carbonyl compound.In addition,the U―O3WBIs of the complexes 9-12 were slightly larger than corresponding complexes5-8,whichwas in accordancewith the change rules of their U―O3 bond lengths.In other words,the shorter the U―O3 bond length,the larger the U―O3WBIs,the more the covalent character of U―O3 would be.With the same receptor,the U―O3WBIs of the R-series complexes became larger from the guests a to d,and the increase of contact area between the hostsand the guestsprobably leads to theenhanced intermolecular forces.

Table4Calculated atom icMulliken popu lation(P)and the per formance before and after uranyl-salophens coordinatingw ith perinaph thenone

Fig.8 Trendsof theWBIs of the U―O 3 bonds for the coordination com plexes
The bonding energy w as calculated by the formula(1).W(R)represents thebonding energy andstands for the totalenergy of the interacting systemandstand for theenergiesof the two separatemoleculesA,B,respectively.

Thebinding energiesof the receptors1,2and 3 coordinated by the fourα,β-unsaturated carbonyl compoundsa,b,c and dwere listed in Table 5.Therewasa general tendency for the binding energy to become larger and larger from receptors 1,2 to 3.W hen the hydrogen atom of receptor 1was replaced by phenyl or pyrenyl, the van derWaals interaction andπ-πstacking effectbetween the receptorsand theguestswould become stronger,whichmakes the guests′degreesof freedom lowerand the receptors combinedwith the guestmore tightly.Consequently,the binding energy ofα,βunsaturated carbonyl compounds has an order of receptor 3> receptor2>receptor1.

Tab le 5Binding energies of 1-12 com p lexes

Fig.9 UV spectra of receptor 3
3.3Mo lecu lar recogn ition o f u ranyl-sa lophen to chira lmo lecu le
As illustrated in Fig.9(curve:a),w hich show s the UV-Vis spectrum of pyrenyl uranyl-salophen receptor 3.There were characteristic absorption peaks around 320and 350nm,which wereassigned to theπ-π*transition of the benzene ringsand the π-π*transition of the C=N,respectively.In the corresponding CD spectraof uranyl-salophen receptor3,as shown in Fig.10(b), the CD absorption displayed a positive Cotton effect(315nm)and a negative Cotton effect(324nm)centered at320nm.Simultaneously,there w ere a positive Cotton effect(337 nm)and a negative Cotton effect(352 nm)centered at350nm.In UV-Vis spectrum,therewas characteristic absorption peak at~393 nm.It could beattributed to the n-π*transition of the lone pairelectrons of the nitrogen atom in C=N.In the corresponding CD spectra, the CD absorption displaysa positive Cotton effect(372 nm)and anegative Cotton effect(410nm)centered at393 nm.From the Cotton effect splitting patterns in the CD spectra,it could be seen that the positive Cotton effect was reflected in the shorter wavelength,meanwhile,the negative Cotton effectwas reflectedin the longer wavelength.From these observations,it could probably deduce that the absolute configuration of uranyl-salophen derivatives is.

Fig.10CD spectra of threemolecu les
Fig.10shows the CD spectraof theuranyl-salophen receptor3 (curve:b)and the complexes13 and 14(curves:c and d)thatwere formed by the coordination between uranyl-salophen receptor3 and two typicalchiralsmallmoleculeseand f(Fig.1),i.e.,(R)-1-(2-n-aphthy)ethylam ine and(S)-1-(2-naphthy)-ethy lam ine,respectively.Itcould be seen that therewere large differencesbetween the CD spectra of the host3 and the CD spectra of complexes 13 and 14.In the CD spectra of host 3,there were the splitting peaks around 320nm,but therewere not the splitting peaks in the CD spectra of complexes13 and 14.It could reflect thatwhen the different guestswere introduced to the host 3,it would have the same influence on theπ-π*transition of the benzene ring.As shown in Fig.10,there was a negative Cotton effect absorption peak around 320nm in the CD spectra of complex 14.However,there was a positive Cotton effect absorption peak in the CD spectra of complex 13.In 340-350nm, the positive Cotton effectabsorption peak value of complex 14was+122 L∙mol-1∙cm-1,but thatof the complex 13 was+438 L∙mol-1∙cm-1and thehost3was+273 L∙mol-1∙cm-1.Furthermore, in 350-360nm,thenegative Cotton effectabsorption peak value of complex 14was-103 L∙mol-1∙cm-1and the complex 13was-358L∙mol-1∙cm-1.TherewasapositiveCottoneffectabsorption peak around 500nm in the CD spectra of complex 14.However, there was a negative Cotton effect absorption peak in the CD spectraof complex 13.
In addition,we calculated the binding energies between the receptor3 and theguesteand f,whichare87.755and 78.160kJ∙mol-1,respectively.Besides,in the CD spectra,the higher the peak of absorption around the same wavelength,the better the recognition selectivity will be13.Theseobservations indicated that the host3 hasa very high recognition selectivity towards the guest(R)-1-(2-naphthy)ethylamine,which is well coincided with the intensity sequence of the CD band in Fig.10.
4Conclusions
Based on the theoretical studies,the following conclusions could bemade.The U atom of the receptors coordinated by the O3 atom ofα,β-unsaturated carbonyl compounds,theα,β-unsaturated carbonyl compoundsw ere located in the inside of the cleft of the uranyl-salophens in the coordination complexes.Compared with corresponding receptors,the bond lengths of N―U and O―U were shortened,the N1―U―N2 bond anglesbecame smallerand O1―U―O2 bond angles became bigger.The calculated IR spectra,electronic structures,andmolecular orbitals indicated that the conjugation effects between C=C and C=O inα,β-unsaturated carbonyl compounds in the coordination complexeswere weakened.Besides,thebond orderanalysesshowed that the U―O3 bonding in complexes had a certain covalent character.In addition,the binding energies ofα,β-unsaturated carbony l compoundsw ith uranyl-salophenswere found to increase with expanding of thearomaticwalls in complexes.The calculated UVVis spectraand CD spectraaswellas thebinding energy further indicated that the recognition selectivity of the uranyl-salophen receptor 3 for the(R)-1-(2-naphthy)ethylam ine was better than that for the(S)-1-(2-naphthy)ethylamine.Therefore,this study threw a new lighton the recognition ability of the uranyl-salophens,which provides clues for design of functional uranyl-salophen complexes in experiments.
References
(1)Zhang,G.L.;Liao,L.F.;Lin,Y.W.;Yang,M.;Xiao,X.L.; Nie,C.M.Anal.Chim.Acta 2013,784,47.doi:10.1016/j. aca.2013.05.002
(2)Zhao,M.M.;Liao,L.F.;Wu,M.L.;Lin,Y.W.;Xiao,X.L.; Nie,C.M.Biosens.Bioelectron.2012,34(1),106.doi: 10.1016/j.bios.2012.01.025.
(3)Matsumoto,K.;Watanabe,A.;Uchida,T.;Ogi,K.;Katsuki,T. Tetrahedron Lett.2004,45(11),2385.doi:10.1016/j. tetlet.2004.01.095
(4)Ohashi,M.;Koshiyama,T.;Ueno,T.;Yanase,M.;Fujii,H.; Watanabe,Y.Angew.Chem.Int.Edit.2003,42(9),1005.doi: 10.1002/anie.200390256
(5)Mirkhani,V.;Tangestaninejad,S.;Moghadam,M.;Moghbel, M.Bioorg.Med.Chem.2004,12(17),4673.doi:10.1016/j. bmc.2004.06.029
(6)Serrette,A.;Carroll,P.J.;Swager,T.M.J.Am.Chem.Soc. 1992,114,1887.doi:10.1021/ja00031a057
(7)Castelli,V.V.;Dalla Cort,A.;Mandolini,L.;Pinto,V.; Reinhoudt,D.N.;Ribaudo,F.;Sanna,C.;Schiaffino,L.; Snellink-Ruël,B.H.M.Supramol.Chem.2002,14(2),211. doi:10.1080/10610270290026112
(8)Dalla Cort,A.;Pasquini,C.;Schiaffino,L.Supramol.Chem. 2007,19(1),79.doi:10.1080/10610270600977714
(9)Shen,X.;Liao,L.F.;Chen,L.;He,Y.F.;Xu,C.H.;Xiao,X. L.;Lin,Y.W.;Nie,C.M.Spectrochim.Acta PartA 2014,123, 110.doi:10.1016/j.saa.2013.12.026
(10)Bodo,E.;Ciavardini,A.;Cort,A.D.;Giannicchi,I.;Yafteh Mihan,F.;Fornarini,S.;Vasile,S.;Scuderi,D.;Piccirillo,S. Chem.Eur.J.2014,20(37),11783.doi:10.1002/chem.201402788
(11)Cametti,M.;Nissinen,M.;Cort,A.D.;Mandolini,L.; Rissanen,K.J.Am.Chem.Soc.2005,127(11),3831.doi: 10.1021/ja042807n
(12)Yang,M.;Liao,L.F.;Zhang,G.L.;Xiao,X.L.;Lin,Y.W.; Nie,C.M.Anal.Bioanal.Chem.2013,405(23),7545.doi: 10.1007/s00216-013-7217-2
(13)Liu,T.;Ruan,W.J.;Nan,J.;Zhu,Z.A.Chin.J.Chem.2003, 21,751.doi:10.1002/cjoc.20030210709
(14)Zhou,N.;Wan,S.G.;Zhao,J.;Lin,Y.J.;Xuan,W.M.;Fang, X.M.;Zhang,H.Sci.China Ser.B 2009,52(11),1851.doi: 10.1007/s11426-009-0261-2
(15)Lombardo,G.M.;Thompson,A.L.;Ballistreri,F.P.; Pappalardo,A.;Sfrazzetto,G.T.;Tomaselli,G.A.;Toscano, R.M.;Punzo,F.Dalton Trans.2012,41(7),1951.doi: 10.1039/C1DT11758K
(16)Mary,Y.S.;Rajub,K.;Panickerc,C.Y.;Al-Saadid,A.A.; Thiemann,T.;Christian,V.A.Spectrochim.Acta PartA 2014, 128,638.doi:10.1016/j.saa.2014.02.194
(17)Frisch,M.J.;Trucks,G.W.;Schlegel,H.B.;etal.Gaussian 09,Revision A.01;Gaussian Inc.:Wallingford,Conn,USA, 2009.
(18)Lee,C.;Yang,W.;Parr,R.G.Phys.Rev.B 1988,37(2),785. doi:10.1103/PhysRevB.37.785
(19)Becke,A.D.J.Chem.Phys.1993,98(7),5648.doi:10.1063/ 1.464913
(20)Elkechai,A.;Mani,Y.;Boucekkine,A.;Ephritikhine,M. Inorg.Chem.2012,51(12),6943.doi:10.1021/ic300811m
(21)Kohn,W.;Sham,L.J.Phys.Rev.1965,140(4A),1133.doi: 10.1103/PhysRev.140.A1133
(22)Gu,J.F.;Lu,C.H.;Chen,W.K.;Chen,Y.;Xu,K.;Huang,X.; Zhang,Y.F.Acta Phys.-Chim.Sin.2012,28(4),792.[辜家芳,陆春海,陈文凯,陈勇,许可,黄昕,章永凡.物理化学学报,2012,28(4),792.]doi:10.3866/PKU. WHXB201201171
(23)Rawat,N.;Bhattacharyya,A.;Tomar,B.S.;Ghanty,T.K.; Manchanda,V.K.Thermochim.Acta 2011,518(1),111.doi: 10.1016/j.tca.2011.02.018
(24)Vetere,V.;Maldivi,P.;Adamo,C.J.Comput.Chem.2003,24(7),850.doi:10.1002/jcc.10228
(25)DiSanto,E.;Santos,M.;Michelini,M.C.;Marcalo,J.;Russo, N.;Gibson,J.K.J.Am.Chem.Soc.2011,133(6),1955.doi: 10.1021/ja109621n
(26)Michelini,M.D.C.;Russo,N.;Sicilia,E.J.Am.Chem.Soc. 2007,129(14),4229.doi:10.1021/ja065683i
(27)Luo,J.;Wang,C.Z.;Lan,J.H.;Wu,Q.Y.;Zhao,Y.L.;Chai, Z.F.;Nie,C.M.;Shi,W.Q.Dalton Trans.2015,44,3227. doi:10.1039/C4DT03321C
(28)Carpenter,J.E.;Weinhold,F.J.Mol.Struc.:Theochem 1988, 169,41.doi:10.1016/0166-1280(88)80248-3
(29)Foster,J.;Weinhold,F.J.Am.Chem.Soc.1980,102(24), 7211.doi:10.1021/ja00544a007
(30)Macka,J.;Otakib,T.;Durfeed,W.S.;Kobayashib,N.; Stillmanc,M.J.J.Inorg.Biochem.2014,136,122.doi: 10.1016/j.jinorgbio.2014.01.001
(31)Zhang,H.;Yan,J.X.;W u,S.T.;Li,D.;Wan,S.G.;D ing,L.; Lin,L.R.Acta Phys.-Chim.Sin.2013,29(12),2481.[章慧,颜建新,吴舒婷,李丹,万仕刚,丁雷,林丽榕.物理化学学报,2013,29(12),2481.]doi:10.3866/PKU. WHXB201310152
(32)Lu,T.;Chen,F.J.Comput.Chem.2012,33(5),580.doi: 10.1002/jcc.v33.5
(33)Asadi,Z.;Shorkaei,M.R.Spectrochim.Acta Part A 2013, 105,344.doi:10.1016/j.saa.2012.12.024
(34)Sessler,J.;Melfi,P.;Pantos,G.Coord.Chem.Rev.2006,250(7),816.doi:10.1016/j.ccr.2005.10.007
(35)Li,X.L.;Luo,J.;Lin,Y.W.;Liao,L.F.;Nie,C.M.J. Radioanal.Nucl.Chem.2016,307,407.doi:10.1007/s10967-015-4326-8
Molecular Recognition of α,β-Unsaturated Carbonyl Compounds and Chiral Guests by Uranyl-Salophen Receptors
GAO Sha LANWen-Bo LINYing-Wu LIAO Li-Fu NIEChang-Ming*
(SchoolofChemistry and Chemical Engineering,University ofSouth China,Hengyang 421001,Hunan Province,P.R.China)
Based on density functional theory(DFT)calculations,themolecular recognition ofα,β-unsaturated carbony lcom pounds and chiralmo lecules by u ranyl-sa lophen recepto rs was investigated theo retica lly.The results showed that the U atom of the receptorswas coordinated by the O3 atom of the guests,and the binding energies between receptors and guests increased with the enlargementof the aromatic substituentof the uranylsalophen receptors.In addition,the U―O3 coordination bonds of R2-and R3-series com p lexes aremore stable than those o f R1-series com p lexes,and the con jugation between the C=C and C=O bonds o f theα,βunsaturated carbonylcom pounds in the coordination com p lexes was weakened.Moreover,according to circular dichroism(CD)spectra and binding-energy calculations,themolecular-recognition selectivity ofan asymmetrical pyrenyluranyl-salophen(receptor3)for(R)-1-(2-naphthyl)ethylam ine wasmuch higher than that for(S)-1-(2-naph thyl)ethylam ine.These results shed new lighton the recognition ability ofasymme tric urany l-sa lophens.
Density functional theory;Uranyl-sa lophen;α,β-Unsaturated carbonylcom pound; Molecular recognition
1 Introduction
U ranyl ion(UO22+)could be coordinated by ligands such as tetradentate schiff derivatives to form a distorted plane structure, inwhich the two oxygen atomsof uranyl ion occupy the two axial positions of the bipyram idal geometry in the uranyl-salophen complex.Moreover,the uranium atom(U)could be coordinated by other ligands at the fifth coordination site1,2resulting in a pentagonal bipyramidal geometryw ith seven coordination atoms.Therefore,the uranyl-salophen complexeshad been found ahost of applications in molecular recognition and enantioselective catalysis3,such as in enzymemodelling4,5and liquid crystals6. Castelli etal.7found thaturanyl-salophen complex can serveasa chiral catalysis to catalyze the conjugate addition of thiophenol w ith cyclohexenone.They also showed thatwhen enlarging the aromatic substituents in the urany l-salophen receptors,as shown in Fig.1 and Fig.2(receptors1,2,and 3),theassociation constants of the receptorswith theguestswillbe increased,and the catalytic effectw ill also be enhanced.In otherwords,the catalytic effectof the uranyl-salophen complexes has an order of receptor 3>receptor 2>receptor 1.
November 10,2015;Revised:December 29,2015;Published on Web:December 30,2015.
O641
10.3866/PKU.WHXB201512302
*Corresponding author.Email:niecm196132@163.com;Tel:+86-13974753172.
The projectwas supported by theNationalNatural Science Foundation of China(11275090),Natural Science Foundation of Hunan Province,China (12JJ9006,2015JJ1012),and Scientific Research Fund of Hunan Provincial Education Department,China(12A 116).
国家自然科学基金(11275090),湖南省自然科学基金(12JJ9006,2015JJ1012)和湖南省教育厅科学研究基金(12A116)资助项目©Editorialofficeof Acta Physico-Chim ica Sinica
