方酸与2,6-二苯并咪唑的超分子自组装及氢键实验与理论研究
2016-09-13胡爱彬潘志权程清蓉周红
胡爱彬 潘志权 程清蓉 周红
(武汉工程大学化学与环境工程学院,武汉430073)
方酸与2,6-二苯并咪唑的超分子自组装及氢键实验与理论研究
胡爱彬潘志权程清蓉周红*
(武汉工程大学化学与环境工程学院,武汉430073)
在氘代的二甲基亚砜的溶剂中合成了方酸与2,6-二苯并咪唑的超分子的化合物,并用X射线单晶衍射对其结构进行了表征。晶体结构分析表明:超分子是通过π-π堆积和分子之间氢键所形成的一维链状的聚合物。探讨了不同温度和不同浓度CCl4溶剂对聚合物中氢键的影响。此外,用密度泛函理论和分子中原子理论对其进行了理论分析,计算结果表明分子间的键能分别是135.65和49.40J∙mol-1。
分子间氢键;变温红外;密度泛函理论;分子中原子理论
A squaric acidmolecule contains two ketonic oxygen atomsand two hydroxylgroups.It iseasy to form hydrogen bond w ith other molecules containing donor or acceptor atoms10-12.2,6-Bis(2-benzimidazolyl)pyridinemolecule contains two secondary amine and two tertiary am ine,which could actas donor and acceptor in the formation of hydrogen bond.When squaric acidmixswith 2, 6-bis(2-benzim idazolyl)pyridine in a suitable solvent,abundant hydrogen bonding interactionsshould be formed.With the purpose of understanding the hydrogen bond interactions between im idazole-based compound and squaric acid,the crystals derived from their m ixture in deuterated DMSO solution were obtained. Moreover,the essence of the hydrogen bonding w as revealed through the electron density.The strength comparison of three kindsof N―H…O hydrogen bondswasperformed and the reason was also analyzed.In thiswork,a new polymer derived from the supramolecular self-assembly of hydrogen bond was obtained and fully characterized.Moreover,the hydrogen bonding interaction was furtherevaluated by the computationalanalysis.
2 Experimental
2.1Mate ria ls and charac terization
Pyridine-2,6-dicarboxylic acid(99%),o-phenylenediamine (98%),and squaric acidwere purchased from Aladdin Industrial Corporation(China).Poly-phosphoric acid(PPA),methanol,and ethanol were analytic reagents and obtained from Sinopharm Chem ical Reagent Co.,Ltd.(China).Deuterated dimethylsulfoxide(d-DMSO,99.9%)was supplied by Cambridge Isotope Laboratories,Inc.(USA).
IR analysiswascarried outusing a Thermo Nicolet8700(USA) Fourier transform spectrometer fittedw ith aglobar source,aGe/ KBr beam splitter,and a DTGS detector.An IR spectrum in the region 400-4000cm-1was recorded ata resolution of4cm-1w ith 128 co-added scans,Happ-Genzelapodized,and Fourier transformed by applying a zero filling factor of 1.The cryostat is optically coupled to the spectrometer using KRS-5w indows.A ll solid-state sam pleswere studied using the KBrmethod.
Diffraction intensity datawereobtained usingaSMART-CCD (SMARTAPEXCCDⅡ,Germany)areadetectordiffractometerat 291KwithgraphitemonochromaticMo-Kαradiation(λ=0.071073 nm).Data reductionand cellrefinementwere carriedoutby SMART and SAINTPrograms13.The structureswere identified by direct methods(BrukerSHELXTL)and refinedon F2by full-matrix least squares(Bruker SHELXTL)using all unique data14.Thenon-H atom sin thestructurewere treatedasanisotropic.Hydrogen atoms were located geometrically and refined in ridingmode.
2.2Syn thes is o f the sup ram o lecu le(1)
Supramolecule1wassynthesized by the reaction of 2,6-bis(2-benzimidazyl)pyridine(L)w ith 1 equivalentof squaric acid in ethanolunder reflux for0.5h.The produced yellow crystalswere purified by recrystallization in methanol.A fter this,the samp le was dissolved in d-DMSO and them ixturewasmaintained for three days.Yellow crystals suitable for the X-ray diffraction measurementwere obtained.
2.3Com putationa ldetails
The initialstructure(IS)w asextracted from the crystalstructure of supramolecule1.Then ISwassubject to optim ization,energy calculation,andwavefunctionanalysis.We firsttried touseB3LYP, BLYP,and revPBE functionals tooptimize thegeometries,however, w e found that thehydroxylhydrogen atom of thecentermolecule transferred to thenitrogenatom opposite to it.Itcould beascribed to the strong hydrogen bonding aswell as the very low transfer barrier.We then tried thePM7method inMOPAC2012 package15,16, which isknown tobeable to reasonably dealw ithnoncovalentinteractions17.Since the resultof the PM7 optim ization was fully consistentwith theactualsituation,nohydrogen transferwasobserved,w e finally adopted the PM7optim ized geometry forall the follow inganalyses.Thecalculationofhydrogenbond energywas performedusing B3LYP-D3(BJ)functionalw ith very highquality basis-setdef2-QZVPP(-g,-f)18,19.ORCA 3.0.2 program20,21wasused to conduct thesingle-pointenergy calculation22,23.AIMtopology analysiswasperformedbyMultiw fn3.3.6program24-26,based on the BLYP/def2-SVPlevelofdensitygeneratedatthePM7geometry.
3 Results and discussion
3.1X-ray s tudy
A perspective view of themolecularunitof 1 is illustrated in Fig.1(a)as well as some atom numbering schemes.Crystallographicdataarepresented in Table1.Themolecularstructureof the supramolecular(1)contains two L,one squaric acid,and two d-DMSOmolecules.Themostreliablecriterionofhydrogen bonding (X―H…Y)formation relies on the van der Waals radius27.According to the previous reported examples,hydrogen bonding distancesbetween X and Yatomswereshorter than thesum of their respectivevan derWaals radii.Thevan derWaals radiiof theatoms, which arepossibly engaged,are0.155nm(N)and 0.152 nm(O)28. Therefore,the presenceof hydrogen bonding between N and O atomsmustbe considered when N…O distance is shorter than 0.307 nm.Due to van derWaals radiusofhydrogenatom ranging from 0.100to 0.120nm,thehydrogenbonding radiiofH…O and H…N are0.252-0.272nm and 0.255-0.275nm,respectively.
Methanolandethanolarethetwosolvents,whichwereused in the synthesisof1,however,therearenomethanolorethanolmolecules involved in the crystal structure,indicating thatmethanol and ethanolhavenohydrogenbonding interactionw itheithersquaric acidorLmolecules.Although themoleculestructureshows thatit containsd-DMSOmolecules,no hydrogenbonding involving d-DMSOexistsin thecrystalstructure.In themolecularunitof1,one squaric acid molecule acts as a bridge group to connect two L molecules through three typesofhydrogenbonding interactions,and twod-DMSOmoleculesarein the lattice(Fig.1(a)).Thesquaricacid moleculealso connectsw ith theadjacentother two Lmolecules through threehydrogenbonding interactions(Fig.1(b)).Onaccount of the symmetry of a squaric acid,the six hydrogen bonding inter-actionsassociatedwithonesquaricacid isgenerally thesameasthe counterparts.Four nitrogen atoms in benzimidazyl groups of L participatein thehydrogenbonding interactionswithsquaricacid, acting aseitheraprotondonororprotonacceptor.Thedistances in thehydrogenbonding interactionarein theregion0.176-0.201nm forH…O,0.115nm forH…N,and 0.265-0.302nm for N…O, alongw ith theangleof thehydrogen bond nomore than 170°.Selected bond lengthsand angles relevant to thehydrogen bonding interactions are listed in Table 2.The length and angle of the hydrogenbondsin1arecomparable to thosereported in literature29,30.
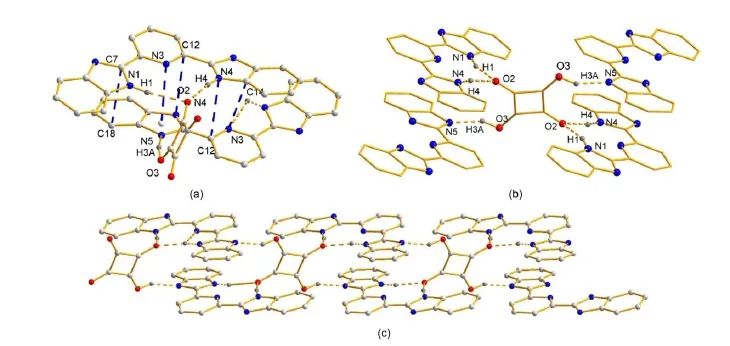
Fig.1(a)A perspectiveofmolecular unitof 1w ithπ-πstacking and hyd rogen bonding interactions,where DMSOmolecu lesareom itted for clarity,(b)the hyd rogen bonding interactionsbetween one squaric acid and four Lmolecules,(c)one dimensional chain polym er assemb led by hydrogen bond ing andπ-πstacking interactions

Table 1 Crystallographic data of supramolecu le 1
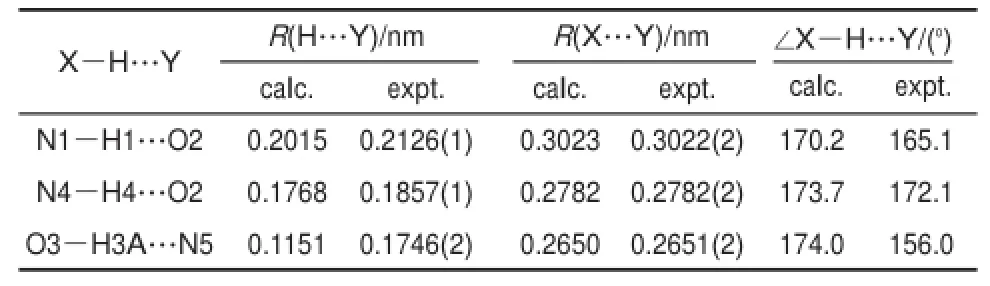
Tab le 2 Bond lengths(R)and angle of hyd rogen bond in 1
Considering the shorter H…O,H…N,and N…O distances betw een donorand acceptatom saswellas large hydrogen bond angles in 1,itcan be concluded that therearevery strong hydrogen bonding interactionsbetweenonesquaricacid and theadjacent four 2,6-bis(2-benzim idazyl)pyridinemolecules.In addition,thereare strongπ-πstacking interactionsbetween thearomaticgroupsin twoadjacentLunits,shown in Fig.1(a),N3and theplanecomposed of an im idazolegroup from theanother Lw ith the distanceof N3 and the centerof theplaneof0.32865nm;and three interactionsof two atoms from different L:C7―C18 0.33409 nm;C12―N40.33234nm,N3―C140.34001 nm,respectively.Thehydrogen bonding interactionsandπ-πstacking interactionsare thedriven force to form one-dimensionalchain(Fig.1(c)).
3.2Vib rationalspec tra ana lysis
Vibrational spectra of the supramolecular(1)at different temperatures and different sample concentrations in CCl4solvent are shown in Fig.2 and Fig.3.In Fig.2,the peaksat3343 and 3149 cm-1at25°C,which can be attributed to the stretching vibration of N-H,shift to 3352 and 3160cm-1at 90°C,respectively31. Additionally,the peak caused by the deformation vibration N-Hshifts from 1481 at 25°C to 1488 cm-1at90°C.The blue-shift caused by the increaseof the temperature can be ascribed to the decrease in thehydrogen bonding interactions32,33.As the temperature increases,the intensity of intermolecular hydrogen bond becomesweakerand the intensity of the related covalentN-H bond increases,resulting in the increasesof thebond energy and wavenumber.Fig.3 shows the IRspectraof thesample1at0.25and 0.50mg∙m L-1in CCl4,respectively.Thecharacteristicabsorption peak intensity increasesw ith the increase of concentration,w hich reflects theconcentration changeson the intensity of theabsorption peak.However,no changehasbeen found in thewavenumberof the characteristic peaksas theconcentrationof thesample increases, suggesting that there is no difference of hydrogen bonding interaction in samp lew ith low erorhigher concentration.
3.3Energy analysis fo r hyd rogen-bond
To clearly illustrate the interaction between the center squaric acid unitw ith theadjacent four2,6-bis(2-benzimidazyl)pyridine molecules,the assembly structure of 1 hasbeen divided into five parts,denoted asC,F1 to F4,shown in Fig.4.In this structure,C is the centermoleculewhile F1,F2,F3,and F4stand for four pieces.Hydrogen bonding energywas calculated using Eq.(1)34:

where E(F1-C)isthehydrogenbondingenergybetween F1and C,E(CF1)isthetotalenergyofCF1,E(C)and E(F1)aretheindividualenergiesof Cand F1,respectively.Thehydrogenbondingenergiesof thecenter moleculeand fouradjacentmolecules(F1-F4)are listed in Table3.

Fig.2 IR spectra of 1 at 25and 90°C
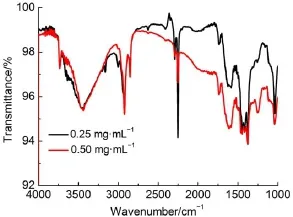
Fig.3 IR spectra of 1 in CCl4solventw ith the concentrationsof 0.25and 0.50mg∙m L-1

Fig.4Divided region diagram for the hyd rogen bonding interactionsofone squaric acid w ith the ad jacen t fou r Lm olecules in 1
Itcan be seen that theenergy valueof C-F1 issamew ith that of C-F3,so are thoseof C-F2 and C-F4,resulting from the symmetric system of 1.There are two O-H…N hydrogen bonds between C-F2and C-F4.Theaverageenergy ofhydrogenbond is 49.4kJ∙mol-1,which isaround 28.4kJ∙mol-1higher than thatof hydrogen bond in water dimer(21 kJ∙mol-1)35,suggesting the presenceofhydrogenbond.ThehydrogenbondingenergiesofCF1 and C-F3 reach up to 135kJ∙mol-1,which ismuch higher than that of the general hydrogen bonding.It could be attributed to strong hydrogen bonding w ith some covalentbond characteristics. The high hydrogen bonding energy can be assigned toπ-conjugation of thehydrogen bondingacceptoratom(N)in the fragment, w hich greatly increases theelectron density of theatom in the receptors.Itbelongs to chargeauxiliary hydrogenbonding(resonance assisted hydrogen bonding,RAHB)36,37.To verify theresults,BLYP/ def2-SVPwasused tooptimize CF1.Thecorrespondinghydrogen bonding energy calculated by Eq.(1)is 119 kJ∙mol-1.Ithas no significant difference from the results obtained from DFT calculation,indicating thatthecalculation resultsare reasonable.
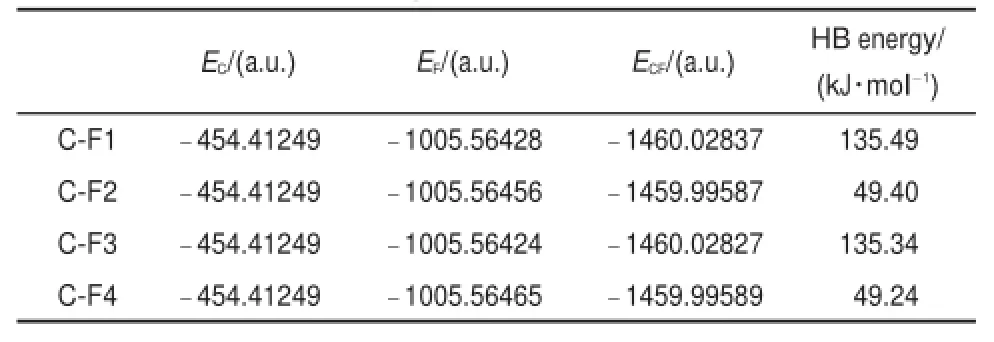
Table3 Hydrogen bond ing(HB)energies of the center m olecu le C and four ad jacentmolecules(F1-F4)
3.4Topo logica lana lys is o fe lec tronic dens ity
Topology analysiswas performed on thebasisof the dataobtained before using Multiw fn 3.3.6program.The topology analysis imagewasdrawn by VMD,presented in Fig.5.As shown in Fig.5,theorange yellow ball represents forbond criticalpoint (BCP),and the violet lines stand for bond path.It can be seen that the hydrogen bonds associated w ith the centermolecule have corresponding BCPand bond path.There is only one hydrogen bond in both C-F1 and C-F3,hence,itownsabond path.Both of C-F2 and C-F4have two hydrogen bonds,they have two bond paths.Besides,the center and the surroundingmolecules have other types of bond paths,such as the bond path between the carbon atoms of the centermoleculeand thehydrogen atoms ofthe surroundingmolecules,which can be attributed to the interaction betweenπ-hydrogen bond interaction38.However,the strength of themutual interaction is very weak because of its long distance and the small positive charge,therefore,this kind of interaction can be ignored.Itwas reported that the topology of the electron density at BCP is an indicator of the strength of intermolecular interations39.The hydrogen bonds in C-F1 and C-F3 have relatively large electron densities(0.0924-0.0922 a.u.), which aremuch stronger than those in C-F2 and C-F4withρBCPin range of 0.0223-0.0349 a.u.It is in agreement with the geometrical findings,the hydrogen bond contacts in the formerare evidently shorter than those in the later.
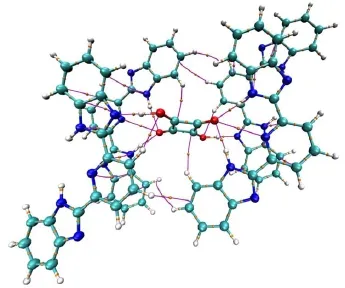
Fig.5Topology analysis diagram for 1 coloronweb version

Table4Information of the corresponding criticalpoint for each hyd rogen bond
Wecan learn from Table4thatC-F1 and C-F3 areequivalent,so areC-F2(1)andC-F4(1).AIManalysisindicates thatthehydrogen bond ofC-F1orC-F3 isverystrong.According to thedataobtained from the literature,theρBCPandvaluesofhydrogenbondare in therangeof0.002-0.04and0.02-0.15a.u.,respectively40-42.And thebiggerρBCPand the smallerthe stronger the hydrogen bond.TheρBCPand theof C-F1were0.092and 0.003 a.u., respectively,whicharemuch largerbeyond the strength rangeof than the corresponding ones of the general hydrogen bond,indicating thatthehydrogenbond strength isverystrong.Generally,the valuesofand HBCPinvan derWaals forcesarepositivevalues while the ones of▽2ρBCPand HBCPin covalent interaction were negative43.For1,the▽2ρBCPvalueofC-F1 ispositiveand the HBCPis negative,whichexplainsthatthishydrogenbondinghastheproperty ofpartialcovalentinteraction.Rybarczyk-Pirek39studied thenature of N-H…Ow ith A IManalysis,the resultsindicated that thehydrogenbondofN-H…O ispartialcovalentinteractionwhen▽2ρBCPand HBCPvaluesof N-H…Oarepositiveandnegative,respectively, whichareconsistentw ithour results.Thevalueof VBCPis related to hydrogenbonding intensity9,44.
The potentialenergy density of BCPcan be used to estimate the bond energy of hydrogen bond using Eq.(2):

Hence,thehydrogen bonding energy is estimated to be118.3 kJ∙mol-1,w hich is comparable to the calculated data of 135k J∙mol-1.Thus,the potential energy density also reveals that the hydrogen bonding isvery strong.ELF isa very important function to evaluate the intensity of covalent interaction.ELF values of BCP for weak interaction is usually quite small,however,the value of ELFBCPfor C-F1 ismore than 0.5,which also shows certain characteristicsof covalentbonding.
From thepointofnumericalvalue,thehydrogenbond ofC-F2(2) isstronger than thatof C-F2(1)butweaker than thatof C-F1.The resultsagreewellw ith the information reflected by the length of hydrogen bonds.The valuesofρBCPand▽2ρBCPare in the rangeof generalhydrogenbonding.Theirvaluesof HBCPare positiveand the valuesof ELFBCParevery small,indicating thatthey areordinary closed shell interactions w ith no covalent interaction character. According to the Eq.(2),the bonding energies of two hydrogen bondsare 34and 19 kJ∙mol-1respectively.The sum of the two hydrogenbond energy is53 kJ∙mol-1,which iscomparable to the calculated valuesof49 kJ∙mol-1.Inaddition,comparing thehydrogen bond parametersbetween calculated valuesand the crystal data,listed in Table2,itwas found thatthecalculated distancesX…Y of X-H…Y aresamew ith those in the relativeones,however, thecalculated distancesH…Yaredifferent from the relativecrystal data.The two hydroxyl groups in squaric acidmoleculeand the surroundingmoleculescan form two extremely strong hydrogen bonds.Meanwhile,two carbonyl oxygen atoms in squaric acid moleculeand the surroundingmoleculesgenerate fourhydrogen bonds thathaveordinary strength,where twoof them area littlebit stronger than theothers.Thebond energy calculation isconsistent w ith the resultsobtained from A IManalysis.
4Conclusions
One-dimensional chain polymer assembled by the hydrogen bonding andπ-πstacking interactionswas obtained from the m ixture of squaric acid and 2,6-bis(2-benzim idazyl)pyridine molecules in d-DMSO.It could be concluded that two kinds of oxygen atom s in a squaric acid molecule can form hydrogen bonding with thenitrogen atom in 2,6-bis(2-benzim idazyl)pyridine.However,the strength of the formed hydrogen bond is different.The hydrogen bond formed between oxygen atom of hydroxyl group and nitrogen atom in 2,6-bis(2-benzimidazyl)pyridine ismuch stronger than that of the oxygen atom in carbony lrom a squaric acid asan acceptor.
References
(1)Majerz,I.J.Phys.Chem.A 2012,116(30),7992.doi:10.1021/ jp300942n
(2)Jeffrey,G.A.;Saenger,W.Hydrogen Bonding in Biological Structures;Springer-Verlag:Berlin,1991.
(3)Scheiner,S.Hydrogen Bonding:a Theoretical Perspective; Ox ford University Press:New York,1997.
(4)Ebrahimi,A.;HabibiKhorassani,S.M.;Delarami,H. Chemical Physics2009,365(1-2),18.doi:10.1016/j. chemphys.2009.09.013
(5)Herrebout,W.A.;Clou,K.;Desseyn,H.O.J.J.Phys.Chem.A 2001,105(20),4865.doi:10.1021/jp004396c
(6)Tonan,K.;Ikawa,S.I.J.Am.Chem.Soc.1996,118(29), 6960.doi:10.1021/JA 953380A
(7)Koch,U.;Popelier,P.L.A.J.Phys.Chem.1995,99(24), 9747.doi:10.1021/j100024a016
(8)Bader,R.F.W.Chem.Rev.1991,91(5),893.doi:10.1021/ cr00005a013
(9)Bader,R.F.W.Atoms inMoleculesa Quantum Theory; Clarendon Press:Oxford,1994.
(10)Ranganathan,A.;Kulkarni,G.U.;Rao,C.N.R.J.Phys. Chem.A 2003,107(31),6073.doi:10.1021/jp030465m
(11)Takasu,I.;Izuoka,A.;Sugawara,T.J.Phys.Chem.B 2004, 108(18),5527.doi:10.1021/030961a
(12)dos Reis,F.D.;Gatti,I.C.;Garcia,H.C.;de Oliveira,V.E.; deOliveira,L.F.C.J.Phys.Chem.A 2014,118(49),11521. doi:10.1021/jp509502w
(13)Smartand Saint,Area DetectorContro land Integration Software;SimensAnalytical XSystems,Inc.:Madison,W I, USA,1996.
(14)Sheldrick,G.M.SHELXTLV5.1 Software Reference Manual; BrukerAXS Inc.:Madison,1997.
(15)Maia,J.D.C.;Carvalho,G.A.U.;Mangueira,C.P.,Jr.; Santana,S.R.;Cabral,L.A.F.;Rocha,G.B.J.Chem.Theory Comput.2012,8(9),3072.doi:10.1021/ct3004645
(16)McNaught,I.J.J.Chem.Educ.2011,88(4),421.doi:10.1021/ ed900038a
(17)Stewart,J.J.P.J.Mol.Model.2013,19(2),1.doi:10.1007/ s00894-012-1667-x
(18)Stewart,J.J.P.J.Comput.Chem.2011,32(7),1456.doi: 10.1002/jcc.21759
(19)Grimme,S.;Antony,J.;Ehrlich,S.;Krieg,H.J.Chem.Phys. 2010,132(15),154104.doi:10.1063/1.3382344
(20)Song,J.S.;Klein,E.L.;Neese,F.;Ye,S.F.Inorg.Chem. 2014,53(14),7500.doi:10.1021/ic500829p
(21)Atak,K.;Golnak,R.;Xiao,J.;Suljoti,E.;Pfluger,M.; Brandenburg,T.;W inter,B.;Azi,E.F J.Phys.Chem.B 2014, 118(33),9938.doi:10.1021/jp505129m
(22)Neese,F.;Wennmohs,F.;Hansen,A.;Becker,U.Chem.Phys. 2009,365(1-3),98.doi:10.1016/j.chemphys.2008.10.036
(23)Weigend,F.;Ahlrichs,R.Phys.Chem.Chem.Phys.2005,7 (18),3297.doi:10.1039/b50841a
(24)Yang,Y.J.Phys.Chem.A 2010,114(50),13257.doi:10.1021/ jp109278v
(25)Zou,W.L.;Nori-Shargh,D.;Boggs,J.E.J.Phys.Chem.A 2013,117(1),207.doi:10.1021/jp3104535
(26)Lu,T.;Chen,F.W.J.Phys.Chem.A 2013,117(14),3100.doi: 10.1021/jp4010345
(27)Arunan,E.;Desiraju,G.R.;Klein,R.A.;Sadle,J.Pure Appl. Chem.2011,83,1619.doi:10.1351/PAC-REP-10-01-01
(28)Hibbert,F.;Emsley,H.Adv.Phys.Org.Chem.1991,26,255. doi:10.1016/S0065-3160(08)60047-7
(29)Akhriff,Y.;Server-Carrio,J.;Garcia-Lozano,J.;Folgado,J.V.; Sancho,S.;Escriva,E.;Vitoria,P.CrystalGrowth Design 2006,6(5),1124.doi:10.1021/cg050543q
(30)Silva,C.E.;DosSantos,H.F.;Speziali,N.L.;Diniz,R.;de Oliveira,L.F.C.J.Phys.Chem.A 2010,114(37),10097.doi: 10.1021/jp105346h
(31)Inkaya,E.;Günnaz,S.;Özdem ir,N.;Dayan,O.;Dinçer,M.; Çetinkaya,B.Spectrochim.Acta A 2013,103,255.doi: 10.1016/j.saa.2012.11.039
(32)Schwager,F.;Marand,E.;Davis,R.M.J.Phys.Chem.1996, 100(50),19268.doi:10.1021/jp9613448
(33)Desseyn,H.O.;C lou,K.;Keuleers,R.;Miao,R.;Van Doren, V.E.;Blaton,N.Spectrochim.Acta A 2001,57(2),231.doi: 10.1016/S1386-1425(00)00370-X
(34)Hao,M.H.J.Chem.Theory Comput.2006,2(3),863.doi: 10.1021/ct0600262
(35)Lane,J.R.J.Chem.Theory Comput.2013,9(1),316.doi: 10.1021/ct300832f
(36)Lipkow ski,P.;Grabow ski,S.J.;Robinson,T.L.;Leszczynski, J.J.Phys.Chem.A 2004,108(49),10865.doi:10.1021/ jp048562i
(37)Grabowski,S.J.;Dubis,A.T.;Palusiak,M.;Leszczynski,J.J. Phys.Chem.B 2006,110(12),5875.doi:10.1021/jp055334v
(38)Grabowski,S.J.J.Phys.Org.Chem.2013,26(6),452.doi: 10.1002/poc.3109
(39)Rybarczyk-Pirek,A.J.Struct.Chem.2012,23(6),1739.doi: 10.1007/s11224-012-9980-7
(40)Chou,P.T.;Ching,Y.W.;Hung,F.T.J.Phys.Chem.B 1997, 101(44),9119.doi:10.1021/jp971824e
(41)Latosinska,J.N.;Latosinska,M.;Seliger,J.;Zagar,V.; Maurin,J.K.;Kazimierczuk,Z.J.Phys.Chem.A 2012,116(5),1445.doi:10.1021/jp210322p
(42)Latosińska,J.N.;Latosińska,M.;Maurin,J.K.;Orzeszko,A.; Kazim ierczuk,Z.J.Phys.Chem.A 2014,118(11),2089.doi: 10.1021/jp411547z
(43)Rozas,I.;Alkorta,I.;Elguero,J.J.Am.Chem.Soc.2000,122 (45),11154.doi:10.1021/ja0017864
(44)Espinosa,E.;Molins,E.;Lecom te,C.Chem.Phys.Lett.1998, 285(3-4),170.doi:10.1016/S0009-2614(98)00036-0
Experimental and Theoretical Evidence for Supramolecular Self-Assembly and Hydrogen Bonding between Squaric Acid and 2,6-Bis(2-benzimidazolyl)pyridine
HU Ai-Bin PAN Zhi-Quan CHENG Qing-Rong ZHOU Hong*
(College ofChemistry and EnvironmentalEngineering,Wuhan Institute ofTechnology,Wuhan 430073,P.R.China)
Sup ramo lecula r com p lex(1)was ob tained from amixture o f squa ric acid and 2,6-bis(2-benzim idazo lyl)pyridine in deuterated dimethylsulfoxide(d-DMSO)and characterized by sing le-crystal X-ray diffraction.The crystalstructure data showed that1 was a one-dimensiona lchain polymerassemb led through π-πstacking and hydrogen bonding interactions.Infra red vibrationalspectra of1 we remeasured atdiffe rent temperatures and sample concentrations in carbon tetrachloride.Moreover,the hydrogen bonding in the crystals was furthe r evaluated by density functional theory(DFT)and atom s inmo lecules(AIM)theory.The calcula ted hydrogen bonding energieswere about135.65and 49.40J∙mol-1,respectively.
Intermolecularhydrogen bonding;Variable temperature infrared;Density functional theory; Atom s inmolecu les theory
1 Introduction
Hydrogen bonding isone kind of interactionsbetween covalent bonds and van derWaals forces1.Due to itsw ide existence in solid,gas,and liquid states,hydrogen bonding playsan important role in the biological,pharmaceutical materials,and chem ical reactions2,3.It iswellknown that thehydrogen bonding interactions are the key factor tomaintain the doublehelix structure of DNA and the secondary structure of protein.To betterunderstand the function of hydrogen bonding in biomacromolecules and other substances,various technologies have been applied to explore hydrogen bonding in molecules4.Among them,X-ray single crystal diffraction can provide information about the distance between the acceptorand donoratoms of thehydrogen bonding aswell as the typesand the contribution of hydrogen bonding to the configuration of crystals.Infrared spectroscopy givesevidence for the hydrogen bonding formation by the shiftof the vibrationbands5,6.Both density functional theory(DFT)and quantum theory of atoms inmolecules(QTAIM)havebeen proved to be themost powerfulmethods to theoretically evaluatehydrogen bonding7-9.
October29,2015;Revised:December24,2015;Published onWeb:December25,2015.
O641
10.3866/PKU.WHXB201512252
*Corresponding author.Email:hzhouh@126.com;Tel:+86-13871359786.
The projectwas supported by the National Natural Science Foundation of China(21171135,21301131).国家自然科学基金(21171135,21301131)资助项目©Editorialofficeof Acta Physico-Chim ica Sinica
