四丁基季鏻羧酸盐离子液体的物理化学性质与CO2溶解度
2016-09-13陈凤凤董艳桑晓燕周言陶端健
陈凤凤 董艳桑 晓燕 周言陶端健
(江西师范大学化学化工学院,南昌330022)
四丁基季鏻羧酸盐离子液体的物理化学性质与CO2溶解度
陈凤凤董艳桑晓燕周言*陶端健*
(江西师范大学化学化工学院,南昌330022)
在298.15-348.15K温度范围内,测定了四种四丁基季鏻羧酸盐离子液体([P4444][CA])的密度、粘度、折射率、电导率等物理化学性质,得出了其随温度的变化关系,并获得了不同温度下该类离子液体的热膨胀系数。其次,在1个大气压和313.15K温度下,测定了CO2在该类离子液体中的溶解度,结果表明,四丁基季鏻丁酸盐吸收CO2的性能最好,吸收量为0.4mo l∙mol-1,平衡时间小于5m in。
离子液体;四丁基季鏻羧酸盐;物理化学性质;CO2吸收
1 Introduction
In recent years,ionic liquids(ILs)have attracted increasing attention as promising catalysts and green solvents.Most ILs possessmany specific properties such as structural designability, low volatility,high thermaland chemicalstability,and outstanding solubility1-3.Thus,these features can afford a lotof opportunities for theapplicationsof ILs inmany fields such asgasabsorption, organic synthesis,catalysis,electrochemistry,biotransformation, etc3-7.
Recently,carboxylate ionic liquids(CAILs),as being a prototype of basic ILs,have been widely reported for Michael addition,Knoevenagel condensation,dissolution of cellulose and other reactions8-14.For exam ple,Liang et al.12reported that the catalysis of 1,1,3,3-tetramethylguanidineacetate([TMG][Ace]) was applied in the alcoholysis of propylene oxide.Cao et al.13reported that1-ethyl-3-methylimidazolium acetate showed amuch higher ability to dissolve cellulose.We also used tetrabuty lphosphonium carboxylate ILs as catalysts for highly efficientsynthesisof propyleneglycolmethylether14.However,in order to develop industrialapplications of CA ILs,there isan urgent need to study the fundamental physicochemical properties of CAILs including density,viscosity,refractive index,conductivity,etc.
Tillnow,a few physicochemicalproperty investigationshave beenmade involving cholinium carboxylate ILs15,tetraalkylammonium carboxylate ILs16,im idazolium carboxylate ILs17-23.The physicochemicalproperties of phosphonium carboxylate ILs have notbeen studied systematically yet.Asour continuouswork on the utilization of phosphonium carboxylate ILs in sustainable catalysis,it is very worth noting that to date the properties of such phosphonium carboxylate ILs remain unknow n.
In this work,four CAILs,tetrabutylphosphonium formate ([P4444][For]),tetrabutylphosphonium acetate([P4444][Ace]),tetrabutylphosphonium propionate([P4444][Prop]),tetrabuty lphosphonium butyrate([P4444][Buty])were prepared,and their physicochemical properties(density,viscosity,refractive index,and conductivity)weremeasured and correlated with thermodynamic and empiricalequations.The CO2solubility of these CAILswas also studied at313.15K and atmosphere pressure.
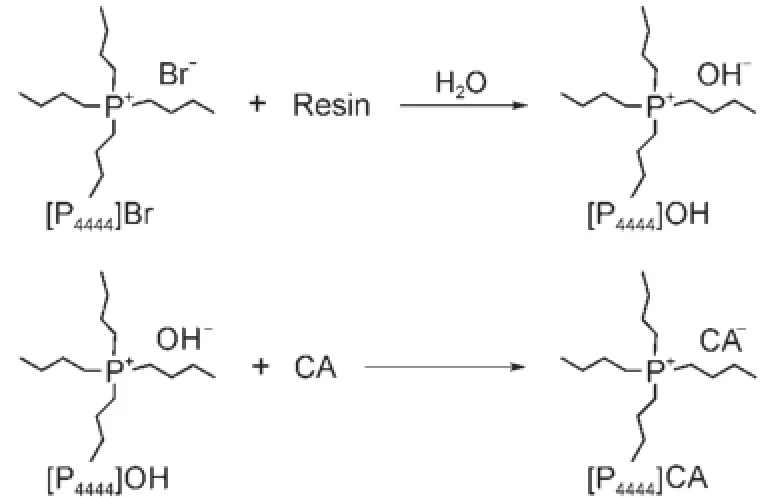
Scheme 1 General route for the synthesisof[P4444][CA]ILs

Fig.1 Device of CO2absorption V:valve,P:pressuremeter,VP:vacuum pump,WT:water trap,GC:gas container,GR:gas reservoir,EC:equilibrium cell,MS:magnetic stirrer,PC: personal computer,NI:num erical indicator,TC:temperature controller
2 Experimental
2.1Materia ls
Tetrabutylphosphonium brom ide(mass purity≥98%)and anion exchange resin Amberlite717were purchased from Aladdin Chem ical Reagent Co.Ltd.(Shanghai,China).CO2w ith amass purity of 99.999%was purchased from JiangxiProvince Specialty Gases.Formic acid,acetic acid,propionic acid,butyric acid,and ethanol w ere of analytical grade and used w ithout any further purification,which were purchased from Tianjin Fuchen Chemical Reagent Co.Ltd.(China).Doubly distilledwaterwasused in all experiments.
The synthesisand characterization of[P4444][CA]ILshad been described in our previouswork(Scheme1)14.Thewater content was less than0.1%(w,mass fraction)in each[P4444][CA]IL,which was analyzed by Karl Fischer titration(Metrohm 756KF coulometer).The concentration of Br-in each[P4444][CA]IL w as measured by Mohr titration,and the related impuritywas less than 0.02%(w).
2.2Physicochem ica lp roperties
Allof[P4444][CA]ILswere dried in vacuum at353.15K before measuring the properties.Densities were measured over the temperature range of 293.15-353.15K by an Anton Paar densimeter(model DMA4500),which was checked by dry air and doubly distilled water.The precision of the density valueswas ±10-5g∙cm-3w ith a temperature accuracy of±0.05K.Viscosities weremeasured by a cone-plate viscometer(Brookfield DV II+ Pro)w ith a temperature controlof±0.05K,and the precision of the viscosity valueswas±10-2mPa∙s.The instrumentwas calibrated using standard calibration fluidssuch asethanoland glycol w ith known viscosities.The temperature rangew as from 298.15to 348.15K,and theattaining thermalequilibrium timewas about 30min.Refractive indiceswere recordedwith a Rudolph research analytical J357 refractometer at temperatures from 298.15to 348.15K.The precision of refractive indices valueswas±10-4w ith a temperature accuracy of±0.05K.Apparatuswas calibrated and checked by pure organic solventswith known refractive index before each series ofmeasurements24.Conductivity was determ ined in the temperature range from 298.15to 348.15K(Conductivity meter DDJS-308A,Shanghai LeiciCompany)with a DJS-1C electrode.Caution was taken to preventevaporation,and the electrode and the solution were sealed in typical glassware, whichwas immersed into a thermostatic bathw ith a temperature accuracy of±0.05K.The precision of the conductivity valueswas ±0.1μS∙cm-1.
2.3Determ ination o f CO2abso rp tion
The apparatus for the determ ination of CO2absorption in [P4444][CA]ILs issimilar to that in the previous reference(Fig.1)25. Thew hole device consistsof tw o 316L stainless steel chambers whose volumesare 128.47 cm3(V1)and 49.67 cm3(V2),respectively.Thebigger chamber,named asgas reservoir,isolatesgases before it contactsw ith the IL samples in the smaller chamber.The smaller chamber used as equilibrium cell is equipped with a magnetic stirrer.The temperatures(T)of both chambers are controlled by awater bath with an uncertainty of±0.1 K.The pressures in the two chambers aremonitored using two pressure transducers of±0.2%uncertainty.The pressure transducers are connected to a numeric instrument to record the pressure changesonline.In a typical run,about1mmol of IL samplewasplaced into the equilibrium cell and the air in the two chamberswas evacuated(<100Pa).The pressure in the equilibrium cellwas recorded to be p0.CO2from itsgas cylinderwas fed into the gas reservior to a pressure of p1.The needle valve between the tw o chamberswas turned on to let gases be introduced to the equilibrium cell.Absorption equilibrium was thought to be reached when the pressures of the two chambers remained constant forat least30min.Theequilibrium pressureswere denoted as p′1for the gas reservior and p2for the equilibrium cell.The gas partial pressure in theequilibrium cellwas pg=p2-p0.Thegasuptake, n(pg),can thusbe calculated using the follow ing equation:
n(pg)=ρg(p1,T)V1-ρg(p′1,T)V1-ρg(pg,T)(V2-w/ρIL)(1) whereρg(pi,T)represents the density of gas inmol∙cm-3at pi(i= 1,g)and T.ρILis the density of the IL in g∙cm-3at T.w is themass of IL sample.V1and V2represent the volumes in cm3of the two chambers,respectively.Duplicate experimentswere run for each IL system to obtain averaged values of gas absorption.The averaged uncertainty of the absorption data in thisw ork w asw ell w ithin±1%.

Fig.2 Densitiyρasa function of tem perature
3 Results and discussion
3.1Density
Theexperimentaldensitiesof four[P4444][CA]ILsare presented in Table S1(Supporting Information),and the density dependence on temperature is plotted in Fig.2.It can be observed that the densities of these CAILs decrease linearly w ith the increase of temperature,and the density order is as follows:[P4444][Prop]< [P4444][Buty]<[P4444][For]≈[P4444][Ace].These results show that an increase in the anion alkyl chain length directly reduces the density values for the presentCAILs.This isbecause as thealkyl chain length of anion prolongs,the volume of CAILs would improve and thus the value of density deceases accordingly.A similar phenomenon forother CAILs reported by Muhammad and co-workers15.However,[P4444][Buty]did notperform the smallest density,which originates from itsbiggestmolecularweight.
3.2Viscosity
The experimental data of the viscosities aregiven in Table S2 in Supporting Information,and Fig.3 shows the effect of temperature on viscositiesof CAILs.It is demonstrated that the high temperature could decrease the viscosities of[P4444][CA]ILs significantly.The viscosity order is as follows:[P4444][Buty]< [P4444][Prop]<[P4444][Ace]<[P4444][For].This shows thatan increase in the alkyl chain length of anions tends to reduce the viscosity of[P4444][CA]ILs.When the volumeof anion in CAILs increases,the interaction between anion and cation w ould be weakened,resulting in a relatively low valueof viscosity.

Fig.3 Viscositiyηasa function of tem perature
3.3Re fractive Index
Themeasured data of the refractive indices are presented in Table S3(Supporting Information),and the effectof temperature on refractive index is shown in Fig.4.It is found that the refractive index is linearly decreasingwith the increaseof temperature,and theorder is[P4444][Buty]<[P4444][Prop]<[P4444][Ace]<[P4444][For], implying thata large anion can lead to a relatively low refractive index.This is explained that according to the Lorentz-Lorenz equation,the volume of its cation and anion is negative to the refractive index of[P4444][CA]ILs26.When the size of anions grows,the value of volume becomes smaller and thus the refractive index decreases.
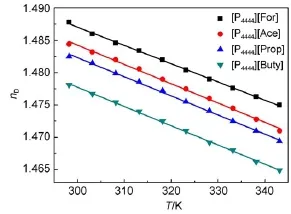
Fig.4Refractive index nDasa function of tem perature
3.4Conduc tivity
The experimental data of conductivity are listed in Table S4(Supporting Information),and the effectof temperature on the conductivity is shown in Fig.5.It is found that the conductivity increases w ith increasing the temperature.Also,it has been pointed out that lower viscosity always leads to higher ionic mobility of the ILs27.Thus,the order of the electrical conductivities of[P4444][CA]ILs is in the opposite sequence of their vis-cosities as follows:[P4444][For]<[P4444][Ace]<[P4444][Prop]. However,it is seen that[P4444][Buty]has a relatively small conductivity,even though itsviscosity is the lowestamong these four [P4444][CA]ILs.Thiscan beexplained that theanion[Buty]-owing too high volume is hard tomove and thus obtains lower ionic mobility,contributing to a relatively low electrical conductivity of the ILs.

Fig.5Conductivityσasa function of tem perature
3.5Em p irical co rre lation
On the basis of the above observations,the physicochemical propertieswere fitted to the follow ing empirical equations as a function of tem perature24,28-30.
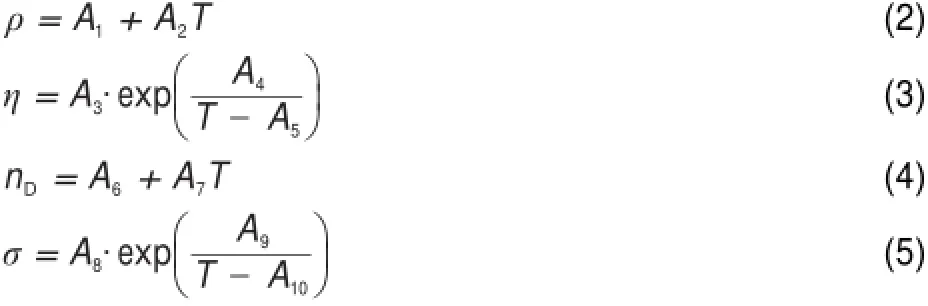
whereρ(g∙cm-3),η,nD,andσdenoteare the density,viscosity, refractive index,and conductivity,respectively.A1,A2,A3,A4,A5, A6,A7,A8,A9,and A10are correlation coefficients.T is the Kelvin temperature.The correlation coefficientswere estimated using linear regression analysis,and the valueswere reported together with the standard deviations(SD)in Tables1-4.The SD values were calculated by using the following expression:

where N is the number of experimental points,Zexpand Zcalare the experimentaland calculated values(using Eqs.(1-4)),respectively. As seen from Tables 1-4,itwas noted that all the correlation coefficientswere over 0.99.The temperature dependence of viscosity and conductivity could be well described by the A rrheniusand Vogel-Tamman-Fulcher(VTF)equations.

Table 1 Fitting parameter valuesof Eq.(1)and the standard deviations

Tab le 2 Fitting parameter valuesof Eq.(2)and thestandard deviations

Tab le 3 Fitting parameter valuesof Eq.(3)and the standard deviations

Tab le 4Fitting parameter valuesof Eq.(4)and thestandard deviations

Tab le 5Thermalexpansion coefficientvaluesof[P4444][CA]ILs
Inaddition,since the temperature-density relationship for these [P4444][CA]ILs was linear,density values as a function of temperaturewere used to calculate the thermalexpansion coefficient (αp)(Table5).The thermalexpansion coefficients(αp),also known asvolumeexpansivity,asa function of temperatureatatmospheric pressurewere determined by using the follow ing Eq.(7):

whereαp,ρ,and T are the thermalexpansion coefficient,density, and absolute temperature,respectively,whereas A1and A2are the fittingparametersof Eq.(2).Asseen from Table5,itisobserved that the values of thermal expansion coefficient do not appreciablychangewith temperature for the range from 298.15to 353.15K. The valueof averaged relativedeviation inαpis found to be less than 3%.Thisdemonstrates thatthe thermalexpansion coefficient of[P4444][CA]ILs isalmost independentof temperature,and this phenomenon is also observed sim ilar to those reported for im idazolium,pyridinium,phosphonium,and ammonium-based ILs31,32.
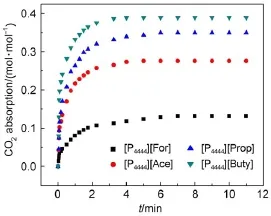
Fig.6CO2absorp tion curves for four[P4444][CA]ILs
3.6CO2abso rp tion
The solubilities of CO2in these four[P4444][CA]ILs were measured ata temperatureof 313.15K and elevated pressuresup to 100kPa.The solubility data are graphically shown in Fig.6.It is significantly noted that theabsorption equilibrium could allbe reached within 5min,much shorter than the timeused inmostof ILs33-35.Thisisprimarily due to theeffectofviscosity on themass transfer of CO2.As seen from Fig.4and Table S2,all the viscositiesof these four[P4444][CA]ILsare lower than 165mPa∙sat 313.15K,and thus low viscosity accelerates the absorption rate. Moreover,it is indicated that the anion of CAILs hasan obvious effecton the CO2absorption capacity.Comparedwith other three CAILs,[P4444][Buty]can absorb CO2with the highestsolubility of 0.4inmolar fraction at 313.15K and 100kPa.It is known that with the prolonging carbon chain length,thebasicity of carboxylic anion gradually increasesbecauseof theelectrondonation of alkyl group.Thus[P4444][Buty]possessing strong basicity can inducea relatively high CO2absorption capacity.This phenomenon isalso observed sim ilar to those reported for im idazolate-based ionic liquids36.
4Conclusions
The physicochemicalproperties of four tetrabutylphosphonium carboxylate ILs including density,viscosity,refractive index, conductivity were determined atatmospheric pressure,and CO2absorption capacities of these CA ILsw ere furthermeasured at 313.15K.It demonstrates that the properties of there carboxylate ILs show simple systematic variationsw ith temperatureandwith the alkyl chain length of the carboxylate anion.The empirical equations can beused for fitting the experimentaldata verywell. Compared with other CAILs,[P4444][Buty]has thehighestcapacity of 0.4mol IL for 1mol CO2.This study will be significant for [P4444][CA]ILs′industrialand engineering applications.
Supporting In formation:The experimentalvalues of density, viscosity,conductivity,and refractive index have been found in this text.This information is available free of charge via the internetathttp://www.whxb.pku.edu.cn.
References
(1)Welton,T.Chem.Rev.1999,99,2071.doi:10.1021/cr980032t
(2)Wasserscheid,P.;Keim,W.Angew.Chem.Int.Edit.2000,39, 3772.doi:10.1002/1521-3773(20001103)39:21<3772::AIDANIE3772>3.0.CO;2-5
(3)Dupont,J.;de Souza,R.F.;Suarez,P.A.Z.Chem.Rev.2002, 102,3667.doi:10.1021/cr010338r
(4)Zhang,X.P.;Zhang,X.C.;Dong,H.F.;Zhao,Z.J.;Zhang,S. J.;Huang,Y.Energy Environ.Sci.2012,5,6668.doi:10.1039/ c2ee21152a
(5)García,S.;Larriba,M.;García,J.;Torrecilla,J.S.;Rodríguez, F.J.Chem.Eng.Data.2010,56,113.doi:10.1021/je100982h
(6)Wang,J.;Sun,G.;Yu,L.;Wu,F.;Guo,X.Bioresour.Technol. 2013,128,156.doi:10.1016/j.biortech.2012.10.098
(7)Mäki-Arvelaa,P.;Anugwom,I.;Virtanen,P.;Sjöholm,R.; Mikkola,J.P.IndustrialCrops and Products 2010,32(3),175. doi:10.1016/j.indcrop.2010.04.005
(8)Yue,C.B.;Mao,A.Q.;Wei,Y.Y.;Lu,M.J.Catal.Commun. 2008,9,1571.doi:10.1016/j.catcom.2008.01.002
(9)Ying,A.G.;Liu,L.;Wu,G.F.;Chen,G.;Chen,X.Z.;Ye,W. D.Tetrahedron Lett.2009,50,1653.doi:10.1016/j. tetlet.2009.01.123
(10)Jiang,T.;Gao,H.X.;Han,B.X.;Zhao,G.Y.;Chang,Y.H.; Wu,W.Z.;Gao,L.;Yang,G.Y.Tetrahedron Lett.2004,45, 2699.doi:10.1016/j.tetlet.2004.01.129
(11)Zhao,H.;Gary,A.B.;Song,Z.Y.;Olarongbe,O.;Tanisha,C.; Darkeysha,P.Green Chemistry 2008,10(6),696.doi: 10.1039/b801489b
(12)Liang,S.G.;Liu,H.Z.;Zhou,Y.X.;Jiang,T.;Han,B.X. New J.Chem.2010,34,2534.doi:10.1039/c0nj00502a
(13)Cao,Y.;Wu,J.;Zhang,J.;Li,H.Q.;Zhang,Y.;He,J.S. Chem.Eng.J.2009,147,13.doi:10.1016/j.cej.2008.11.011
(14)Tao,D.J.;Ouyang,F.;Li,Z.M.;Hu,N.;Yang,Z.;Chen,X.S. Industrial&Engineering Chemistry Research 2013,52(48), 17111.doi:17111.10.1021/ie402250e
(15)Muhammad,N.;Hossain,M.I.;Man,Z.;El-Harbaw i,M.; Bustam,M.A.;Noaman,Y.A.;Alitheen,N.B.M.;Hefter,G.; Yin,C.Y.J.Chem.Eng.Data 2012,57,2191.doi:10.1021/ je300086w
(16)Talavera-Prietoa,N.M.C.;Ferreiraa,A.G.M.;Simões,P.N.; Carvalho,P.J.;Mattedi,S.;Coutinho,J.A.P.J.Chem. Thermodyn.2014,68,221.doi:10.1016/j.jct.2013.09.010
(17)Xu,A.;Zhang,Y.;Li,Z.;Wang,J.J.Chem.Eng.Data 2012, 57(11),3102.doi:10.1021/je300507h
(18)Xu,A.;Wang,J.;Zhang,Y.J.;Chen,Q.T.Industrial& Engineering Chemistry Research 2012,51(8),3458.doi:10.1021/ie201345t
(19)Li,C.;Yang,H.X.;Liu,R.J.;Yang,Q.;Tong,J.;Yang,J.Z. Acta Phys.-Chim.Sin.2015,31(1),11.[李驰,杨宏旭,刘入境,杨奇,佟静,杨家振.物理化学学报,2015,31(1), 11.]doi:10.3866/PKU.WHXB201411063
(20)Li,C.P.;Li,Z.;Zou,B.X.;Liu,Q.S.;Liu,X.X.Acta Phys.-Chim.Sin.2013,29(10),2157.[李长平,李琢,邹本雪,刘青山,刘晓霞.物理化学学报,2013,29(10), 2157.]doi:10.3866/PKU.WHXB201307293
(21)Guan,W.;Ma,X.X.;Li,L.;Tong,J.;Fang,D.W;Yang,J.Z. J.Phys.Chem.B 2011,115,12915.doi:10.1021/jp207882t
(22)Tong,J.;Ma,X.;Kong,Y.X.;Chen,Y.;Guan,W.;Yang,J.Z. J.Phys.Chem.B 2012,116,5971.doi:10.1021/jp301985g
(23)Ma,X.X.;Wei,J.;Zhang,Q.B.;Tian,F.;Feng,Y.Y.;Guan, W.Industrial&Engineering Chemistry Research 2013,52, 9490.doi:10.1021/ie401130d
(24)Muhammad,N.;Man,Z.B.;Bustam,M.A.;Mutalib,M.I.A.; Wilfred,C.D.;Rafiq,S.J.Chem.Eng.Data 2011,56,3157. doi:10.1021/je2002368
(25)Zhang,X.M.;Huang,K.;Xia,S.;Chen,Y.L.;Wu,Y.T.;Hu, X.B.Chem.Eur.J.2009,274,30.doi:10.1016/j. cej.2015.03.052
(26)Wang,Z.X.;Chen,J.S.;Gu,M.X.;He,G.T.;Dai,P.F.;Li, M.JournalofFunctionalMaterials2012,16(43),2251.[王仲勋,陈吉胜,谷明信,何国田,戴鹏飞,李明.功能材料, 2012,16(43),2251.]doi:10.3969/j.issn.1001-9731.2012.16.032
(27)Liu,Q.S.;Yan,P.F.;Yang,M.;Tan,Z.C.;Li,C.P.;Welz-Biermann,U.Acta Phys.-Chim.Sin.2011,27(12),2762.[刘青山,颜佩芳,杨淼,谭志诚,李长平,Welz-Biermann,U.物理化学学报,2011,27(12),2762.]doi:10.3866/PKU. WHXB20112762
(28)Machanová,K.;Boisset,A.;Sedláková,Z.;Anouti,M.; Bendová,M.;Jacquemin,J.J.Chem.Eng.Data 2012,57, 2227.doi:10.1021/je300108z
(29)Ziyada,A.K.;Bustam,M.A.;Wilfred,C.D.;Murugesan,T. J.Chem.Eng.Data 2011,56,2343.doi:10.1021/je101316g
(30)Yunus,N.M.;Mutalib,M.I.A.;Man,Z.;Bustam,M.A.; Murugesan,T.J.Chem.Thermodyn.2010,42,491.doi: 10.1016/j.jct.2009.11.004
(31)Gu,Z.;Brennecke,J.F.J.Chem.Eng.Data 2002,47,339. doi:10.1021/je010242u
(32)Tariq,M.;Forte,A.S.;Gomes,F.C.;Lopes,N.C.;Rebelo,P. N.J.Chem.Thermodyn.2009,41,790.doi:10.1016/j. jct.2009.01.012
(33)Wang,C.M.;Luo,H.M.;Li,H.R.;Zhu,X.;Yu,B.;Dai,S. Chem.Eur.J.2012,18,2153.doi:10.1002/chem.201103092
(34)Wang,C.M.;Luo,H.M.;Luo,X.Y.;Li,H.R.;Dai,S.Green Chem.2010,12,2019.doi:10.1039/c0gc00070a
(35)Jiang,Y.Y.;Wang,G.N.;Zhou.Z.;Wu,Y.T.;Geng.J.; Zhang,Z.B.ChemicalCommunications 2008,4,505.doi: 0.1039/B713648J
(36)Zhang,Y.;Wu,Z.K.;Chen,S.L.;Yu,P.;Luo,Y.B.Industrial &Engineering Chemistry Research 2013,52(18),6069.doi: 10.1021/ie302928v
Physicochemical Properties and CO2Solubility of Tetrabutylphosphonium Carboxylate Ionic Liquids
CHEN Feng-Feng DONG Yan SANG Xiao-Yan ZHOU Yan*TAO Duan-Jian*
(College ofChemistry and Chemical Engineering,JiangxiNormalUniversity,Nanchang 330022,P.R.China)
Four tetrabutylphosphonium carboxylate ionic liquids([P4444][CA])were prepared,and their densities, viscosities,re fractive indices,and conductivities were measured and correlated w ith thermodynam ic and em piricalequations in the tem perature range of298.15-348.15K.The influence of tem peratu re on these four properties of[P4444][CA]was discussed,and their thermalexpansion coefficientvalueswere calculated.The CO2absorp tion capacity o f[P4444][CA]was studied at313.15K and 100kPa.The results indicated that[P4444][Buty] had the highestCO2capture capacity among these ionic liquids,with an absorption capacity of0.4mo l∙mol-1and balance time of less than 5m in.
Ionic liquid;Te trabutylphosphonium ca rboxyla te;Physicochem icalprope rty;CO2captu re
September11,2015;Revised:December23,2015;Published onWeb:December24,2015.
O642
10.3866/PKU.WHXB201512241
*Corresponding authors.TAO Duan-Jian,Email:djtao@jxnu.edu.cn.ZHOU Yan,Email:anitachow@jxnu.edu.cn;Tel:+86-791-88120317.
The projectwas supported by theNationalNatural Science Foundationsof China(21206063,21566011,31570560),Natural Science Foundationsof JiangxiProvincialDepartmentof Scienceand Technology,China(20151BAB213016),and Sponsored Program for Cultivating Youthsof
Outstanding Ability in JiangxiNormal University,China.
国家自然科学基金(21206063,21566011,31570560),江西省自然科学基金(20151BAB213016)及江西师范大学青年英才计划项目资助©Editorialofficeof Acta Physico-Chim ica Sinica
