放线菌JXJ-0136对白菜和豇豆生长的影响及其解磷作用
2016-09-09张炳火李汉全罗娟艳杨建远石红璆孙凤珍
张炳火,李汉全,罗娟艳,杨建远,石红璆,孙凤珍
(九江学院药学与生命科学学院,江西九江 332000)
放线菌JXJ-0136对白菜和豇豆生长的影响及其解磷作用
张炳火,李汉全,罗娟艳,杨建远,石红璆,孙凤珍
(九江学院药学与生命科学学院,江西九江 332000)
【目的】确定放线菌JXJ-0136的分类地位,分析其溶解不溶性磷的能力,在作物根际土壤定殖的情况及对蔬菜种子萌发、幼苗生长和蔬菜产量的影响,评价该菌在研制微生物肥料方面的潜在应用价值。【方法】利用培养特征、形态学特征和16S rRNA基因序列系统发育分析,初步确定菌株JXJ-0136的分类学地位;以白菜和豇豆为指示植物,采用琼脂平板法,研究该菌对蔬菜种子萌发和幼苗生长的影响;采用田间栽培试验,研究菌株对作物生长和产量的影响,测定栽培前后土壤总磷的含量,分析该菌对作物利用土壤磷效率的影响,并对作物根际土壤微生物进行分离纯化,分析该菌在根际土壤中的定殖情况;采用液体纯培养方式,研究菌株对不溶性无机磷和有机磷的溶解效率,分析其解磷机理。【结果】放线菌JXJ-0136在6—45℃、pH 4.0—13.0和0—4%(w/v)的盐浓度下均能生长,其中最适生长温度、pH和盐浓度分别约为28℃、pH 8.0和1%(w/v),在ISP2培养基上该菌气丝较发达,灰白色,孢子丝簇生,孢子长卵圆形;该菌16S rRNA基因序列与链霉菌Streptomyces violascens、S. somaliensis、S. hydrogenans、S. albidoflavus和S. daghestanicus的亲缘关系最近,相似性依次为 97.98%、97.71%、97.30%、97.23%和 97.03%,但在系统进化树上与这些菌聚在不同分支上;该菌培养液能够显著提高作物种子萌发率,促进幼苗生长,在0.2%—0.8%的剂量下,白菜种子萌发率、幼苗株高和根长分别比对照组增加3.55%—12.61%、13.91%—53.03%和7.37%—51.92%,豇豆种子萌发率、幼苗株高和根长分别比对照组增加4.71%—21.18%、3.60%—22.33%和2.37%—20.08%;田间栽培试验显示,该菌能够定殖于根际土壤,促进作物对土壤磷的利用,提高作物产量,当每穴施加5 mL该菌培养液,试验结束时,白菜和豇豆试验组的土壤总磷含量分别下降(23.56±2.65)%和(37.10±1.98)%,分别为对照组的(1.77±0.29)和(2.70±0.15)倍(P<0.01),而白菜和豇豆的产量却分别比对照组增加(27.59±6.15)%和(70.29±5.15)%(P<0.01);液体纯培养条件下接种培养5 d后,无机磷和有机磷培养基pH值由起始的7.0分别降至5.0和6.0,有效磷元素含量比对照组分别增加(73.94±0.94)和(7.12±0.28)mg(P<0.01)。【结论】放线菌JXJ-0136是链霉菌属的成员,能够显著提高作物种子的萌发率,增加幼苗株高和根长,并定殖于根际土壤中,增加土壤可溶性磷的含量,提高作物对土壤磷的利用效率,促进它们的生长,增加其产量,在微生物肥料研制中具有较大的潜在应用价值。
微生物肥料;放线菌JXJ-0136;链霉菌;白菜;豇豆;解磷
0 引言
【研究意义】农业可持续发展已成为全球特别是中国的一个重要议题[1]。然而,长期大量使用化学肥料导致土壤有机物质减少、质量恶化[2-3]、微生物区系发生变化[1,4]、土壤生态遭受严重破坏,肥力降低[5-6]。因此,减少化学肥料的用量已得到广泛提倡[7]。许多微生物具有促进植物生长、提高营养成分的可用性及其摄取率、维持植物健康等多种功能,因此,微生物肥料成为可替代或部分替代化学肥料的理想候选者[8-11]。放线菌通常能够产生抗逆性较强的孢子,并能够定殖于植物根部土壤,产生各种抗生素,抑制多种植物病原体[12-14],分泌生长调节物质[15],同时这类微生物还具有解磷、解钾等各种功能[12,16-18],是研制微生物肥料的理想材料,因此,开展放线菌菌肥的研制对农业可持续发展具有重要意义。【前人研究进展】放线菌菌肥的研究受到国内外广泛关注,在实验室[17]和野外[12-16,18]开展了许多工作,特别是尹莘耘等[13-16]对“5406”放线菌菌肥开展了一系列系统研究,在20世纪50—70年代,全国各地曾进行了大面积推广应用“5406”菌肥,取得了良好的增产效果,该菌肥的有效微生物为泾阳链霉菌(Streptomyces jingyangensis)[19]。近年来,国内放线菌菌肥的研究主要集中在生物防治方面,同时放线菌对作物种子萌发、幼苗生长、作物产量和品质的影响也日益受到关注。赵娟等[20]筛选到3株广谱拮抗放线菌Act1、Act11和Act12,它们对甜瓜和西瓜枯萎病菌具有拮抗活性,并对甜瓜幼苗具有促生作用,且Act12对土传植物病原真菌镰刀菌,如木贼镰孢、接骨木镰孢、尖镰孢西瓜专化型、尖镰孢棉花专化型和尖镰孢黄瓜专化型等,均具有较好的抑制作用[21],该菌与腐植酸钾配施,能够提高丹参出苗率,减少植株死亡率,改善丹参生长微环境,促进其生长[22]。吴艳辉等[23]筛选到一株对多种植物病原真菌具有抑制作用的放线菌 W04,该菌株对西瓜枯萎病的抑制作用较强,在80%左右;王兰英等[24]研究发现金黄垂直链霉菌 HN6对香蕉枯萎病的防治效果达72.02%,极显著高于95%恶霉灵1 200倍液,并能提高香蕉生物量;张艳杰等[25]田间试验表明玫瑰黄链霉菌Men-myco-93-63固体发酵物在对番茄连作障碍防效达46%;沈婷等[26]研究表明吸水链霉菌B04固体发酵物能有效降低草莓发病率,促进草莓生长,改善草莓品质;王兰英等[27]研究表明放线菌HN20对生菜具有显著促生作用,提高其各项生长指标;王世强等[28]发现链霉菌JD211能够显著提高土壤速效N、P含量,显著促进水稻幼苗生长,改善其生长微环境。然而,目前多数研究是在实验室条件下进行,而野外田间试验研究则较少开展。因此,亟需加强野外试验研究,以便真实评价放线菌菌肥在实际应用中的效果。【本研究切入点】放线菌菌肥的作用机制多种多样,包括防治植物病害、增加土壤有效磷和钾等矿物质元素的含量、提高种子发芽率和促进作物生长等[14-15,18,20-31]。菌株 JXJ-0136是笔者实验室筛选的一株具有肥料效应的放线菌,本文将以2种常见蔬菜为指示植物,结合实验室和野外田间栽培试验,研究菌株JXJ-0136对作物生长的影响及其机制。【拟解决的关键问题】明确 JXJ-0136菌株的分类学地位及促进作物生长的机制,分析其潜在应用价值,为放线菌菌肥的研制提供新材料。
1 材料与方法
试验于2013—2014年在九江学院完成。
1.1 菌株及培养基
菌株 JXJ-0136由笔者实验室分离自庐山土样的一株放线菌。供试植物白菜,特纯矮抗青(江西洪城种业有限公司);豇豆,美国无架豆(徐州市新天地种业有限公司)。
放线菌培养基:葡萄糖4 g,酵母浸粉4 g,麦芽浸粉5 g,微量盐1 mL(微量盐组成:FeSO4·7H2O 0.2 g,MnCl2·4H2O 0.1 g,ZnSO4·7H2O 0.1g,蒸馏水100 mL),H2O 1 L,pH 7.2,121℃灭菌30 min。解磷培养基:采用中华人民共和国农业行业标准NY/T 1847 —2010的配方[32]。葡萄糖10 g,(NH4)2SO40.5 g,NaCl 0.3 g,MgSO4·7H2O 0.3 g,KCl 0.3 g,FeSO4·4H2O 0.036 g,MnSO4·4H2O 0.03 g;有机磷(卵磷脂,2.0 g)或无机磷(Ca3(PO4)210 g);去离子水1 L;pH 7.0,121℃灭菌30 min。种子萌发培养基:参考文献[33],Ca(NO3)20.8207 g,KNO30.5056 g,MgSO4·7H2O 0.6162 g,KH2PO40.2722 g,Na2-EDTA 0.0745 g,FeSO4·7H2O 0.0557 g,H3BO30.02860 g,MnSO40.01105 g,CuSO4·5H2O 0.00092 g,ZnSO4·7H2O 0.0022 g,H2MoO40.0009 g,蒸馏水1 L,琼脂15—18 g,pH 7.0,121℃灭菌30 min。
1.2 放线菌JXJ-0136的鉴定
[34]对JXJ-0136进行初步鉴定。采用插片法[35]获得菌丝,用光学显微镜和扫描电镜观察菌丝形态特征。以ISP 2培养基[36]为基础培养基,采用不同的pH(2.0—14.0)、NaCl含量(m/v,0—10%)和培养温度(4—50℃)培养该菌,其中pH和NaCl耐受性试验培养温度为28℃,生长温度试验的培养基pH为7.4,NaCl含量为0。采用溶菌酶法提取基因组DNA,用细菌通用引物 primer A(5′-AGAGTTTG ATCCTGGCTCAG-3′)和primer B(5′-TACGGCTAC CTTGTTACGACTT-3′)进行PCR扩增,扩增产物送上海生工测序部测序,并与数据库 EzTaxon Server version 2.1(http://www.ezbiocloud.net/ eztaxon)的16S rRNA基因序列进行比较,调出相似性高的菌株序列,利用软件CLUSTAL_X1.83进行多重序列比对,采用MEGA软件,以邻接法构建16S rRNA基因序列系统进化树。
1.3 放线菌JXJ-0136对种子萌发及幼苗生长的影响
将菌株 JXJ-0136接种于放线菌培养基中,置于180 r/min、28℃的条件下培养3 d,再在种子萌发培养基中分别加入0.2%、0.4%、0.6%和0.8%(v/v)的放线菌培养液,摇匀后倒入无菌组培瓶中,冷却凝固后将经表面消毒的蔬菜种子置于培养基中,用组培膜封瓶口,于28℃、光照强度为3 000 lx,光暗比为12 h∶12 h的条件下培养,3—5 d后观察种子萌发及幼苗生长情况,并计算种子萌发率,测量幼苗株高和根长。另外,将未接种放线菌的培养基按照0.2%、0.4%、0.6% 和 0.8%(v/v)的量,加入种子萌发培养基中,作为培养基对照组;空白对照组加无菌水。
1.4 田间试验
利用实验地土壤直接做营养钵,并培育豇豆和白菜幼苗,将生长一致的幼苗进行移栽,移栽处每穴施加5 mL放线菌培养液,移栽后整个生长过程中不施加任何其他肥料,统一管理,定时观察作物生长状况,及时收获豆荚,测量并记录其长度和质量,取平均值;白菜45 d后收获,测量其株高和质量,取平均值。对照组施加 5 mL未接菌的放线菌培养基。田间试验于2013年4—10月在九江学院浔东校区实验地进行。
1.5 放线菌JXJ-0136对土壤总磷的影响
田间试验结束后,取植物根部土壤烘干并粉碎,用100目筛子过筛,称取0.5000 g置于锥形瓶中,参考汪小兰等[37]的方法提取土壤总磷。以少许蒸馏水润湿,加10 mL浓硫酸和1 mL高氯酸,于电炉上加热消煮,至瓶内溶液变为白色后继续消煮30 min,消煮液冷却后洗入200 mL容量瓶,并定容,采用钼锑抗比色法[32]测定溶液中可溶性磷的含量。
1.6 放线菌JXJ-0136在植物根部土壤的定殖
田间试验结束后,取试验组和对照组的植物根部,将根部土样用无菌水进行梯度稀释,取稀释液在ISP 2琼脂平板上涂布,置于28℃条件下培养3—7 d,观察平板上生长的放线菌形态特征,将JXJ-0136的疑似放线菌菌落进一步纯化,并与 JXJ-0136的菌落形态特征、色素产生情况、菌丝形态特征等进行比较,判断所分离在放线菌是否可能为放线菌JXJ-0136。
1.7 放线菌JXJ-0136纯培养解磷效果
取放线菌JXJ-0136培养液按照5%(v/v)的接种量,接入不溶性有机或无机磷液体培养基中,于 180 r/min、28℃的条件下培养,每24 h取样1次,共取样5次,样品溶液于4 500 r/min离心20 min,取上清,采用钼锑抗比色法[32]测定上清液中有效磷含量。第 5次取样后用pH计检测各样品的pH。
1.8 统计学处理
采用SPSS19.0统计软件对数据进行统计处理。确定3次重复实验的平均值(mean value)和标准偏差(standard deviation,SD),绘图数据取3次试验的平均值,试验组与对照组比较,采用单因素方差分析,P≤0.05为差异显著,P≤0.01为差异极显著。
2 结果
2.1 放线菌JXJ-0136初步鉴定
菌株JXJ-0136在6—45℃、pH 4.0—13.0和0—4%的盐浓度下均能生长,其中最适生长温度、pH和盐浓度分别为28℃、pH 8.0和1%。该菌气丝较发达,灰白色,孢子丝簇生,孢子长卵圆形(图1),16S rRNA基因序列(1 463 bp)分析表明,菌株JXJ-0136是链霉菌属的成员,与Streptomyces violascens ISP 5183T( AY999737) 、 S. somaliensis NBRC 12916T(AB184243)、S. hydrogenans NBRC 13475T(AB184868)、S. albidoflavus DSM 40455T(Z76676)和S. daghestanicus NRRL B-5418T(DQ442497)的相似性依次为 97.98%、97.71%、97.30%、97.23%和97.03%,但在系统进化树上后5株菌聚在单独一支,而菌株JXJ-0136形成单独一支(图2);该菌与其他有效发表种的16S rRNA基因序列相似性均低于97%。

图1 放线菌JXJ-0136孢子丝Fig. 1 Spore chains of actinomycete strain JXJ-0136
2.2 对种子萌发、幼苗株高和根长的影响
添加 0.2%—0.8%的无菌培养基对白菜和豇豆种子萌发率、幼苗株高和根长均无明显影响;而添加0.2%—0.6%的放线菌JXJ-0136培养液时,白菜种子萌发率、幼苗株高和根长均随培养液剂量的增加而增加,在剂量为 0.6%时试验组比对照组分别高(12.61± 2.93)%、(53.03±5.72)%和(51.92±10.13)%(P<0.01),但继续增加放线菌培养液剂量时,白菜种子萌发率、幼苗株高和根长均略有减少;在试验浓度下(0.2%—0.8%),豇豆种子萌发率、幼苗株高和根长均随放线菌培养液剂量的增加而增加,在剂量为0.8%时,试验组分别比对照组高(21.18±2.04)%、(22.33± 5.56)%和(20.08±4.32)%(P<0.01)(图3)。
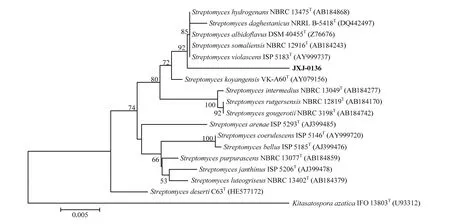
图2 菌株JXJ-0136及其相关种属的系统进化树Fig. 2 Phylogenetic tree based on 16 s rRNA gene sequences analysis of strain JXJ-0136 and related taxa
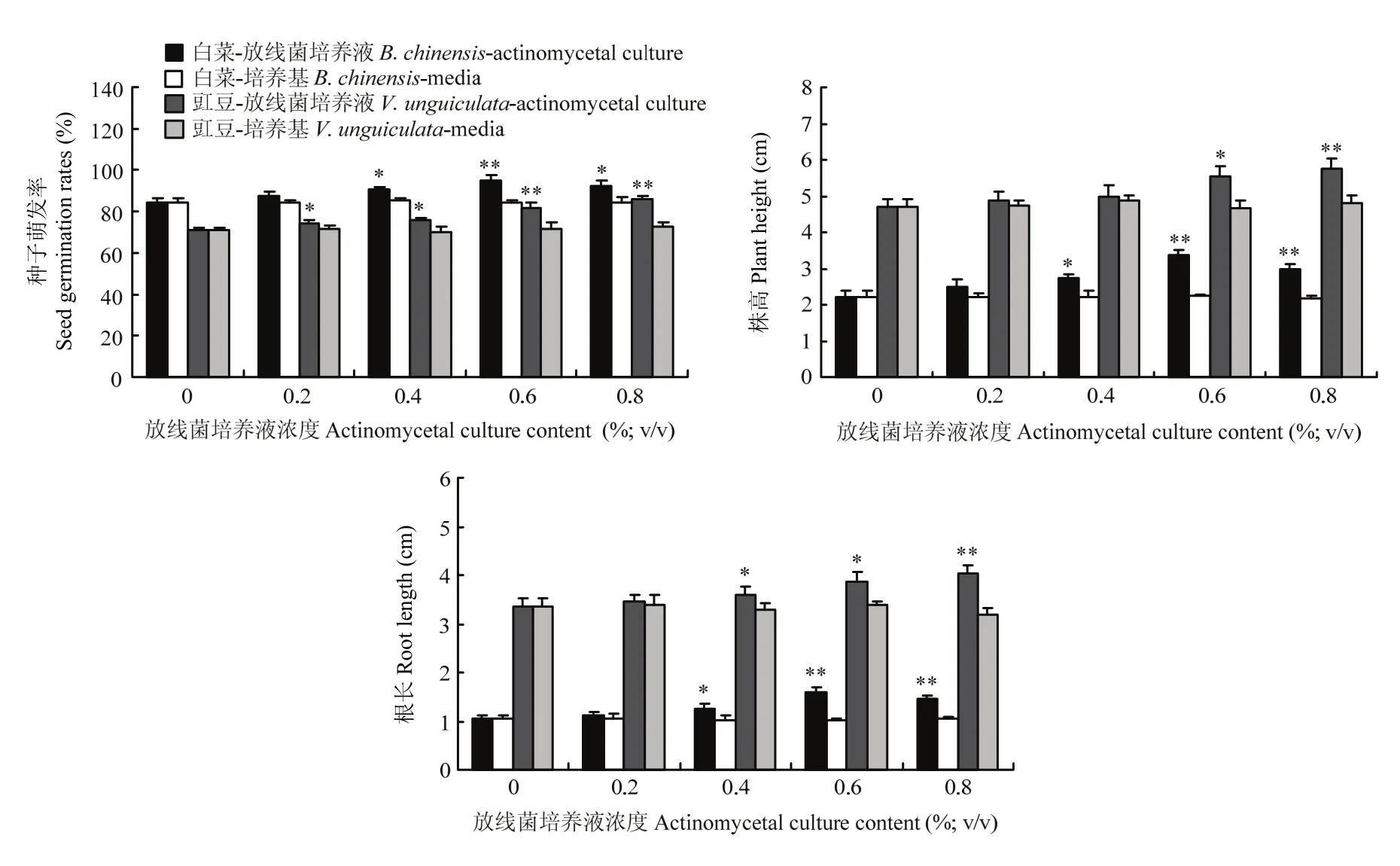
图3 放线菌JXJ-0136培养液对白菜和豇豆种子萌发率、幼苗株高和根长的影响Fig. 3 Influences of actinomycete strain JXJ-0136 culture on the germination rates, heights and root lengths of seedlings of B. chinensis and V. unguiculata
2.3菌株对栽培作物生长及产量的影响
放线菌 JXJ-0136对试验蔬菜的生长和产量均有较大影响。试验组豆荚平均长度为(34.28±1.10)cm,比对照组(31.51±0.96)cm增加(8.79±3.48)%(P <0.05);单株豇豆的平均产量为(195.98±5.92)g,比对照组(115.08±6.17)g增产(70.29±5.15)%(P <0.01);白菜平均高度为(13.65±0.26)cm,比对照组(10.45±0.38)cm增加(30.86±2.49)%(P<0.01);白菜每株平均质量为(205.63±9.92)g,比对照组(161.17±2.07)g增加(27.59 ± 6.15)%(P<0.01)。
2.4 放线菌JXJ-0136对土壤总磷的影响
无论是否施加放线菌JXJ-0136,栽培作物后土壤总磷含量均会显著降低(P<0.05,P<0.01),但对照组白菜土壤总磷含量比栽培前(1.44±0.06)mg·g-1降低了(13.32±2.75)%,而放线菌试验组白菜土壤总磷含量却比栽培前降低了(23.56±2.65)%,是对照组的(1.77±0.29)倍(P<0.01);对照组(未施加放线菌)豇豆土壤总磷含量只比栽培前(1.43±0.07)mg·g-1降低了(12.76±3.34)%,而放线菌试验组豇豆土壤总磷含量却比栽培前降低了(37.10±1.98)%,是对照组的(2.70±0.15)倍(P<0.01),这说明放线菌促进了作物对土壤磷的利用(图4)。
2.5 放线菌JXJ-0136在植物根部的定殖
从施加放线菌 JXJ-0136培养液的白菜和豇豆根部土样中,都分离到了与JXJ-0136菌落形态特征、产生的色素颜色、菌丝形态特征等均一致的放线菌,其中白菜根际土样中为(10.33±2.52)×103CFU/g干土,豇豆根际土样中为(8.33±2.08)×103CFU/g干土;而在未施加放线菌 JXJ-0136培养液的对照组植物根部土样中,未分离到类似放线菌,说明放线菌JXJ-0136能够在白菜和豇豆根部土壤定殖。
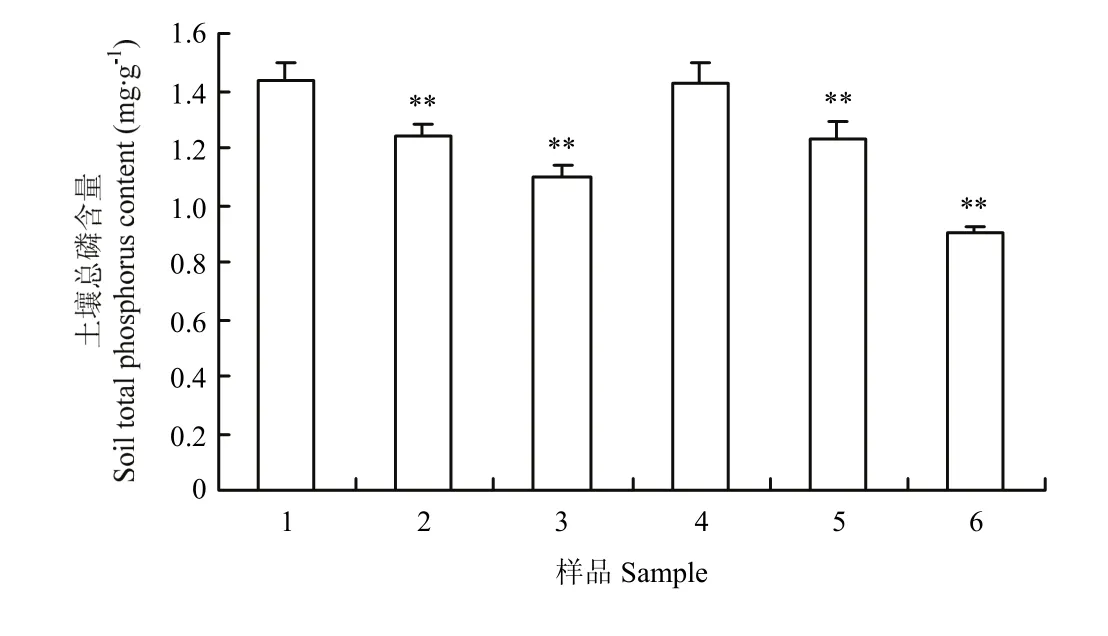
图4 放线菌JXJ-0136对土壤总磷含量的影响Fig. 4 Influences of actinomycete strain JXJ-0136 on the soil total phosphorus contents
2.6 放线菌JXJ-0136液体解磷效果
在纯培养条件下,放线菌JXJ-0136对不溶性有机磷和无机磷均具有较好的降解作用,培养液中有效磷的含量随培养时间的延长而显著增加(P<0.01)(图5),接种之前,培养基中有效磷的含量分别只有(0.86 ±0.05)和(1.26±0.18)mg,接种后5 d,分别增加到(73.94±0.94)和(7.12 ± 0.28)mg;而未接菌的无机磷培养基中有效磷浓度基本未发生变化,有机磷培养基中有效磷浓度稍有上升,但其浓度增加速率远低于试验组(P<0.01)(图5)。试验结束时,无机磷和有机磷培养基pH由起始pH 7.0分别降至5.0和6.0。
3 讨论
放线菌JXJ-0136与Streptomyces violascens ISP 5183T亲缘关系最近。然而,S. violascens的孢子丝为2—8圈螺旋形[38],而菌株JXJ-0136孢子丝簇状生长,长曲状,不形成螺旋。一般认为,16S rRNA基因序列相似性在95%以下可定为新属,98%以下可定为新种[34]。放线菌JXJ-0136与S. violascens ISP 5183T相似性为97.98%,略小于98%,因此,初步确定JXJ-0136是链霉菌属的一个潜在新种,但需要DNA-DNA分子杂交等试验结果的进一步确认。

图5 放线菌JXJ-0136对不溶性磷的降解活性Fig. 5 Degradation activities of actinomycete strain JXJ-0136 on insoluble phosphorus
放线菌菌肥“5406”对不同农作物生长的影响不同,其增产率在 5%—45%[15]。王兰英等[27]研究表明灰色链霉菌HN20对黄瓜促生作用不明显,但在低剂量条件下却显著促进生菜种子胚芽和胚根生长,增加其生物量,而在高浓度下则有抑制作用。李堆淑[29]研究发现细黄链霉菌培养液低浓度促进黄芩种子萌发和幼苗生长,高浓度则表现抑制作用。本研究也发现类似现象,在试验条件下,豇豆种子的萌发率、幼苗株高和根长,均随放线菌JXJ-0136使用剂量的增加而增加;但白菜种子的萌发率、幼苗株高和根长在剂量<0.6%时,随使用剂量增加而增加,>0.6%时则随着剂量增加而降低;同时该菌对白菜和豇豆的增产效果也具有显著差异,这说明放线菌JXJ-0136对不同农作物的影响不同。
生长调节物质能够刺激植物种子萌发,提高发芽率[39]。放线菌菌肥“5406”能够产生多种植物生长调节物质,提高作物种子萌发率,促进根的生长[15]。放线菌 JXJ-0136制剂能够显著增加白菜和豇豆种子萌发率、幼苗株高及根长,这说明该菌能够产生植物生长调节物质,但这些生长调节物质的理化性质及类型等尚需要进一步研究。
溶解土壤中不溶性元素,为植物提供可吸收的矿物质营养,是微生物肥料促使农作物增产的主要机制之一。许多放线菌能够有效提高土壤中有效磷的含量,如放线菌菌肥“5406”[16]、Micromonospora aurantiaca MAMPM和灰色链霉菌SGMPM[12]、玫瑰黄链霉菌Menmyco-93-63[25]和链霉菌JD21[28]等。本研究表明,放线菌JXJ-0136在纯培养条件下,接种于不溶性无机磷和有机磷培养基并培养,其有效磷含量均随着培养时间的延长而迅速增加;在土壤中使用放线菌JXJ-0136制剂后,土壤中总磷含量显著降低,这说明该菌能够增加土壤中有效磷含量,促进植物对磷的吸收。因此,溶解不溶性磷,为植物提供可吸收的有效磷,是放线菌JXJ-0136促进植物生长的重要机制之一。
分泌小分子有机酸是解磷微生物溶解不溶性无机磷的重要方式,这些小分子有机酸主要包括草酸、乙酸、乳酸、酒石酸、琥珀酸、柠檬酸、丁二酸、丙酸和苹果酸等,由于有机酸的产生,往往导致pH降低[40-42]。放线菌JXJ-0136接种于不溶性无机磷培养基,培养5 d后,培养液的pH比空白对照组降低了2.0,这说明产生有机酸是该菌溶解不溶性无机磷的机制之一,然而该菌分泌何种有机酸尚需要进一步研究。
JXJ-0136菌株对不溶性有机磷也具有一定的降解作用。许多微生物在环境中缺少正磷酸盐时,可诱导其细胞产生磷酸酶,将有机磷等降解为可溶性正磷酸盐,满足其生长需要。但由于不溶性有机磷培养基接种该菌培养5 d后,培养液的pH比空白对照组降低了0.98,pH降低可促进卵磷脂水解。因此,该菌对有机磷的降解作用是由于其分泌了有机酸,有机酸再促使卵磷脂的水解作用,还是由于该菌能够产生水解卵磷脂的磷酸酶,尚需要进一步研究。
有效微生物能否在施用的植物根际土壤中长期存活,并与植物形成特定的互利关系,是微生物肥料肥效能否持久的关键。放线菌菌肥“5406”肥效持久与其有效菌在很多作物根际土壤中能够长期、大量定殖有关[14]。张艳杰等[25-28]研究也发现,具有肥料效应的链霉菌能在作物根部土壤定殖良好。本研究在整个栽培试验中,只在栽培时施加一次放线菌 JXJ-0136制剂,但其肥效却持续至白菜和豇豆整个生长周期,这与该菌能够在作物根际土壤中定殖有一定联系。
4 结论
放线菌JXJ-0136是链霉菌属的一个潜在新种,该菌能够产生未知生长调节物质,提高白菜和豇豆种子萌发率,增加幼苗株高和根长,并定殖于作物根际土壤,溶解土壤中不溶性磷,为植物提供可吸收的有效磷,促进作物生长,提高作物产量,在微生物肥料研制方面具有一定的潜在应用价值。
References
[1] LIU M, KLEMENS E, ZHANG B, STEPHANIE I J H, LI Z P,ZHANG T L, SABINE R. Effect of intensive inorganic fertilizer application on microbial properties in a paddy soil of subtropical China. Agricultural Sciences in China, 2011, 10(11): 1758-1764.
[2] 徐明岗. 中国土壤肥力演变. 北京: 中国农业科学与技术出版社,2006. XU M G. The Evolution of China’s Soil Fertility. Beijing: China Agricultural Science and Technology Press, 2006. (in Chinese)
[3] KIBBLEWHITE M G, RITZ K, SWIFT M J. Soil health in agricultural systems. Philosophical Transactions of the Royal Society B: Biological Sciences, 2008, 363: 685-701.
[4] GEISSELER D, SCOW K M. Long-term effects of mineral fertilizers on soil microorganisms-A review. Soil Biology & Biochemistry, 2014,75: 54-63.
[5] AYALA S, RAO E V. Perspective of soil fertility management with a focus on fertilizer use for crop productivity. Current Science, 2002,82(7): 797-807.
[6] HU J L, LIN X G, WANG J H, DAI J, CUI X C, CHEN R R,ZHANG J B. Arbuscular mycorrhizal fungus enhances crop yield and P-uptake of maize (Zea mays L.): A field case study on a sandy loam soil as affected by long-term P-deficiency fertilization. Soil Biology and Biochemistry, 2009, 41(12): 2460-2465.
[7] WILLIAMS A, BÖRJESSON G, HEDLUND K. The effects of 55 years of different inorganic fertiliser regimes on soil properties and microbial community composition. Soil Biology and Biochemistry,2013, 67: 41-46.
[8] KAUR G, REDDY M S. Effects of phosphate-solubilizing bacteria,rock phosphate and chemical fertilizers on maize-wheat cropping cycle and economics. Pedosphere, 2015, 25(3): 428-437.
[9] WELLER D M. Pseudomonas biocontrol agents of soilborne pathogens: Looking back over 30 years. Phytopathology, 2007, 97(2):250-256.
[10] SINGH H, REDDY M S. Effect of inoculation with phosphate solubilizing fungus on growth and nutrient uptake of wheat and maize plants fertilized with rock phosphate in alkaline soils. European Journal of Soil Biology, 2011, 47: 30-34.
[11] ADESEMOYE A O, TORBERT H A, KLOEPPER J W. Enhanced plant nutrient use efficiency with PGPR and AMF in an integrated nutrient management system. Canadian Journal of Microbiology,2008, 54: 876-886.
[12] HAMDALI H, HAFIDI M, VIROLLE M J, OUHDOUCH Y. Growth promotion and protection against damping-off of wheat by two rock phosphate solubilizing actinomycetes in a P-deficient soil under greenhouse conditions. Applied Soil Ecology, 2008, 40(3): 510-517.
[13] 尹莘耘, 荀培琪, 林声远, 邱桂英, 张均康. 5406抗生菌肥料作用机制的研究Ⅱ. 产生抗菌物质的分析研究. 微生物学报, 1965,11(2): 270-274. YIN S Y, XUN P Q, LIN S Y, QIU G Y, ZHANG J K. Studies on the mechanisms of antagonistic fertilizer “5406” II. Analysis of the antibiotic substances. Acta Microbiologica Sinica, 1965, 11(2):270-274. (in Chinese)
[14] 尹莘耘, 张均康, 荀培琪. 5406抗生菌肥料作用机制的研究IV. 抗生菌在土壤中和作物根周围活动情况的研究. 微生物学报, 1965,11(2): 281-287. YIN S Y, ZHANG J K, XUN P Q. Studies on the mechanisms of antagonistic fertilizer “5406” IV. The status of the antagonist in the soil and around the crop root. Acta Microbiologica Sinica, 1965, 11(2):281-287. (in Chinese)
[15] 尹莘耘, 荀培琪, 邱桂英, 林声远, 张均康. 5406抗生菌肥料作用机制的研究I. 5406号抗生菌产生刺激物质的分析研究. 微生物学报, 1965, 11(2): 259-269. YIN S Y, XUN P Q, QIU G Y, LIN S Y, ZHANG J K. Studies on the mechanisms of antagonistic fertilizer “5406” I. Isolation of the stimulating substances. Acta Microbiologica Sinica, 1965, 11(2):259-269. (in Chinese)
[16] 尹莘耘, 张均康, 荀培琪. 5406抗生菌肥料作用机制的研究Ⅲ. 抗生菌在不同土类中的适应性及其转化氮、磷元素的分析. 微生物学报, 1965, 11(2): 275-280. YIN S Y, ZHANG J K, XUN P Q. Studies on the mechanisms of antagonistic fertilizer “5406” III. Analysis of the adaptability of antagonist “5406” in different types of soils and the conversion ofnitrogen and phosphorus. Acta Microbiologica Sinica, 1965, 11(2):275-280. (in Chinese)
[17] HAMDALI H, BOUIZGARNE B, HAFIDI M, LEBRIHI A,VIROLLE M J, OUHDOUCH Y. Screening for rock phosphate solubilizing Actinomycetes from Moroccan phosphate mines. Applied Soil Ecology, 2008, 38: 12-19.
[18] HAMDALI H, HAFIDI M, VIROLLE M J, OUHDOUCH Y. Rock phosphate solubilizing Actinomycetes: screening for plant growth promoting activities. World Journal of Microbiology & Biotechnology,2008, 24(11): 2565-2575.
[19] 陶天申, 岳莹玉, 梁绍芬, 桑金隆, 尹莘耘. 5406抗生菌——泾阳链霉菌新种. 微生物学报, 1979, 19(3): 249-254. TAO T S, YUE Y Y, LIANG S F, SANG J L, YIN S Y. The antibiotic strain 5406-Streptomyces jiangyangensis n. sp. Acta Microbiologica Sinica, 1979, 19(3): 249-254. (in Chinese)
[20] 赵娟, 杜军志, 薛泉宏, 段春梅, 王玲娜, 申光辉, 陈秦, 薛磊. 3株放线菌对甜瓜幼苗的促生与抗性诱导作用. 西北农林科技大学学报 (自然科学版), 2010, 38(2): 109-116. ZHAO J, DU J Z, XUE Q H, DUAN C M, WANG L N, SHEN G H,CHEN Q, XUE L. The growth-promoting effect and resistance induction of 3 antagonistic actinomyces on Cucumis melo L.. Journal of Northwest A &F University (Natural Science Edition), 2010, 38(2):109-116. (in Chinese)
[21] 赵娟, 薛泉宏, 王玲娜, 段春梅, 薛磊, 毛宁. 多功能放线菌Act12对土传病原真菌的拮抗性及其鉴定. 中国生态农业学报, 2011,19(2): 394-398. ZHAO J, XUE Q H, WANG L N, DUAN C M, XUE L, MAO N. Antagonistic effect of multifunctional actinomycete strain Act12 on soil-borne pathogenic fungi and its identification. Chinese Journal of Eco-Agriculture, 2011, 19(2): 394-398. (in Chinese)
[22] 段佳丽, 薛泉宏, 舒志明, 王东胜, 何斐. 放线菌 Act12与腐植酸钾配施对丹参生长及其根域微生态的影响. 生态学报, 2015, 35(6):1807-1819. DUAN J L, XUE Q H, SHU Z M, WANG D S, HE F. Effects of combined application of actinomycetes Act12 bio-control agents and potassium humate on growth and microbial flora in rooting zone of Salvia miltiorrhiza Bge. Acta Ecologica Sinica, 2015, 35(6):1807-1819. (in Chinese)
[23] 吴艳辉, 赵春田, 裘娟萍. 植物病原菌拮抗放线菌的分离筛选与鉴定. 农药, 2010, 49(2): 146-149. WU Y H, ZHAO C T, QIU J P. Isolation, screening and identification of antagonistic actinomycetes inhibiting plant pathogens. Agrochemicals,2010, 49(2): 146-149. (in Chinese)
[24] 王兰英, 王琴, 骆焱平. 金黄垂直链霉菌 HN6对香蕉的防病促生作用. 西北农林科技大学学报 (自然科学版), 2015, 43(5): 163-167. WANG L Y, WANG Q, LUO Y P. Disease preventing and growth promoting effects of Streptomyces aureoverticillatus strain HN6 on banana. Journal of Northwest A & F University (Natural Science Edition), 2015, 43(5): 163-167. (in Chinese)
[25] 张艳杰, 杨淑, 陈英化, 沈凤英, 乔丹娜, 李亚宁, 刘大群. 玫瑰黄链霉菌防治番茄连作障碍及对土壤微生物区系的影响. 西北农业学报, 2014, 23(8): 122-127. ZHANG Y J, YANG S, CHEN Y H, SHEN F Y, QIAO D N, LI Y N,LIU D Q. Efficiency of Streptomyces roseoflavus against tomato continuous cropping obstacle and effects to soil microflora. Acta Agriculturae Boreali-occidentalis Sinica, 2014, 23(8): 122-127. (in Chinese)
[26] 沈婷, 杨华, 戴乐天, 邓照亮, 王世梅. 吸水链霉菌(Streptomyces hygroscopicus) B04固体菌剂对草莓生长及果实品质影响的研究.农业资源与环境学报, 2016, 33(1): 49-54. SHEN T, YANG H, DAI L T, DENG Z L, WANG S M. Effects of solid fermentation agent of Streptomyces hygroscopicus B04 on strawberry growth and fruit quality. Journal of Agricultural Resources and Environment, 2016, 33(1): 49-54. (in Chinese)
[27] 王兰英, 姚明燕, 骆焱平. 放线菌HN20对黄瓜和生菜的促生作用.贵州农业科学, 2016, 44(1): 98-100. WANG L Y, YAO M Y, LUO Y P. Growth-promoting effect of actinomycete HN20 strain on cucumber and lettuce. Guizhou Agricultural Science, 2016, 44(1): 98-100. (in Chinese)
[28] 王世强, 魏赛金, 杨陶陶, 李庆蒙, 涂国全, 倪国荣, 潘晓华. 链霉菌 JD211对水稻幼苗促生作用及土壤细菌多样性的影响. 土壤学报, 2015, 52(3): 673-681. WANG S Q, WEI S J, YANG T T, LI Q M, TU G Q, NI G R, PAN X H. Effect of Streptomyces JD211 promoting growth of rice seedlings and diversity of soil bacteria. Acta Pedologica Sinica, 2015, 52(3):673-681. (in Chinese)
[29] 李堆淑. 细黄链霉菌对黄芩种子萌发特性的影响. 种子, 2015,34(9): 24-27. LI D S. Effects of Streptomyces microflavus on seed germination characteristics of Scutellaria baicalensis. Seed, 2015, 34(9): 24-27. (in Chinese)
[30] 张忠良, 何斐, 马军妮, 薛泉宏, 楚金强. 放线菌剂及其与有机肥配施对魔芋的促生作用. 西北农林科技大学学报 (自然科学版),2016, 44(3): 173-180. ZHANG Z L, HE F, MA J N, XUE Q H, CHU J Q. Growthpromoting effect of actinomycetes agents and organic fertilizer onAmorphophallus konjac. Journal of Northwest A & F University (Natural Science Edition), 2016, 44(3): 173-180. (in Chinese)
[31] 朱金英, 王友平, 郭建军, 张书良, 高春华. 细黄链霉菌 (AMCC 400001) 与有机肥配合施用对设施番茄生长和产量的影响. 北方园艺, 2015(22): 177-181. ZHU J Y, WANG Y P, GUO J J, ZHANG S L, GAO C H. Effect of Streptomyces microflavus (AMCC 400001) and organic fertilizer combined application on the growth and yield of tomato in greenhouse. North Horticulture, 2015(22): 177-181. (in Chinese)
[32] 中华人民共和国农业部种植业管理司. 微生物肥料生产菌株质量评价通用技术要求: NY/T 1847—2010. 2011: 4-5. [2011-10-13]. Planting Management, Ministry of Agriculture of the People’s Republic of China. General technical requirements for production strain quality of microbial fertilizer: NY/T 1847-2010, 2011: 4-5. [2011-10-13]. (in Chinese)
[33] 申建波, 毛达如. 植物营养研究方法. 3版. 北京: 中国农业大学出版社, 2011. SHEN J B, MAO D R. Research Methods of Plant Nutrition. 3rd ed. Beijing: China Agricultural University Press, 2011. (in Chinese)
[34] 徐丽华, 李文均, 刘志恒, 蒋成林. 放线菌系统学——原理、方法及实践. 北京: 科学出版社, 2007. XU L H, LI W J, LIU Z H, JIANG C L. Actinomycete Systematic -Principle, Methods and Practice. Beijing: Science Press, 2007. (in Chinese)
[35] 黄秀梨, 辛明秀. 微生物学实验指导. 2版. 北京: 高等教育出版社,2008. HUANG X L, XIN M X. Microbiology Experiment Guidance. 2nd ed. Beijing: Higher Education Press, 2008. (in Chinese)
[36] SHIRLING E B, GOTTLIEB D. Methods for characterization of Streptomyces species. International Journal of Systematic Bacteriology,1966, 16(3): 313-340.
[37] 汪小兰, 田荷珍, 耿承延. 基础化学. 北京: 高等教育出版社, 1995. WANG X L, TIAN H Z, GENG C Y. Basic Chemistry. Beijing:Higher Education Press, 1995. (in Chinese)
[38] TRESNER H D, HAYES J A, BACKUS E J. Streptomyces wistariopsis sp. nov. a new violet-spored species. International Journal of Systematic Bacteriology, 1969, 19(2): 141-152.
[39] 宋文坚, 曹栋栋, 金宗来, 周伟军. 影响根寄生植物列当种子萌发因素的研究. 种子, 2005, 24(2): 44-47. SONG W J, CAO D D, JIN Z L, ZHOU W J. The factors of influencing seed germination of root parasitic plants broomrape. Seed,2005, 24(2): 44-47. (in Chinese)
[40] CHEN Y P, PEKHA P D, ARUN A B, SHEN F T, LAI W A,YOUNG C C. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Applied Soil Ecology, 2006, 34: 33-41.
[41] HAMEEDA B, REDDY Y H, RUPELA O P, KUMAR G N, REDDY G. Effect of carbon substrates on rock phosphate solubilization by bacteria from composts and macrofauna. Current Microbiology, 2006,53(4): 298-302.
[42] 张英, 芦光新, 谢永丽, 姚拓, 荣良燕, 朱颖. 溶磷菌分泌有机酸与溶磷能力相关性研究. 草地学报, 2015, 23(5): 1033-1038. ZHANG Y, LU G X, XIE Y L, YAO T, RONG L Y, ZHU Y. The relationship between organic acid secreted from phosphorus-solubilizing bacteria and the phosphate-solubizing ability. Acta Agrestia Sinica,2015, 23(5): 1033-1038. (in Chinese)
(责任编辑 岳梅)
Influences of Actinomycete Strain JXJ-0136 on the Growth of Brassica chinensis and Vigna unguiculata and Its Phosphate Solubilization
ZHANG Bing-huo, LI Han-quan, LUO Juan-yan, YANG Jian-yuan, SHI Hong-qiu, SUN Feng-zhen
(College of Pharmacy and Life Sciences, Jiujiang University, Jiujiang 332000, Jiangxi)
【Objective】The objective of this study is to determine the taxonomic status of an actinomycete strain JXJ-0136,investigate its ability of dissolving insoluble phosphorus, the colonization in the rhizospheric soil of crops, and its influences on the seed germination, seedling growth and yield of vegetables, and to evaluate the application value of strain JXJ-0136 in developingmicrobial fertilizer.【Method】Taxonomic status of strain JXJ-0136 was determined on the basis of the cultural and morphological characteristics, and the phylogenetic analysis of 16S rRNA gene sequence. Influences of strain JXJ-0136 on the seed germination and seedling growth were studied using agar plate. The field cultivation tests were carried out to investigate the influences of strain JXJ-0136 on the growth and yield of vegetables. The total contents of phosphorus in the soil before and after the field trial were measured to investigate the influence of strain JXJ-0136 on the utilization of phosphorus in the soil by crops. The colonization of strain JXJ-0136 in the rhizospheric soil of the plants was investigated by isolation of the microorganisms in rhizosphere soil. The efficiencies of strain JXJ-0136 to dissolve insoluble inorganic and organic phosphorus were investigated using liquid pure culture. The model vegetables of the study were Brassica chinensis and Vigna unguiculata. 【Result】 Growth of actinomycete strain JXJ-0136 was observed at 6-45℃, pH 4.0-13.0 and 0-4% (w/v) NaCl, with optimal growth at 28℃, pH 8.0 and 1% (w/v) NaCl. Strain JXJ-0136 developed well-branched aerial mycelia on ISP 2 medium. The aerial mycelia was off-white in color. Its spore chains were fascicular with elliptical spores. The 16S rRNA gene sequence was closest to Streptomyces violascens, S. somaliensis, S. hydrogenans, S. albidoflavus and S. daghestanicus with the similarities of 97.98%, 97.71%, 97.30%, 97.23% and 97.03%,respectively. However, strain JXJ-0136 formed different clades on phylogenetic tree. The culture broth of strain JXJ-0136 enhanced the seed germination and the seedling growth significantly. After addition of 0.2%-0.8% broth culture of strain JXJ-0136, the seed germination rate, plant height and root length of B. chinensis were 3.55%-12.61%, 13.91%-53.03% and 7.37%-51.92% higher than those of the controls, respectively. The seed germination rate, plant height and root length of V. unguiculata were 4.71%-21.18%,3.60%-22.33% and 2.37%-20.08% higher than these of the controls, respectively. The field cultivation tests indicated that strain JXJ-0136 could colonize in the rhizospheric soil of the plants, and promoted crops to utilize phosphorus in the soil, and enhanced the yields of the crops. After inoculating with 5 mL broth culture of strain JXJ-0136 to each plant, the soil total phosphorus contents of B. chinensis and V. unguiculata decreased by (23.56±2.65)% and (37.10±1.98)%, respectively, at the end of the tests, which were (1.77±0.29) and (2.70±0.15) times of the controls (P<0.01). The yields of B. chinensis and V. unguiculata increased by (27.59±6.15)% and (70.29±5.15)% (P<0.01) than the controls, respectively. After inoculating strain JXJ-0136 and culturing for 5 days under liquid pure culture condition, the pH values of inorganic and organic phosphorus cultures decreased to 5.0 and 6.0 initially from pH 7.0, respectively, and available phosphorus in the cultures of inorganic and organic phosphorus increased by (73.94±0.94) and (7.12±0.28) mg (P<0.01), respectively. 【Conclusion】Actinomycete JXJ-0136 is a member of the genus Streptomyces. With good properties including increasing the seed germination, plant height and root length of seedling, colonizing in rhizospheric soil, increasing the content of the available phosphorus in the soil, enhancing the crops to utilize the phosphorus in the soil and promoting the growth and yields of crops, strain JXJ-0136 has a potential application value in developing microbial fertilizer.
microbial fertilizer; actinomycete JXJ-0136; Streptomyces; Brassica chinensis; Vigna unguiculata; phosphoric solubilization
2016-03-23;接受日期:2016-05-05
国家自然科学基金(31060010)、江西省科技支撑计划(20121BBF60048)
联系方式:张炳火,E-mail:binghuozh@126.com。通信作者李汉全,Tel:0792-8565939;E-mail:lihanquan62@126.com
