LiBr-[BMIM]Cl/H2O工质对的饱和蒸气压、结晶温度和腐蚀性
2016-07-12罗春欢张渊苏庆泉北京科技大学机械工程学院北京00083北京科技大学北京市高校节能与环保工程研究中心北京00083
罗春欢,张渊,苏庆泉(北京科技大学机械工程学院,北京 00083;北京科技大学北京市高校节能与环保工程研究中心,北京 00083)
LiBr-[BMIM]Cl/H2O工质对的饱和蒸气压、结晶温度和腐蚀性
罗春欢1,2,张渊1,苏庆泉1,2
(1北京科技大学机械工程学院,北京 100083;2北京科技大学北京市高校节能与环保工程研究中心,北京 100083)
摘要:为了解决LiBr/H2O工质对易结晶和腐蚀性强的问题,提出了LiBr-[BMIM]Cl/H2O和LiBr-[BMIM]Br/H2O新型工质对,研究了离子液体 [BMIM]Cl和[BMIM]Br对LiBr/H2O结晶温度和饱和蒸气压的影响。对质量比为2.5的LiBr-IL(ionic liquids)/H2O的饱和蒸气压、结晶温度和腐蚀性进行了测定并与LiBr/H2O的进行了比较,结果表明[BMIM]Cl/H2O和[BMIM]Br/H2O的饱和蒸气压与质量分数低8%~9%的LiBr/H2O的饱和蒸气压基本相同。在常用工作浓度范围内,LiBr-[BMIM]Cl/H2O的结晶温度比相同吸收能力的LiBr/H2O的低约30℃。在相同的腐蚀条件下,LiBr-[BMIM]Cl/H2O对碳钢的腐蚀性明显小于LiBr/H2O,对紫铜的腐蚀性与LiBr/H2O的基本相同。因此,采用LiBr-[BMIM]Cl/H2O作为替代工质对具有一定的应用潜力。
关键词:工质对;离子液体;饱和蒸气压;结晶温度;腐蚀
2015-02-13收到初稿,2015-10-11收到修改稿。
联系人:苏庆泉。第一作者:罗春欢(1983—),男,博士研究生,讲师。
Received date: 2015-02-13.
Foundation item: supported by the Fundamental Research Funds for the Central Universities (FRF-TP-14-022A1) and the Key Project of the Ministry of Education and Guangdong Province (2009A090100032).
引 言
吸收式热泵可以通过余热驱动来提高热量的品位或制冷,从而有效利用余热,因而在节能领域具有广阔的发展前景。LiBr/H2O是一种应用广泛的工质对,但存在易结晶和高温腐蚀性强的问题,严重制约了吸收式热泵的发展。
近年来,基于离子液体(ionic liquid,IL)工质对的吸收式热泵正受到越来越多的关注。现有离子液体中,咪唑类离子液体最为稳定,其主要与H2O、NH3、CO2、醇类和HFCs等制冷剂组成新型的工质对[1-5]。Kim等[6]的研究结果表明,H2O作为制冷剂可以获得较高的COP,同时具有天然环保,容易获得优良特性,因此,IL/H2O工质对成为当今研究的一个热点。皇甫立霞等[7-9]对[BMIM]BF4/H2O、[EMIM]Ac/H2O和[HMIM]Cl/H2O的热物性进行了系统的测定,并对基于这些工质对的制冷循环性能进行了理论分析,结果表明IL/H2O工质对不会出现结晶问题,在较高的发生温度下[EMIM]Ac/H2O获得的COP最大且与LiBr/H2O相当,但在常规工况下[EMIM]Ac/H2O达不到LiBr/H2O系统的性能系数。Zhang等[10]对基于[EMIM]DMP/H2O和[DMIM]DMP/H2O工质对的吸收式热泵系统的性能进行了模拟计算,并与LiBr/H2O和TFE/E181系统的性能进行了比较,发现[DMIM]DMP/H2O系统的COP比TFE/E181的大,但是小于LiBr/H2O系统。Dong等[11-14]对阴离子为Cl、Br、BF4和DMP的咪唑类离子液体展开了一系列的研究,确定了离子液体吸收剂的选择标准,测定了IL/H2O的饱和蒸气压和比热容等热物性,并同样对基于[DMIM]DMP/H2O的吸收式制冷系统的性能进行了计算,结果发现[DMIM]DMP/H2O在结晶温度和腐蚀性上优于LiBr/H2O,但COP略有降低。以上研究表明IL/H2O工质对具有不易结晶和腐蚀性小的优势,但其吸收能力弱于LiBr/H2O,实际工况中所需浓度较高,导致溶液黏度和循环倍率过大,降低了系统的COP。
为了解决现有工质对存在的易结晶和腐蚀性强的问题,本文通过在LiBr/H2O的基础上添加稳定性和亲水性好的离子液体,形成新型的LiBr-IL/H2O工质对。根据Nie等[15]的研究结果,阴离子为卤族元素的咪唑类离子液体具有较强的稳定性和亲水性,因此,本文对LiBr-[BMIM]Cl/H2O和LiBr-[BMIM]Br/H2O的饱和蒸气压、结晶温度和腐蚀性进行测定并与LiBr/H2O的进行比较。
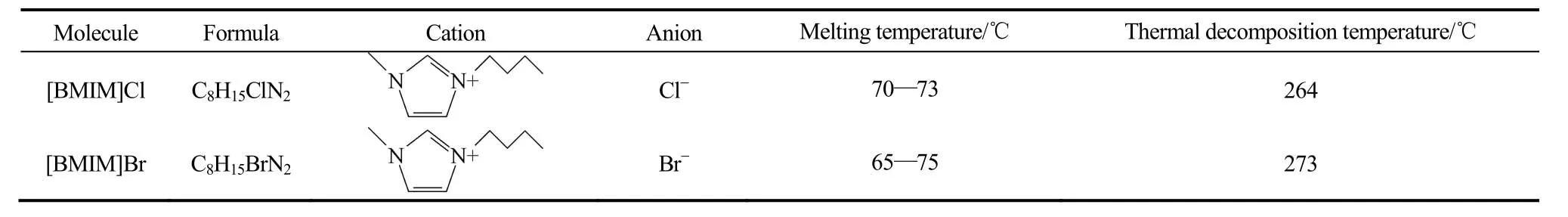
表1 [BMIM]Cl和[BMIM]Br的分子结构与性质参数Table 1 Molecule structure and properties of [BMIM]Cl and [BMIM]Br
1 实验材料和方法
1.1实验材料
实验用溴化锂(GR级,≥99%)和铬酸锂(AR级,≥98%)为天津市津科精细化工研究所生产,盐酸(AR级,36%~38%)和丙酮(AR级,≥98%)为北京化工厂生产,所用超纯水的纯度为18.2 MΩ·cm-1。实验所用离子液体[BMIM]Cl(AR级,≥99%)和[BMIM]Br(AR级,≥99%)为上海成捷化学有限公司生产,分子结构与性质参数见表1。
1.2实验装置和方法
1.2.1结晶温度结晶温度采用动态变温法测定,具体测定方法:用精密天平(梅特勒-托利多PL2002,0.1 mg)称取并配制待测溶液,放置于已设定为一定温度的精密恒温循环器(JDC-1006,0.1℃)中,保持恒温状态24 h后,观察其是否析出晶体。如果未结晶,降低设定温度,继续观察;如果结晶,则适当提高精密恒温循环器的设定温度,并将结晶加热溶解后放置其中,继续观察。重复以上操作,直至精密恒温循环器变温幅度≤1.0℃,此时所测溶液析出晶体的温度即为结晶温度,测定误差小于1.0℃。
1.2.2饱和蒸气压溶液饱和蒸气压采用静态法测定,即把待测溶液放在一个封闭系统中,抽真空后,在不同的温度下,测定与液相达到蒸发平衡的蒸气的压力。实验装置主要包括恒温系统、测温系统、测压系统及真空系统等,具体测定装置和实验方法见文献[16]。本文测压系统采用量程分别为0~20 kPa和0~110 kPa的精密数字绝压表(AX-110,0.05级)。通过对超纯水的饱和蒸气压进行测定,并与文献值[17]比较,得到该系统的不确定度为≤ ±1% (P≤20 kPa)和≤ ±3% (P>20 kPa)。
1.2.3腐蚀性基于拟应用的吸收式热泵循环的工况条件,采用高温浸泡法对Q235碳钢和T6紫铜在LiBr-IL/H2O和LiBr/H2O中的腐蚀速率进行测定。具体测定装置和实验方法见文献[18]。
2 实验结果与分析
2.1IL对LiBr/H2O结晶温度和饱和蒸气压的影响2.1.1IL对LiBr/H2O结晶温度的影响表2为总浓度为70%,不同质量比(mLiBr/mIL)的LiBr-IL/H2O的结晶温度。从表2中可以看出,LiBr-[BMIM]Cl/ H2O和LiBr-[BMIM]Br/H2O的结晶温度明显低于同浓度下LiBr/H2O的结晶温度[19],且随着质量比mLiBr/mIL的减小,溶液结晶温度会进一步降低,这有利于解决吸收式热泵的结晶问题。在总浓度和质量比相同的条件下,LiBr-[BMIM]Br/H2O的结晶温度高于LiBr-[BMIM]Cl/H2O,这可能是由[BMIM]Br 和LiBr中的阴离子所产生的共离子效应导致的。
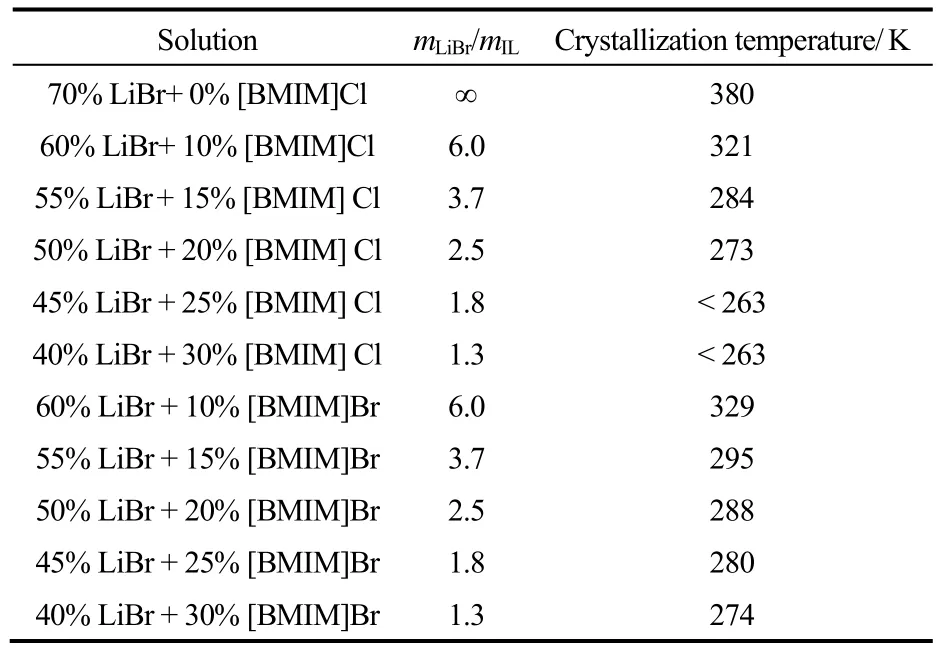
表2 LiBr-[BMIM]Cl /H2O和LiBr-[BMIM]Br/H2O溶液的结晶温度Table 2 Crystallization temperatures of LiBr-[BMIM]Cl/ H2O and LiBr-[BMIM]Br/H2O
2.1.2IL对LiBr/H2O饱和蒸气压的影响图1为总浓度为70%,质量比mLiBr/mIL分别为1.8、2.5和6.0的LiBr-[BMIM]Cl/H2O及LiBr-[BMIM]Br/H2O的饱和蒸气压。
从图1可以看出,总浓度为70%的LiBr-[BMIM]Cl/H2O和LiBr-[BMIM]Br/H2O的饱和蒸气压明显大于同浓度下LiBr/H2O的饱和蒸气压。LiBr-IL/H2O的饱和蒸气压随着质量比mLiBr/mIL的减小而增加,因此,质量比mLiBr/mIL不能太小。但是,如果质量比mLiBr/mIL太大,又不利于LiBr-IL/H2O结晶温度的降低。综合分析发现,当质量比mLiBr/mIL为2.5时,总浓度70%的LiBr-[BMIM]Cl/H2O和LiBr-[BMIM]Br/H2O溶液的吸收能力与62%的LiBr溶液相当,且具有较低的结晶温度。因此,以下对质量比mLiBr/mIL为2.5的LiBr-[BMIM]Cl/H2O与LiBr-[BMIM]Br/H2O的饱和蒸气压、结晶温度和腐蚀性进行研究,并与LiBr/H2O工质对的进行比较。
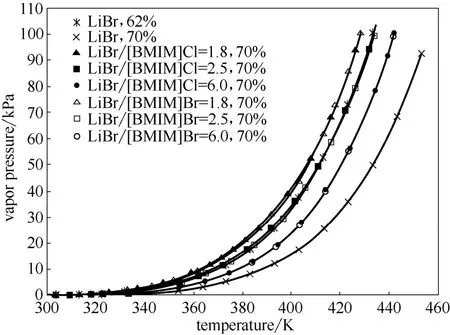
图1 不同质量比的LiBr-IL/H2O三元系饱和蒸气压Fig.1 Saturated vapor pressures of 70%(mass) LiBr-IL/H2O ternary systems under different mass ratios
2.2LiBr-IL(2.5:1)/H2O新型工质对的饱和蒸气压
表3和表4分别为LiBr-[BMIM]Cl(2.5:1)/H2O (60%~75%,296.2~445.7 K)和LiBr-[BMIM]Br (2.5:1)/H2O(60%~75%,296.4~449.6 K)的饱和蒸气压。
LiBr-BMIM]Cl(2.5:1)/H2O和LiBr-[BMIM]Br (2.5:1)/H2O的饱和蒸气压实验数据采用安托万方程拟合成温度和浓度的函数[20-22]
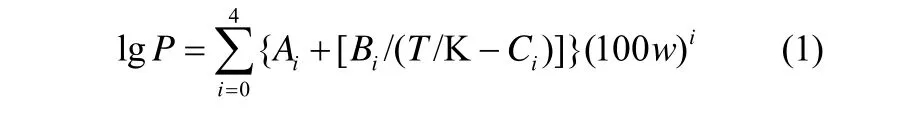
式中,P为溶液的饱和蒸气压,kPa;Ai、Bi、Ci是回归参数;T为热力学温度,K;w为溶液质量分数,%。实验数据和拟合计算数据之间的平均绝对相对偏差AARD值由式(2)计算得到
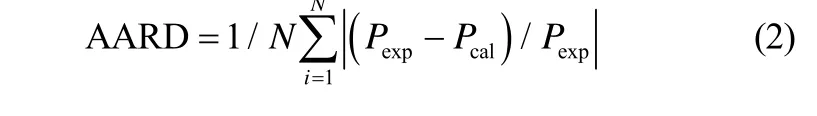
式中,N为实验点数,Pexp为实验值,Pcal为拟合计算值。拟合系数和 AARD值见表5和表6。
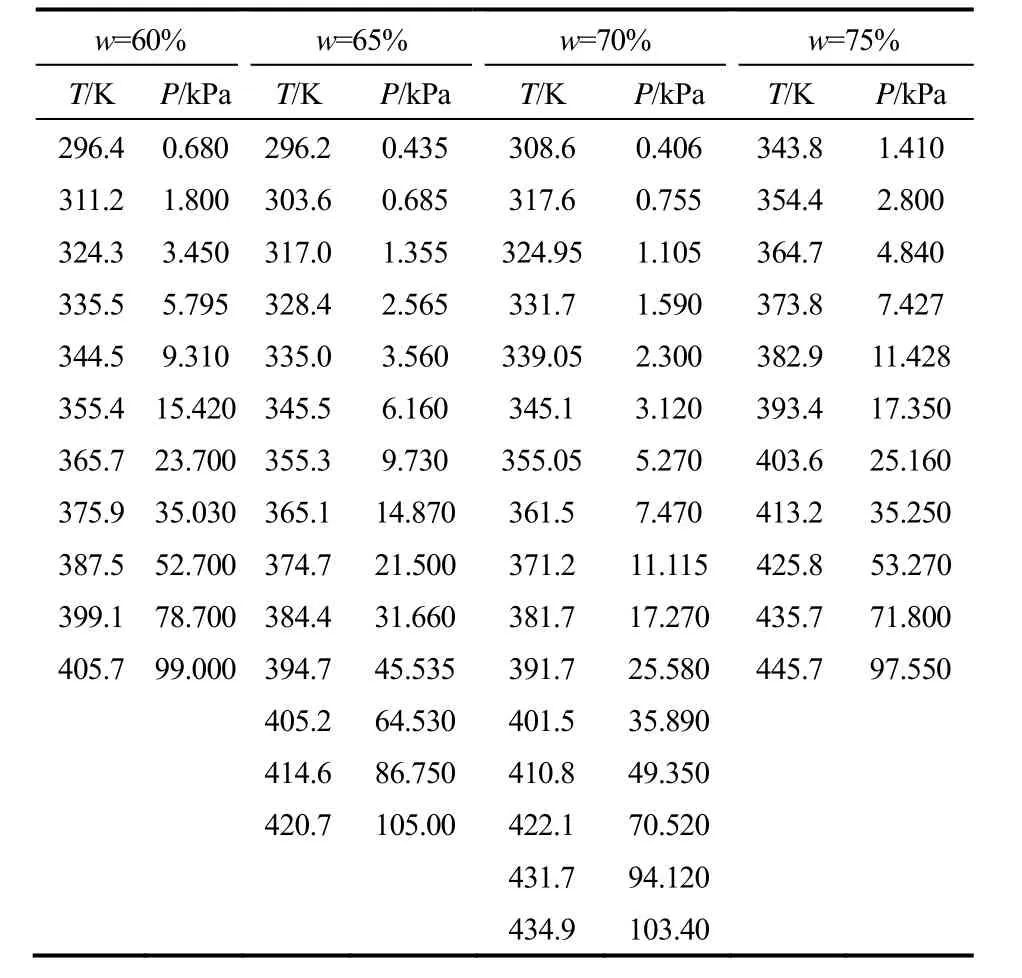
表3 LiBr-[BMIM]Cl(2.5:1)/H2O三元系饱和蒸气压Table 3 Saturated vapor pressure of LiBr-[BMIM]Cl(2.5:1)/H2O ternary system
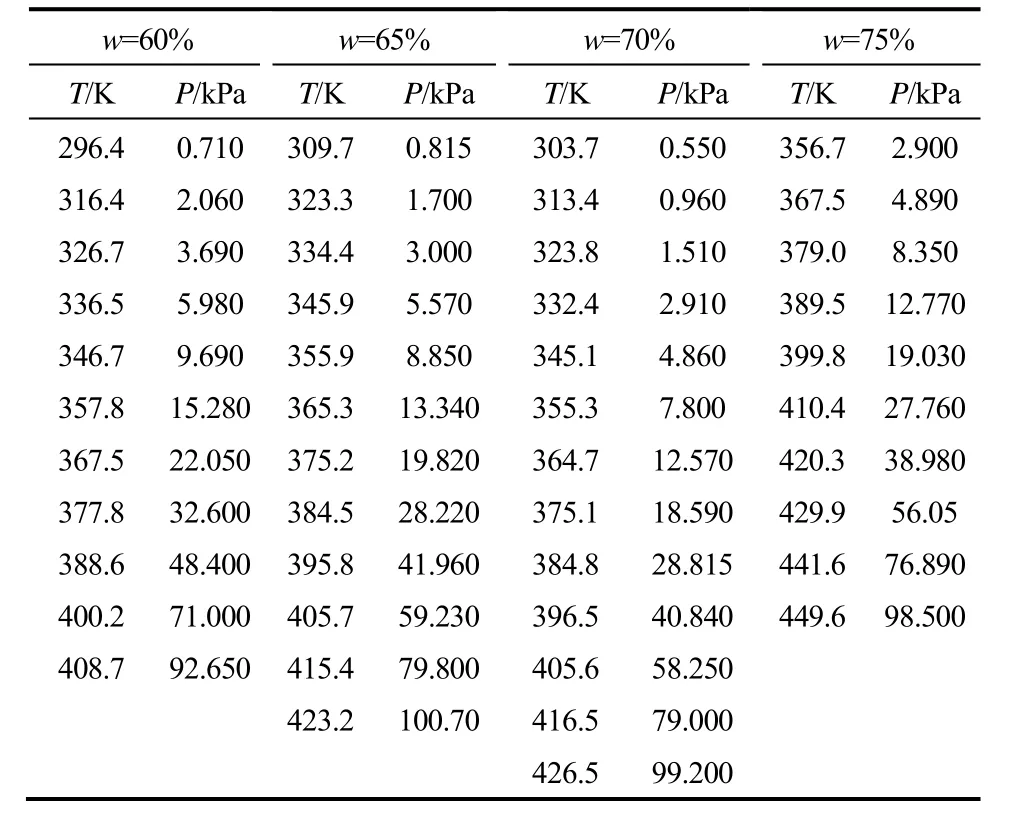
表4 LiBr-[BMIM]Br(2.5:1)/H2O三元系饱和蒸气压Table 4 Saturated vapor pressure of LiBr-[BMIM]Br(2.5:1)/H2O ternary system
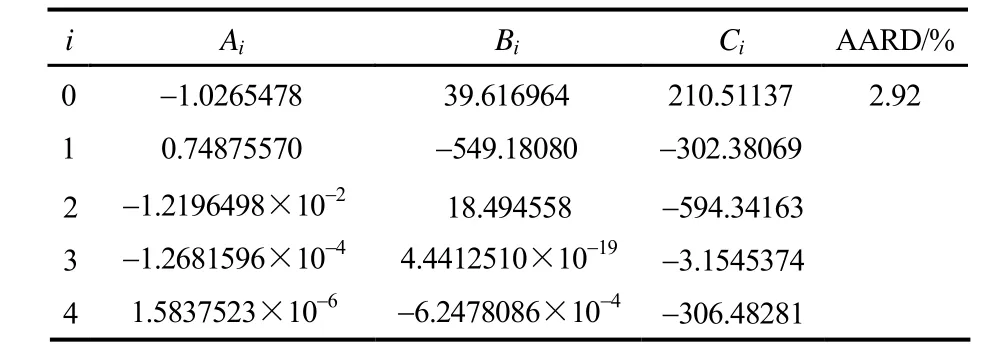
表5 LiBr-[BMIM]Cl(2.5:1)/H2O饱和蒸气压回归参数和AARD值Table 5 Regression parameters and AARD value of saturated vapor pressure for LiBr-[BMIM]Cl(2.5:1)/H2O

表6 LiBr-[BMIM]Br(2.5:1)/H2O饱和蒸气压回归参数和AARD值Table 6 Regression parameters and AARD value of saturated vapor pressure for LiBr-[BMIM]Br(2.5:1)/H2O
图2给出了LiBr-[BMIM]Cl/H2O和LiBr-[BMIM]Br/H2O体系的饱和蒸气压实验值与计算值的关系,并与LiBr/H2O的饱和蒸气压进行了比较。从图中可以看出,在实验测定范围内,实验值与拟合方程式(1)计算值吻合良好,两者的平均绝对相对偏差分别为2.92%和2.04%。LiBr-BMIM]Cl/H2O和LiBr-[BMIM]Br/H2O体系的饱和蒸气压P随温度T呈指数增长,且随质量分数的增加而减小。与LiBr/H2O的饱和蒸气压比较发现,LiBr-BMIM]Cl/H2O和LiBr-[BMIM]Br/H2O的吸收能力基本相同,两者的饱和蒸气压都与质量分数低8%~9%的LiBr/H2O的饱和蒸气压相当。
2.3LiBr-IL(2.5:1)/H2O新型工质对的结晶温度
表7为LiBr-[BMIM]Cl(2.5:1)/H2O(68%~75%)和LiBr-[BMIM]Br(2.5:1)/H2O(68%~75%)的结晶温度。
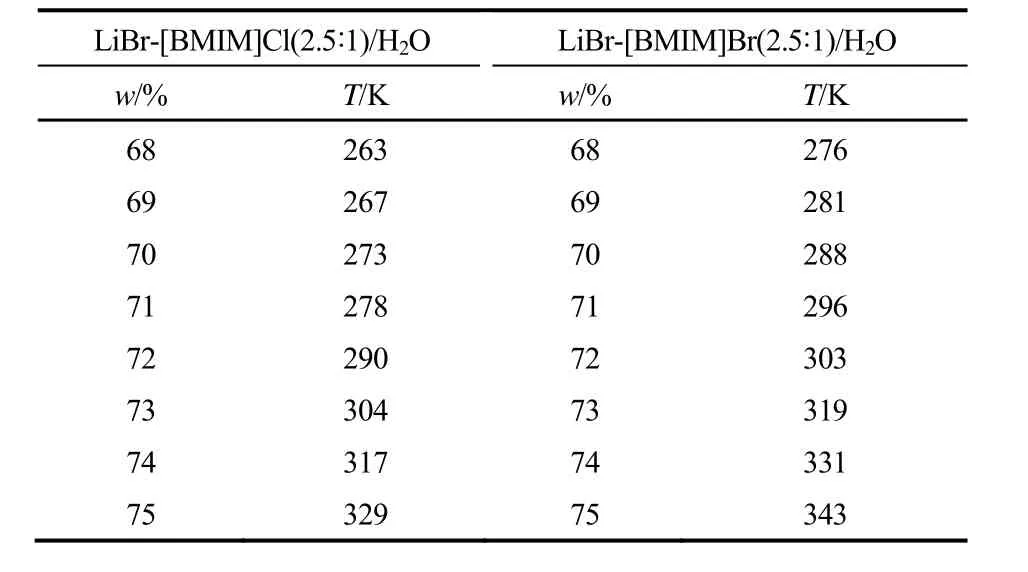
表7 LiBr-[BMIM]Cl/H2O和LiBr-[BMIM]Br/H2O的结晶温度Table 7 Crystallization temperatures of LiBr-[BMIM]Cl/H2O and LiBr-[BMIM]Br/H2O
采用最小二乘法将结晶温度实验数据拟合成
采用最小二乘法将结晶温度实验数据拟合成溶液浓度的函数[20]
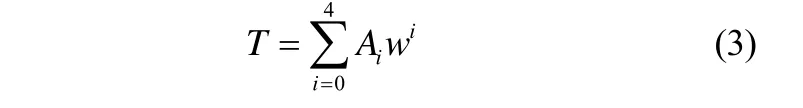
式中,w是LiBr-IL/H2O三元体系的浓度,%;算值的关系,并与相同吸收能力的LiBr/H2O的结晶温度进行了比较。从图中可以看出,在实验测定的范围内,实验值与拟合方程式(3)计算值吻合良好,T是结晶温度。拟合系数Ai和 AARD值见表8。
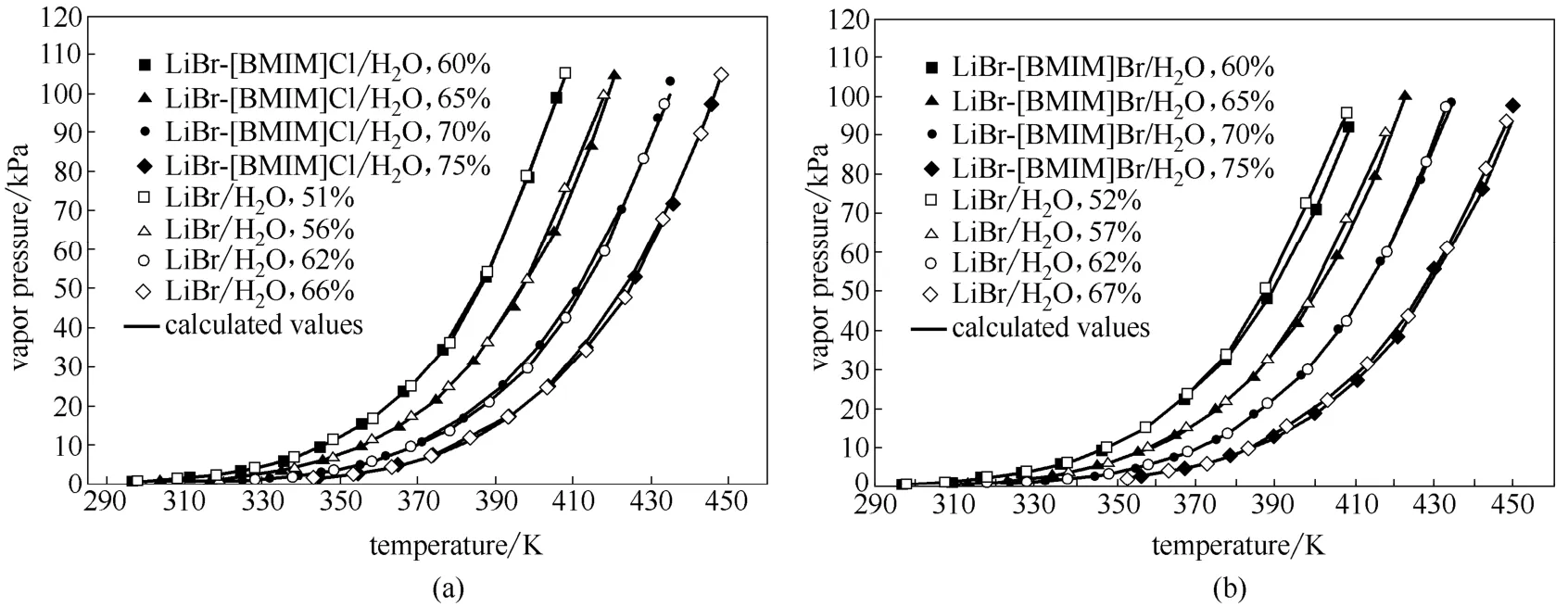
图2 LiBr-IL/H2O与LiBr /H2O体系饱和蒸气压的比较Fig.2 Comparison of saturated vapor pressures between LiBr-IL/H2O and LiBr/H2O
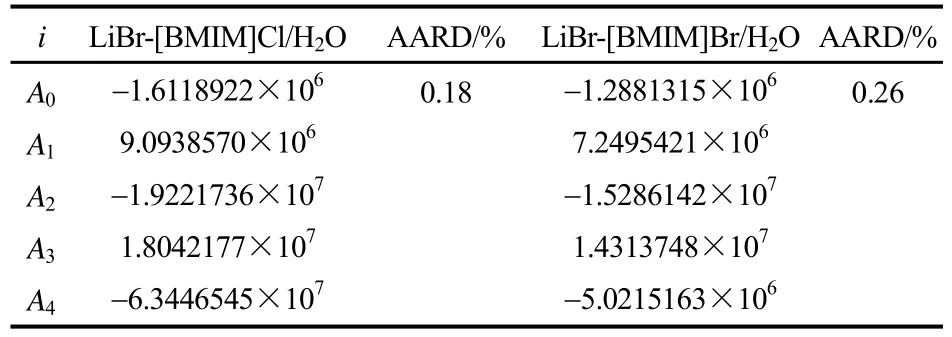
表8 拟合方程(3)系数和AARD值Table 8 Regression parameters and AARD value of fitting equation (3)

图3 相同吸收能力的LiBr-IL/H2O与LiBr/H2O结晶温度的对比Fig.3 Comparison of crystallization temperature between LiBr-IL/H2O and LiBr/H2O of the same absorption ability
图3给出了LiBr-[BMIM]Cl/H2O和LiBr-[BMIM]Br/H2O体系的结晶温度的实验值与计两者的平均绝对相对偏差分别为0.18%和0.26%。LiBr-BMIM]Cl/H2O和LiBr-[BMIM]Br/H2O的结晶温度T随质量分数w呈近似线性增长,且前者结晶温度明显低于后者。在相同吸收能力的条件下,当质量分数低于71%时,LiBr-[BMIM]Cl/H2O和LiBr-[BMIM]Br/H2O的结晶温度分别比LiBr/H2O的低约30℃和15℃。当质量分数低于72%时,采用LiBr-[BMIM]Cl/H2O作为替代工质对在室温下不易结晶,可以有效解决LiBr/H2O工质对的结晶问题。
2.4LiBr-IL(2.5:1)/H2O的腐蚀性研究
为了研究LiBr-IL(2.5:1)/H2O对吸收式系统的腐蚀性,本文采用高温浸泡法对结构材料碳钢和换热材料紫铜在相同吸收能力的LiBr-IL(2.5:1)/H2O 和LiBr/H2O溶液中的腐蚀速率进行了测定,结果如图4所示。
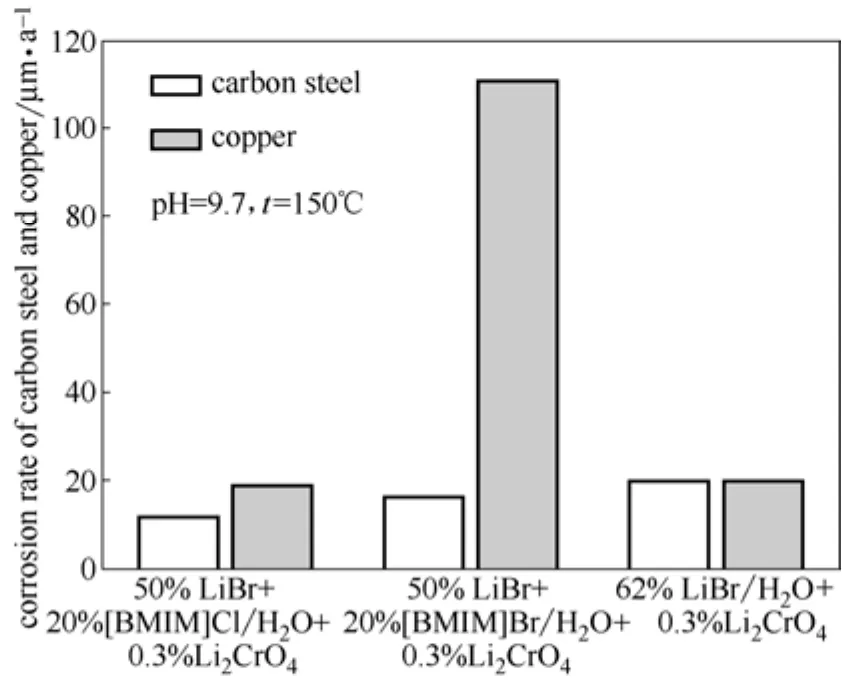
图4 碳钢和紫铜在相同吸收能力的LiBr-IL/H2O与LiBr/H2O中的腐蚀速率Fig.4 Corrosion rates of carbon steel and copper in LiBr-IL/H2O and LiBr/H2O at same absorption ability
从图4可以看出,在LiBr/H2O中添加[BMIM]Cl 和[BMIM]Br两种离子液体,可以有效抑制LiBr/H2O对碳钢的腐蚀,且添加[BMIM]Cl时的效果更加明显,其主要原因是离子液体中阳离子咪唑环上氮原子含有的孤对电子可提供给铁原子空轨道,与水合氢离子H3O+在碳钢表面形成化学吸附的竞争,从而抑制了析氢反应[23];其次,离子液体中的有机阳离子[BMIM]+能够吸附在碳钢表面形成一层有机膜,阻碍了金属离子从表面向溶液本体扩散,也在一定程度上抑制了腐蚀的进行。从图4还可以看出,紫铜在LiBr-[BMIM]Cl/H2O中的腐蚀速率与LiBr/H2O中的腐蚀速率基本相同,而LiBr-[BMIM]Br/H2O对紫铜的腐蚀更为剧烈。这可能是由于Br-的摩尔分数增大,促进了Br-对紫铜表面氧化膜的侵蚀导致的。因此,采用LiBr-[BMIM]Cl/H2O作为替代工质对,还可以改善LiBr/H2O工质对的腐蚀性问题。
3 结 论
(1)用[BMIM]Cl和[BMIM]Br取代LiBr/H2O中部分LiBr可以有效降低溶液的结晶温度,且结晶温度随着离子液体质量比的增加而明显下降。可是,吸收能力随着离子液体质量比的增加而明显减小。
(2)对质量比mLiBr/mIL为2.5的LiBr-[BMIM]Cl/H2O和LiBr-[BMIM]Br/H2O的饱和蒸气压和结晶温度进行了系统的测定,并与LiBr/H2O的进行了比较。结果表明LiBr-[BMIM]Cl/H2O和LiBr-[BMIM]Br/H2O的吸收能力与质量分数低8%~9%的LiBr/H2O的吸收能力相当。在常用工作浓度范围内,LiBr-[BMIM]Cl/H2O的结晶温度最低,比相同吸收能力的LiBr/H2O的低约30℃。采用LiBr-[BMIM]Cl/H2O作为LiBr/H2O的替代工质对可以有效解决吸收式热泵系统的结晶问题。
(3)在相同的腐蚀条件下,LiBr-[BMIM]Cl/H2O对碳钢的腐蚀速率明显小于LiBr/H2O对碳钢的腐蚀速率,而LiBr-[BMIM]Cl/H2O对紫铜的腐蚀速率与LiBr/H2O对紫铜的腐蚀速率基本相同。LiBr-[BMIM]Cl/H2O作为LiBr/H2O的替代工质对还可以改善吸收式热泵系统的腐蚀问题,因而具有一定的应用潜力。
References
[1] KIM K S,SHIN B K,LEE H,et al. Refractive index and heat capacity of 1-butyl-3-methylimidazolium bromide and 1-butyl-3-methylimidazolium tetrafluoroborate,and vapor pressure of binary systems for 1-butyl-3-methylimidazolium bromide + trifluoroethanol and 1-butyl-3-methylimidazolium tetrafluoroborate + trifluoroethanol [J]. Fluid Phase Equilibria,2004,218(2): 215-220.
[2] WANG J,ZHENG D,FAN LI,et al. Vapor pressure measurement for the water + 1,3-dimethylimidazolium chloride system and 2,2,2-trifluoroethanol + 1-ethy1-3-methylimidazlium [J]. Journal of Chemical and Engineering Data,2010,55(6): 2128-2132.
[3] YOKOZEKI A,SHIFLETT M B. Vapor-liquid equilibria of ammonia+ ionic liquid mixtures [J]. Applied Energy,2007,84(12): 1258-1273.
[4] MARTÍN A,BERMEJO M D. Thermodynamic analysis of absorption refrigeration cycles using ionic liquid + supercritical CO2pairs [J]. The Journal of Supercritical Fluids,2010,55(2): 852-859.
[5] 赵杰,梁世强,王立,等. [bmim]Cl-CH3OH 作为吸收式制冷工质对的潜能分析 [J]. 化工学报,2009,60(12): 2957-2962. ZHAO J,LIANG S Q,WANG L,et al. Potential analysis of [bmim]Cl-CH3OH as working fluid for absorption refrigeration [J]. CIESC Journal,2009,60(12): 2957-2962.
[6] KIM Y J,KIM S,JOSHI Y K,et al. Thermodynamic analysis of an absorption refrigeration system with ionic-liquid/refrigerant mixture as a working fluid [J]. Energy,2012,44(1): 1005-1016.
[7] 关婷婷,孙立,皇甫立霞,等. 离子液体[BMIM]BF4+ H2O汽液相平衡实验研究 [J]. 低温物理学报,2011,33(3): 194-198. GUAN T T,SUN L,HUANGFU L X,et al. Experiment on vapor-liquid phase equilibrium of [BMIM]BF4+ H2O system [J]. Chinese Journal of Low Temperature Physics,2011,33(3): 194-198.
[8] 孙立,郭开华,皇甫立霞. EMIMAc和HMIMCl及其水溶液热力学特性实验研究 [J]. 低温物理学报,2011,33(6): 467-473. SUN L,GUO K H,HUANGFU L X. Experiments on thermodynamic properties of ionic liquid [EMIM]Ac and [HMIM]Cl as well as aqueous solution [J]. Chinese Journal of Low Temperature Physics,2011,33(6): 467-473.
[9] 粟航,郭开华,皇甫立霞,等.强吸水性离子液体-水工质对吸收式制冷循环性能分析 [J]. 制冷学报,2013,34(3):24-27. SU H,GUO K H,HUANGFU L X,et al. Study on absorption refrigeration cycle with a new working pair of ionic liquid and water [J]. Journal of Refrigeration,2013,34(3):24-27.
[10] ZHANG X,HU D A. Performance analysis of the single-stage absorption heat transformer using a new working pair composed of ionic liquid and water [J]. Applied Thermal Engineering,2012,37:129-135.
[11] DONG L,ZHENG D X,WEI Z,et al. Synthesis of 1,3-dimethylimidazolium chloride and volumetric property investigations of its aqueous solution [J]. International Journal of Thermophysics,2009,30(5): 1480-1490.
[12] DONG L,ZHENG D,NIE N,et al. Performance prediction of absorption refrigeration cycle based on the measurements of vapor pressure and heat capacity of H2O + [DMIM]DMP system [J]. Applied Energy,2012,98: 326-332.
[13] DONG L,ZHENG D,LI J,et al. Suitability prediction and affinity regularity assessment of H2O + imidazolium ionic liquid working pairs of absorption cycle by excess property criteria and UNIFAC model [J]. Fluid Phase Equilibria,2013,348: 1-8.
[14] ZHENG D,DONG L,HUANG W,et al. A review of imidazolium ionic liquids research and development towards working pair of absorption cycle [J]. Renewable and Sustainable Energy Reviews,2014,37:47-68.
[15] NIE N,ZHENG D,DONG L,et al. Thermodynamic properties of the water + 1-(2-hydroxylethyl)-3-methylimidazolium chloride system [J]. Journal of Chemical and Engineering Data,2012,57(12): 3598-3603. [16] LUO C,SU Q,MI W.Solubilities,vapor pressuress,densities,viscosities,and specific heat capacities of the LiNO3/H2O binary system [J]. Journal of Chemical and Engineering Data,2013,58(3): 625-633.
[17] 刘光启,马连湘,刘杰. 化学化工物性数据手册(无机卷) [M]. 北京:化学工业出版社,2006: 17. LIU G Q,MA L X,LIU J. Handbook of Chemical and Engineering Property Data (Inorganic Volume) [M]. Beijing: Chemical Industry Press,2006: 17.
[18] LUO C,SU Q. Corrosion of carbon steel in concentrated LiNO3solution at high temperature[J]. Corrosion Science,2013,74(9): 290-296.
[19] 陈东,谢继红. 热泵技术及其应用[M]. Beijing: Chemical Industry Press,2006: 197. CHEN D,XIE J H. Heat Pump Technology and Application [M]. Beijing: Chemical Industry Press,2006: 197.
[20] PARK Y,KIM J S,LEE H,et al. Density,vapor pressure,solubility,and viscosity for water + lithium bromide + lithium nitrate + 1,3-propanediol [J]. Journal of Chemical & Engineering Data,1997,42(1): 145-148.
[21] SAFAROV J T. Vapor pressure of heat transfer fluids of absorption refrigeration machines and heat pumps: binary solutions of lithium nitrate with methanol [J]. Journal of Chemical Thermodynamics,2005,37(12): 1261-1267.
[22] VEREVKIN S,SAFAROV J,BICH E,et al. Study of vapour pressure of lithium nitrate solutions in ethanol [J]. Journal of Chemical Thermodynamics,2006,38(5): 611-616.
[23] 袁小丽. 金属材料在离子液体型新工质中的腐蚀性能[D]. 大连:大连理工大学,2013. YUAN X L. Corrosion of metal materials in ionic liquid working fluids[D]. Dalian: Dalian University of Technology,2013.
Saturated vapor pressure,crystallization temperature and corrosivity of LiBr-[BMIM]Cl/H2O working pair
LUO Chunhuan1,2,ZHANG Yuan1,SU Qingquan1,2
(1School of Mechanical Engineering,University of Science and Technology Beijing,Beijing 100083,China;2Beijing Engineering Research Center for Energy Saving and Environnental Protection,University of Science and Technology Beijing,Beijing 100083,China)
Abstract:In order to solve the problems of crystallization and corrosion for LiBr/H2O,LiBr-[BMIM]Cl/H2O and LiBr-[BMIM]Br/H2O were proposed as new working pairs. The influences of ionic liquids on crystallization temperatures and saturated vapor pressures of LiBr/H2O were investigated. The saturated vapor pressures,crystallization temperatures and corrosivity of LiBr-IL/H2O with a mass ratio of 2.5 were measured and compared with that of LiBr/H2O. The results showed that the saturated vapor pressures of [BMIM]Cl/H2O and [BMIM]Br/H2O were almost the same as that of LiBr/H2O with a 8%—9% lower concentration. In general operation concentration range,the crystallization temperatures of LiBr-[BMIM]Cl/H2O were about 30℃ lower than that of LiBr/H2O with the same absorption ability. Under the same corrosion conditions,the corrosion rate of carbon steel for LiBr-[BMIM]Cl/H2O was obviously smaller than that for LiBr/H2O,and the corrosion rate of copper for LiBr-[BMIM]Cl/H2O was nearly the same as that for LiBr/H2O. As an alternative working pair,LiBr-[BMIM]Cl/H2O has a great potential for absorption heat pump systems.
Key words:working pair; ionic liquids; saturated vapor pressure; crystallization temperature; corrosion
DOI:10.11949/j.issn.0438-1157.20150230
中图分类号:TK 01+9
文献标志码:A
文章编号:0438—1157(2016)04—1110—07
基金项目:中央高校基本科研业务费专项资金项目(FRF-TP-14-022A1);广东省教育部产学研结合项目(2009A090100032)。
Corresponding author:Prof. SU Qingquan,suqingquan@ustb.edu.cn
