四元体系LiCl-LiBO2-Li2SO4-H2O在298.15 K时的相平衡
2016-07-04曹丽娜李珑张楠郭亚飞邓天龙天津科技大学化工与材料学院天津市海洋资源与化学重点实验室天津300457
曹丽娜,李珑,张楠,郭亚飞,邓天龙(天津科技大学化工与材料学院,天津市海洋资源与化学重点实验室,天津 300457)
四元体系LiCl-LiBO2-Li2SO4-H2O在298.15 K时的相平衡
曹丽娜,李珑,张楠,郭亚飞,邓天龙
(天津科技大学化工与材料学院,天津市海洋资源与化学重点实验室,天津 300457)
摘要:采用等温溶解平衡法开展了四元体系LiCl-LiBO2-Li2SO4-H2O在298.15 K时相平衡实验研究,测定体系溶解度和平衡溶液的折光率、密度、pH和电导率。根据实验数据,分别绘制该四元体系的干基图、水图以及相对应的物化性质–组成图。研究结果表明:在该四元体系298.15 K相图中,有2个无变度共饱点分别为相称共饱点(Li2SO4·H2O + LiCl·H2O + LiBO2·2H2O)和不相称共饱点(Li2SO4·H2O + LiBO2·2H2O + LiBO2·8H2O)、5条溶解度曲线和4个单盐结晶相区(Li2SO4·H2O、LiCl·H2O、LiBO2·8H2O和LiBO2·2H2O),无复盐和固溶体产生,属于简单四元体系水合物Ⅱ型相图。实验研究中,发现2种偏硼酸锂水合矿物存在(LiBO2·8H2O和LiBO2·2H2O),LiCl对Li2SO4的盐析效应显著;四元体系平衡溶液物化性质随着溶液中氯化锂浓度的变化呈现规律性的变化。
关键词:水盐体系;相平衡;水溶液;化学分析;硼酸盐;硫酸锂;等温溶解平衡法
2015-07-27 收到初稿,2015-11-11收到修改稿。
联系人:邓天龙。第一作者:曹丽娜(1990—),女,硕士研究生。
Received date: 2015-07-27.
Foundation item: supported by the National Natural Science Foundation of China (21276194,41306136,U14076113).
引 言
我国盐湖资源主要分布在西部四省区,尤其青海和西藏盐湖富含锂、硼资源,开发前景广阔[1-4]。水体中硼主要以硼氧酸盐形式存在,硼酸盐的形态随溶液浓度、pH以及共存离子的不同而不同,如[B(OH)4]−、[B3O3(OH)5]2−、[B3O4(OH)5]2−、[B4O5(OH)4]2−、[B5O6(OH)4]−、[B6O7(OH)6]2−、[B6O9(OH)2]2−、[B4O3(OH)4]2−等硼酸盐物种[5-7]。水盐体系相平衡关系作为研究和预测盐类析出、溶解等相转化规律的理论,是盐湖卤水资源开发的必要工具[8-12]。因此,开展含锂、硼组成特征的卤水体系相平衡研究,对于揭示盐湖卤水锂、硼蒸发富集行为与析盐规律,指导盐湖卤水锂、硼资源开发利用具有重要意义。
宋彭生等[13]和李明等[14]报道了四元体系LiCl-Li2B4O7-Li2SO4-H2O在298.15 K和288.15 K下的相关系,两体系平衡固相有Li2B4O7·3H2O、Li2SO4·H2O和LiCl·H2O,且LiCl对Li2SO4具有强烈的盐析作用。本课题组[15-16]研究了Li2SO4-LiBO2-H2O体系在288.15 K时的稳定和介稳相图,体系平衡固相为Li2SO4·H2O和LiBO2·8H2O,发现在硫酸锂和偏硼酸锂共存体系,偏硼酸锂只有1种水合物存在(LiBO2·8H2O)。在进一步的三元体系LiCl-LiBO2-H2O在288.15、298.15和308.15 K时相平衡研究中[17-18],发现在含氯化锂三元体系中,偏硼酸锂有2种水合物共存在(LiBO2·2H2O和LiBO2·8H2O),首次发现LiBO2·2H2O矿物形成。本文进一步深入探究同时含有氯化锂、硫酸锂和偏硼酸锂的四元体系LiCl-LiBO2- Li2SO4-H2O在298.15 K下相平衡,力求揭示氯化锂、硫酸锂和偏硼酸锂共存体系相关系,以期为盐湖锂硼资源开发利用提供化工热力学基础数据。
1 实 验
1.1仪器与药品试剂
主要仪器:HXG-500-12A型恒温磁力搅拌器(控温精度±0.1 K,江苏省金坛市);Orion 3-Star型电导率仪(±0.01 mS·cm-1,美国Thermo Scientific);DMA4500型高精度密度计(±0.01 mg·cm-3,±0.01 K,奥地利Anton Paar);pH7310型精密pH计(±0.001,上海精密科学仪器公司);WYA-2S型数字阿贝折光率仪(±0.0001,上海精密科学仪器公司);K20-cc-NR恒温循环水浴(±0.1 K,德国Huber);BX51-P研究型偏光显微镜(日本Olympus);X射线粉晶衍射仪(MSAL XD-3,北京普析);艾科浦超纯水机(AWL-0502-U,重庆)。
主要药品试剂:Li2SO4·H2O和LiCl(新疆有色金属研究所)、LiBO2·8H2O(国药集团化学试剂公司)均为分析纯,重结晶备用。实验过程中药品重结晶、复体配制及平衡液相分析所用水均为二次去离子水(DDW),去离子水电导率小于1×10-4S·m-1,pH=6.60。
1.2实验方法
采用等温溶解平衡法[19]。在一系列的250 cm3的聚四氟乙烯瓶中,从三元子体系共饱点开始加入第三种盐配制复体[20],如从LiBO2·8H2O和Li2SO4·H2O共饱点开始质量梯度加入LiCl和适量的水,将其置于(298.15±0.1)K的磁力搅拌恒温水浴槽中搅拌。定期取上层清液进行分析。取样时需停止搅拌,静置2 h后,取上层清液进行化学分析,以液相组成不变作为达到平衡的标志。
当达到平衡后,固相用X射线粉晶衍射法和偏光显微镜油浸法进行鉴定[19];液相中Cl-和BO-
2测定分别采用汞量法和甘露醇-碱滴定法[21],误差均≤0.3%;SO42-采用硫酸钡重量法[21],误差≤0.05%;液相物化性质测定需在恒温循环水浴控温(298.15±0.1)K条件下,采用相应仪器分别进行测定。
2 实验结果与讨论
2.1稳定相平衡研究
该四元体系溶解度数据及干基组成数据见表1。根据表1中干基组成绘制相应的干基图(图1)及水图(图2)。
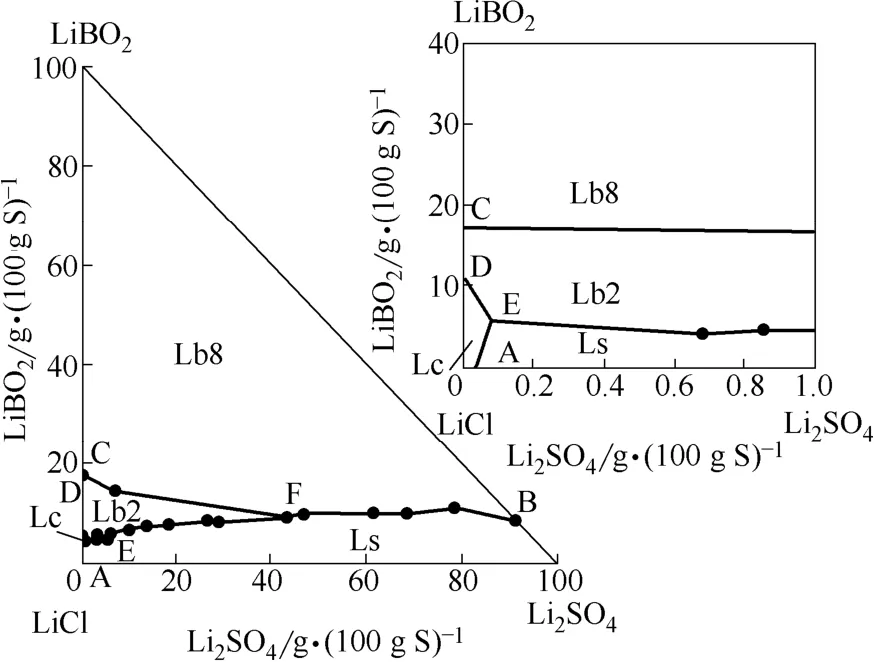
图1 四元体系LiCl-LiBO2-Li2SO4-H2O 298.15 K干基图Fig. 1 Dry-salt phase diagram of quaternary system(LiCl-LiBO2-Li2SO4-H2O) at 298.15 KLs—Li2SO4·H2O; Lc—LiCl; Lb2—LiBO2·2H2O; Lb8—LiBO2·8H2O

图2 四元体系298.15 K水图Fig. 2 Water-phase diagram of quaternary system(LiCl-LiBO2-Li2SO4-H2O) at 298.15 K(2个共饱点C、D,即 LiCl·H2O + LiBO2·8H2O
由图1可见:①四元体系干基图上有4个边界点A、B和C、D,分别就是对应的3个三元子体系LiCl-Li2SO4-H2O(共饱点A,即LiCl·H2O + Li2SO4·H2O)、Li2SO4-LiBO2-H2O(共饱点B,即Li2SO4·H2O + LiBO2·8H2O)和LiCl-LiBO2-H2O(2个共饱点C、D,即 LiCl·H2O + LiBO2·8H2O和LiBO2·8H2O + LiBO2·2H2O);值得一提的是,在三元子体系LiCl-Li2SO4-H2O共饱点A的组成[3次测定结果为:w(LiCl)= 44.54%,w(Li2SO4)= 0.020%],与文献值[22]吻合;②四元体系有5条单变量溶解度曲线,分别对应为AE、DE、EF、CF、BF;③2个无变度共饱点分别为相称共饱点E (Li2SO4·H2O + LiCl·H2O + LiBO2·2H2O),其液相组成为w(LiCl)= 44.72%,w(Li2SO4)= 0.04%,w(LiBO2)= 2.68%,不相称共饱点F(Li2SO4·H2O + LiBO2·2H2O + LiBO2·8H2O),其平衡液相组成为w(LiCl)= 10.49%,w(Li2SO4)=9.84%,w(LiBO2)= 2.10%;④4个单盐结晶区,分别对应为Li2SO4·H2O、LiCl·H2O、LiBO2·8H2O和LiBO2·2H2O。四元体系由于同时有LiBO2·2H2O、LiBO2·8H2O结晶区,属简单四元体系水合物Ⅱ型相图。由表1和图1可见,LiCl的溶解度最大,所以LiCl·H2O结晶区面积最小;LiCl对Li2SO4有很强的盐析作用,导致Li2SO4·H2O相区增大;随着溶液中LiCl的浓度增大体系出现新固相LiBO2·2H2O。由图2可见,水含量随着溶液中Li2SO4干盐组成含量增加呈现规律性变化。

表2 四元体系LiCl-LiBO2-Li2SO4-H2O 298.15 K物化性质Table 2 Physicochemical properties of system (LiCl-LiBO2-Li2SO4-H2O) at 298.15 K
2.2稳定相平衡物化性质研究
平衡液相的折光率、pH、电导率和密度测定结果见表2。由表2物化性质数据绘制了物化性质–组成图(图3)。
由图3(a)、(b)可见,四元体系中溶液的密度和折光率变化趋势相近。在4条单变量双固相共饱和线BF(Li2SO4·H2O+LiBO2·8H2O)、DE(LiCl·H2O+ LiBO2·2H2O)、CF(LiBO2·8H2O + LiBO2·2H2O)和AE(Li2SO4·H2O + LiCl·H2O)上,均呈现随着溶液中氯化锂浓度的增加而减小,但在单变量双固相共饱和线EF(LiBO2·2H2O + Li2SO4·H2O)随着溶液中氯化锂浓度的增加先微弱减小再增大。在四元体系2个无变度点F(LiBO2·8H2O + LiBO2·2H2O + Li2SO4·H2O)和E(LiCl·H2O + LiBO2·2H2O + Li2SO4·H2O)时取得极值。由图3(c)可见,四元体系pH总体上呈现为随着溶液中氯化锂浓度增大而减小,在有偏硼酸锂存在的结晶区域,pH介于9.80~6.18,在与一水氯化锂共存区域为酸性,pH介于4.56~6.18。由图3(d)可见,四元体系中溶液的电导率与密度呈现相反的变化趋势,即随着溶液中氯化锂浓度增大,总体上呈现先增大后减小。在四元体系的三元子体系研究中也发现,对于含氯化锂强电解质溶液,在浓度不大时,电导率随浓度增大而明显增大,可能原因是单位体积溶液中导电粒子数增多,但当浓度超过一定浓度时,数据。
(2)四元体系LiCl-LiBO2-Li2SO4-H2O相图,有2个无变度共饱点、5条单变量溶解度曲线和4个单盐结晶相区(LiCl·H2O,LiBO2·2H2O,由于离子间相互作用增大,导致导电能力减小大于导电粒子增多而引起的电导率增大,致使电导率随氯化锂浓度增大而减小[17-18]。在四元体系的电导率也呈现相近的特点。
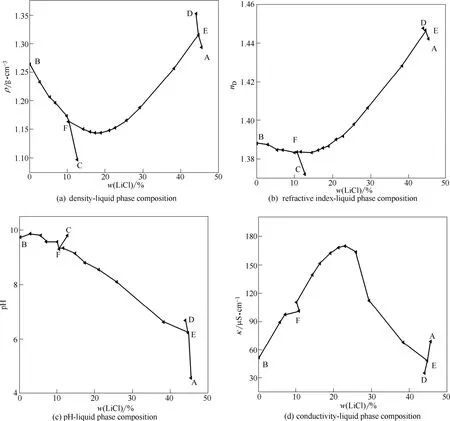
图3 四元体系LiCl-LiBO2-Li2SO4-H2O 298.15 K物化性质与溶液氯化锂含量Fig. 3 Physicochemical properties versus composition of lithium chloride concentration in quaternary system (LiCl-LiBO2-Li2SO4-H2O) at 298.15 K
3 结 论
(1)采用等温溶解平衡法对LiCl-LiBO2-Li2SO4-H2O在298.15 K时的稳定相平衡进行了研究,测得了相应的溶解度数据及平衡液相物化性质LiBO2·8H2O和Li2SO4·H2O),属于水合物Ⅱ型相图,无复盐和固溶体生成。
(3)该四元体系的物化性质随着氯化锂浓度变化呈现规律性变化。折光率和密度随着氯化锂浓度的变化规律一致。电导率和pH随着氯化锂浓度在不同的共饱线上呈现不同的变化规律。
References
[1] 郑绵平. 中国盐湖资源与生态环境 [J]. 地质学报,2010,84(11): 1613-1621.ZHENG M P. Salt lake resources and eco-environment in China [J]. Acta Geol. Sin.-Engl.,2010,84(11): 1613-1621.
[2] 程芳琴,成怀刚,崔香梅. 中国盐湖资源的开发历程及现状 [J]. 无机盐工业,2011,43(7): 1-12. DOI: 10.3969/j.issn.1006-4990. 2011.07.001. CHENG F Q,CHENG H G,CUI X M. Development course and status quo of China’s salt lake resources [J]. J. Inorg. Salt Ind.,2011,43(7): 1-12. DOI: 10.3969/j.issn.1006-4990.2011.07.001.
[3] 王伟,王刚,赵元艺. 西藏盐湖硼矿资源特点与开发利用 [J]. 盐业化工,2013,42(8): 9-13. WANG W,WANG G,ZHAO Y Y. The characteristics and utilization of boron resource in Tibet salt lake [J]. J. Salt. Chem. Ind.,2013,42(8): 9-13.
[4] DENG T L. Phase equilibrium for the aqueous system containing lithium,sodium,potassium,chloride,and borate ions at 298.15 K [J]. J. Chem. Eng. Data,2004,49(5): 1295-1299. DOI: 10.1021/je049975f.
[5] 张爱芸,姚燕. 硼酸盐水溶液中硼物种的存在形式及影响因素 [J].盐湖研究,2007,15(2): 50-55. DOI: 10.3969/j.issn.1008-858X. 2007.02.011. ZHANG A Y,YAO Y. The existence form and influence factors of boron species in boric acid solution [J]. J. Salt Lake Res.,2007,15(2): 50-55. DOI: 10.3969/j.issn.1008-858X.2007.02.011.
[6] 张林进,叶旭初. 水溶液中硼氧配阴离子的存在形式及影响因素[J]. 无机盐工业,2008,40(2): 4-9. DOI: 10.3969/j.issn.1006-4990. 2008.02.002. ZHANG L J,YE X C. The existing forms and influencing factors of the poly borate anions in aqueous solution [J]. Norg. Chem. Ind.,2008,40(2): 4-9. DOI: 10.3969/j.issn.1006-4990.2008.02.002.
[7] 周永全,房艳,房春晖. 硼酸盐水溶液结构及研究方法 [J]. 盐湖研究,2010,18(2): 65-73. ZHOU Y Q,FANG Y,FANG C H. Boric acid salt solution structure and research methods [J]. J. Salt Lake Res.,2010,18(2): 65-73.
[8] GUO Y F,LIU Y H,WANG Q,et al. Phase equilibria and phase diagrams for the aqueous ternary system (Na2SO4-Li2SO4-H2O) at (288 and 308) K [J]. J. Chem. Eng. Data,2013,58(10): 2763-2767. DOI: 10.1021/je4004146.
[9] DENG T L,WANG S Q,SUN B. Metastable phase equilibrium in the aqueous quaternary system (KCl-K2SO4-K2B4O7-H2O) at 308.15 K [J]. J. Chem. Eng. Data,2007,53(2): 411-414. DOI: 10.1021/je700472p.
[10] DENG T L,LI D C,WANG S Q. Metastable phase equilibrium in the aqueous ternary system (KCl-CaCl2-H2O) at (288.15 and 308.15) K [J]. J. Chem. Eng. Data,2008,53(4): 1007-1011. DOI: 10.1021/je700753g.
[11] DENG T L,LI D. Solid-liquid metastable equilibria in the quaternary system (NaCl-KCl-CaCl2-H2O) at 288.15 K [J]. Fluid Phase Equilib.,2008,269(1): 98-103. DOI: 10.1016/j.fluid.2008.05.005.
[12] WANG S Q,DENG T L. (Solid+ liquid) isothermal evaporation phase equilibria in the aqueous ternary system (Li2SO4-MgSO4-H2O) at T= 308.15 K [J]. J. Chem. Thermodyn.,2008,40(6): 1007-1011. DOI: 10.1016/j.jct.2008.02.008.
[13] 宋彭生,杜宪惠. 四元体系Li2B4O7-Li2SO4-LiCl-H2O 25℃相关系和溶液物化性质的研究 [J]. 科学通报,1986,(3): 209-213. SONG P S,DU X H. Phase relation and solution research of physical and chemical properties of the quaternary system of Li2B4O7-Li2SO4-LiCl-H2O at 298.15 K [J]. Chin. Sci. Bull.,1986,(3): 209-213.
[14] 李明,桑世华,张振雷,等. 四硼酸锂-硫酸锂-氯化锂-水四元体系288 K相平衡研究 [J]. 无机工业,2009,41(5): 21-24. DOI:10.3969/j.issn.1006-4990.2009.05.007. LI M,SANG S H,ZHANG Z L,et al. Study on phase equilibrium of the quaternary system Li2B4O7-Li2SO4-LiCl-H2O at 288.15 K [J]. Norg. Chem. Ind.,2009,41(5): 21-24. DOI:10.3969/j.issn.1006-4990. 2009.05.007.
[15] GAO D L,WANG S Q,GUO Y F,et al. Solid-liquid phase equilibria in the aqueous ternary system Li2SO4-LiBO2-H2O at T = 288.15 and 298.15 K [J]. Fluid Phase Equilib.,2014,371: 121-124. DOI:10.1016/j.fluid.2014.03.019.
[16] 赵美玲,王士强,韩徐年,等. 三元体系Li2SO4-LiBO2-H2O 在288.15 K时介稳相平衡 [J]. 化学工程,2015,43(3): 37-45. DOI: 10.3969/j.issn.1005-9954.2015.03.010. ZHAO M L,WANG S Q,HQN X N,et al. Metastable equilibria of the ternary system Li2SO4-LiBO2-H2O at 288.15 K [J]. Chem. Eng.,2015,43(3): 37-45. DOI: 10.3969/j.issn.1005-9954.2015.03.010.
[17] GAO D L,GUO Y F,YU X P,et al. Solubilities,densities and refractive indices of the salt-water system (LiCl-LiBO2-H2O) at 288.15 and 298.15 K and 0.1 MPa [J]. J. Chem. Eng. Data,2015,60(9): 2594-2599. DOI: 10.1021/acs.jced.5b00121.
[18] ZHANG N,GUO Y F,LIU Y H,et al. Thermodynamic phase equilibria of the aqueous ternary system (LiCl-LiBO2-H2O) at 308 K: experimental data and predictions using the Pitzer model [J]. J. Chem. Eng. Jpn.,(in press).
[19] 邓天龙,周桓,陈侠. 水盐体系相图及应用[M]. 北京: 化学工业出版社,2013. DENG T L,ZHOU H,CHEN X. Salt-Water System Phase Diagrams and Applications[M]. Beijing: Chemical Industry Press,2013.
[20] MENG L Z,LI D,GUO Y F,et al. Stable phase equilibrium of the aqueous quaternary system (MgCl2-MgSO4-MgB6O10-H2O) at 323.15 K [J]. J. Chem. Eng. Data,2011,56(12): 5060-5065. DOI: 10.1021/je2006852.
[21] 中国科学院青海盐湖研究所分析室. 盐湖卤水分析[M]. 2版. 北京:科学出版社,1988. Qinghai Institute of Salt Lakes of CAS. Analytical Methods of Brines and Salts[M]. 2nd ed. Beijing: Science Press,1988.
[22] SILCOCK H. Solubilities of Inorganic and Organic Compounds [M]. New York: Pergamon Press,1979.
Phase equilibria of quaternary system LiCl-LiBO2-Li2SO4-H2O at 298.15 K
CAO Lina,LI Long,ZHANG Nan,GUO Yafei,DENG Tianlong
(College of Chemical Engineering and Material Sciences,Tianjin University of Science and Technology,Tianjin Key Laboratory of Marine Resources and Chemistry,Tianjin 300457,China)
Abstract:The phase equilibria and phase diagram of the quaternary system (LiCl-LiBO2-Li2SO4-H2O) at 298.15 K,which was not reported in the literature,were studied with the isothermal dissolution equilibrium method. Solubilities and physicochemical properties including refractive index (nD),density (ρ),pH and conductivity (κ) in the quaternary system were determined experimentally. According to the experimental data,the dry-salt diagram,water-phase diagram and the diagram of physicochemical properties versus lithium chloride concentration in the quaternary system were plotted,respectively. The experimental results showed that there were two invariant points named as invariant co-saturated point (Li2SO4·H2O + LiCl·H2O + LiBO2·2H2O) and incommensurate co-saturated point (Li2SO4·H2O + LiBO2·2H2O + LiBO2·8H2O),five univariant curves and four crystalline regions corresponding to Li2SO4·H2O,LiCl·H2O,LiBO2·8H2O and LiBO2·2H2O in the quaternary system at 298.15 K. Neither double salt nor solid solution was formed,and the phase diagram of this system at 298.15 K belonged to hydrate-type Ⅱ. The two kinds of hydrate lithium-containing minerals (LiBO2·2H2O and LiBO2·8H2O) were found for the first time. The salting-out effect of LiCl in the solution was obvious for the composition of Li2SO4. The diagram of physicochemical properties including nD,ρ,pH and κ versus composition shows that the physicochemical properties were changed regularly with increasing lithium chloride concentration in the solution and the singular values were achieved at the invariant points of the quaternary system at 298.15 K.
Key words:salt-water system; phase equilibria; aqueous solution; chemical analysis; borate; lithium sulfate; isothermal dissolution equilibrium method
DOI:10.11949/j.issn.0438-1157.20151204
中图分类号:O 642.4
文献标志码:A
文章编号:0438—1157(2016)04—1117—06
基金项目:国家自然科学基金项目(21276194,41306136,U14076113)。
Corresponding author:Prof. DENG Tianlong,tldeng@tust.edu.cn
