Investigation of Ultrafast Electronic Transfer Process on Organic/Inorganic Heterojunction by Femtosecond Transient Absorption
2016-07-05XuecongLiNingSuiQinghuiLiuQilinYuanYinghuiWangDepartmentofPhysicsTianjinPolytechnicUniversityTianjin300387ChinaFemtosecondlaserlaoratoryCollegeofPhysicsJilinUniversityChangchun130012China
Xue-cong Li,Ning Sui,Qing-hui Liu,Qi-lin Yuan,Ying-hui Wang∗a. Department of Physics,Tianjin Polytechnic University,Tianjin 300387,China . Femtosecond laser laoratory,College of Physics,Jilin University,Changchun 130012,China
Investigation of Ultrafast Electronic Transfer Process on Organic/Inorganic Heterojunction by Femtosecond Transient Absorption
Xue-cong Lia∗,Ning Suib,Qing-hui Liub,Qi-lin Yuanb,Ying-hui Wangb∗
a. Department of Physics,Tianjin Polytechnic University,Tianjin 300387,China b. Femtosecond laser laboratory,College of Physics,Jilin University,Changchun 130012,China
(Dated:Received on December 8,2015;Accepted on January 25,2016)
We detect a relaxation process of excited SQ02 dye in the chlorobenzene solution and anchor SQ02 on Al2O3and TiO2film,so as to investigate the photophysical properties of pristine SQ02 in the monodisperse system,aggregation state,and the corresponding interfacial electron transfer process. The experimental data show that the lifetime of SQ02 in the monondisperse system is~2.0 ns,but that of SQ02 anchored on the Al2O3film could obviously decrease to~21 ps. The time of electron transfer from excited SQ02 to TiO2film is estimated to be~2.6 ps and the yield of electron injection is estimated to be~89.1%,which matches the incident photon to current efficiency of dye-sensitized solar cell based on SQ02. In addition,some dyes are found to pack on the other dyes anchored on the nanocrystal film,and their relaxation time could reach~60 ps. They couldn't participate in the interfacial electron transfer,since they are far away from the TiO2interface.
Key words:SQ02 dye,Femtosecond transient absorption,Photoexcitation dynamics D
∗Authors to whom correspondence should be addressed. E-mail:lixcyy@163.com,ying_hui wang@jlu.edu.cn
I. INTRODUCTION
The dye-sensitized solar cell(DSSC)is one of the most attractive next generation solar cells,due to its potential low cost,variable architecture,and flexibility [1-5]. The power conversion efficiency(PCE)of DSSC based on Ru complexes[6]has reached~11%. Based on multi-organic dyes anchored on TiO2film,the PCE of organic DSSC could achieve~12.3%under simulated air mass 1.5 Global sunlight and reach an even higher value of 13.1%at 509 W/m2(50.9%Sun)[7]. In comparison with the expensive ruthenium based sensitizers,many metal-free organic sensitizers have been synthesized[8,9],which own outstanding photophysical properties and especially high molar extinction coefficients. In order to further improve the performance of DSSC,people gradually broaden the harvesting region of sunlight through narrowing the bandgap of organic dyes[10,11]. Meanwhile,it is necessary to carry out a high efficient vectorial electron transfer from the lightharvesting dye toward the semiconductor surface,which acts as another important factor for improvement of the short current of photovoltaic devices. In order to obtain high performance of photovoltaic device,people need to achieve a balance between the narrowing of bandgap and the high efficiency of electron injection. Note that the latter is an important step for converting from photon to electron[12,13],which is composed of multidynamics,such as the relaxation of excited dyes and the interfacial electron transfer process. Generally speaking,modulating the interfacial electron transfer process based on the low bandgap dye could be a tendency for improving the performance of DSSC. The DSSCs associated to ruthenium-based dyes[14,15],porphyrin dyes[16],and triphenylamine dyes[17],etc. have been reported. Transient absorption(TA)spectroscopy and femtosecond fluorescence up-conversion techniques,acting as power instruments,are much useful for understanding these fast dynamic processes,since these relative processes occur on a sub-picosecond or even faster time scale.
An unsymmetrical squaraine dye,5-carboxy-2-[[3-[(2,3-dihydro-1,1-dimethyl-3-ethyl-1H-benzo[e]indol-2-ylidene)methyl]-2-hydroxy-4-oxo-2-cyclobuten-1-yliden e]methyl]-3,3-dimethyl-1-octyl-3H-indolium with carboxylic acid as anchoring group(SQ02,shown in Fig.1),is one kind of indole type organic dyes and its absorption edge could reach up to~700 nm in chlorobenzene(CB)solution,which is benefitical for harvesting many photons in the visible region. The PCE of DSSC based on SQ02 could be up to 5.4%and the IPCE may reach 85%[18]. This kind of indole type sensitizers have a huge potential in the development of organic DSSC,however there have been very few reports on its photo-physical characteristics and the corresponding interfacial electron transfer process. In order to further increase the PCE of DSSC based on metal-free dyes,it is necessary to understand the basic photo-physical processes occurring at the interface in DSSC[19]. Femtosecond TA spectroscopy[20]is one ofthe time-resolved spectroscopy techniques,which could offer the dynamic information of interfacial electron transfer occurring in the DSSC based on organic dye [21,22].
Herein,we detect the photoexcitation dynamics of SQ02 in CB solution,and anchor SQ02 on the Al2O3and TiO2film by using femtosecond TA spectroscopy with a time resolution of~150 fs. Firstly,we offer the photo-physical behavior of SQ02 in the solution. And then,we use the relaxation process of SQ02 anchoring on the Al2O3as counterpoint to investigate the electron injection process occurring on the SQ02/TiO2interface,which could be beneficial for us to understand the structure-dynamic relationship.

FIG. 1 Molecular structure of SQ02.
II. EXPERIMENTS
The chemicals were all purchased from Lumtec Technology without further purification. A 5-µm-thick transparent layer of 20-nm-sized TiO2nanocrystal film is used in the layer film and a transparent Al2O3film with similar thickness is used as reference film. The concentration of SQ02 in CB solution is~10µmol/L for spectral measurement and~1.5×10-4mol/L for sensitizing the Al2O3and TiO2nanocrystal films. The corresponding semiconductor films may be washed by the CB solvent and dried in air after sensitization. The sensitization time was~10 h.
Steady-state absorption measurements were carried out using a UV-Vis spectrophotometer(Purkinje,TU-1810PC). Photoluminescence(PL)spectra were recorded by a fiber optic spectrometer(Ocean Optics,USB4000)with excitation pulse at 600 nm. The femtosecond TA measurement has been reported anywhere. Herein,we employed a mode-lock Ti:sapphire femtosecond laser system(Coherent),which offers 2.0 mJ,130 fs pulses at 800 nm with a repetition rate of 1 kHz. The output of femtosecond laser beam was split into two parts,the major one pumps the optical parametric amplifier(TOPAS,Coherent),which provides an excitation pulse with wavelength of 600 nm. The minor one was focused into a 3 mm quartz cell filled with water to generate a white light continuum as the probe beam. The pump beam was continuously chopped by the optical chopper and finally focused into the sample cell after passing through an optical delay line,while the probe beam was also focused onto the sample to overlap with the pump beam before it travels through a monochromator. The time-dependent transmittance change was detected by a photomultiplier tube(Zolix,PMTH-S1-CR131)connected to the lock-in amplifier (SR830,DSP). The solution is filled with a 1 mm quartz cell. In the wavelength-dependent dynamic measurement,the excitation intensity is 20µJ/cm2. All measurements were carried out at room temperature.

FIG. 2 Steady-state absorption and PL spectra of(a)SQ02 in CB solution and(b)SQ02 aggregated together on Al2O3and TiO2films.
III. RESULTS AND DISCUSSION
As seen in Fig.2(a),the absorption spectrum of SQ02 in a monodisperse system obviously consists of a main absorption peak at 664 nm and a shoulder at 613.5 nm,which shows a narrow absorption band. As a consequence of the extended π-conjugated system,its absorption edge in CB solution is closed to 700 nm,indicating it could harvest a large number of photons in the red part of visible region. The photoluminescence (PL)spectrum of SQ02 in CB solution shows a good enantiomorphous feature in comparison with its absorption spectrum,and is also composed of a main PL peak (678.5 nm)and a shoulder(734.5 nm). The bandgap of SQ02 could be estimated from the intersection point between the normalized absorption band and PL band,which is~1.847 eV. Meanwhile,the Stokes shift,given by the frequency difference between the emission maximum and the absorption peak,is~339 cm-1,indicating that the configuration is not much altered when the sensitizer going from the ground state to the excited state[10]. When the SQ02 is anchored on Al2O3film (as seen in Fig.2(b)),its absorption band may broaden in comparison with that in CB solution due to intermolecular interaction and is also composed of two ab-sorption bands at 614 and 670 nm. In addition,its absorption band edge could reach~750 nm,so the region of light harvesting obviously improves due to the molecular aggregation. Since the conduction band of Al2O3is higher than the LUMO of SQ02,no injection electron could occur at the interface of Al2O3. Its PL spectrum is presented in Fig.2(b),which also contains a main peak at 705 and a shoulder at 807.5 nm,respectively. Herein,the bandgap and the Stokes shift of SQ02 anchored on transparent Al2O3nanocrystal film could be 1.787 eV and~729.3 cm-1. When the SQ02 is anchored on TiO2film,its absorption spectrum is much similar to that anchored on Al2O3film in the region from 550 nm to 750 nm,indicating that the molecular gesture of SQ02 anchored on TiO2should be similar to that on Al2O3film,and the photophysical behavior of SQ02 anchored on Al2O3film could act as a reference in comparison with that on TiO2film[23]. Since the conduction band of TiO2film is lower than the LUMO of SQ02,the interfacial electron transfer could occur in the interface between the sensitizer and the TiO2nanocrystal after photoexcitation,which leads to the PL quenching of SQ02 anchored on TiO2nanocrystal and the corresponding PL spectrum is not presented in this work.
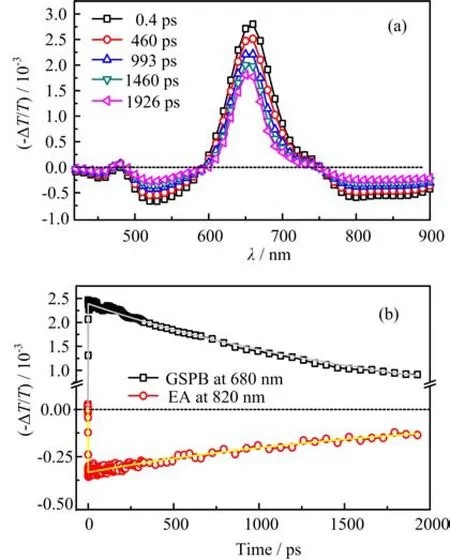
FIG. 3 (a)Time-dependent TA spectra of SQ02 in CB solution,(b)the TA curves at 680 and 820 nm. The excitation intensity is~30µJ/cm2.
So as to understand the relaxation character of SQ02 in the excited state,the time-dependent TA spectra of SQ02 in CB solution are presented in Fig.3(a). These TA spectra are complex,which consists of one positive band and two negative bands. The positive spectral feature and its shoulder at~655 and 750 nm should be assigned to the ground state photo-bleaching (GSPB)and stimulated emission(SE),since their positions are overlapped with the ground state absorption and PL. According to the steady-state absorption spectrum,two broad negative bands at 520 and 850 nm should be assigned to the excited states absorption(EA). In addition,their similar relaxation behaviors suggest that they should be originated from the same transient species(TS)generated after photoexcitation. The corresponding GSPB and EA curves at 680 and 820 nm are shown in Fig.3(b)and both of them are fitted by mono-exponential function. The fitted results show that their lifetimes are 1.9 ns(GSPB)and 2.0 ns (EA). The similar relaxation lifetime indicates that the TS should come from single-single transition and the localized exciton relaxation is dominant in the relaxation process of excited SQ02 in a monodisperse system.
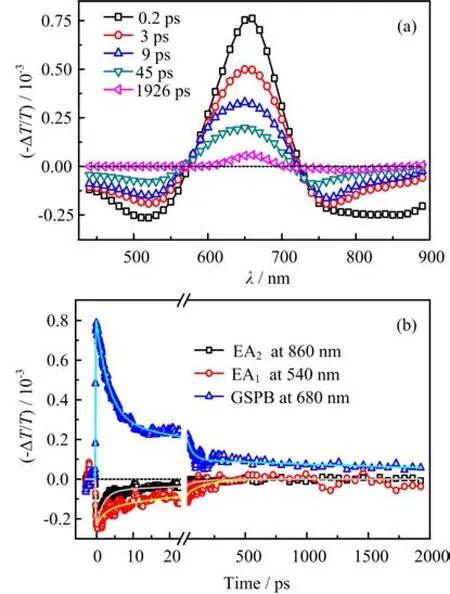
FIG. 4 (a)Time-dependent TA spectra of SQ02 anchored on Al2O3film,(b)the TA curves at 540,680,and 860 nm fitted by multi-exponential function(Eq.(1))and their mean lifetimes calculated as Eq.(2). And the excitation intensity is~30µJ/cm2.
In the condensed phase,we offer the TA spectra of SQ02 anchored on Al2O3nanocrystal film at different delay time,which also contains one positive spectral band at 660 nm and two negative bands at 520 and 850 nm,as seen in Fig.4(a),moreover,the shape of TA spectra is similar to that of SQ02 in CB solution,but the GSPB becomes broader due to molecular aggregation,which is similar to the variation of their absorption spectrum. It is noted that the relaxation process of ex-cited SQ02 in aggregation state obviously accelerates in comparison with that in CB solution. The TA curves at 540 nm(EA1),680 nm(GSPB)and 860 nm(EA2)nm are presented in Fig.4(b)and fitted by using the multi-exponential function:

Their mean lifetimes are calculated as below:

It works by taking a weighted average of the lifetime. The fitted results show that their mean lifetimes of TS are 62.1 ps(540 nm)and 21.3 ps(860 nm),which should depend on transient species after photoexcitation. The intensity-dependent TA curves at 540 and 860 nm are presented in Fig.5,which demonstrates that the slope of the TA curves increases as the intensity increases. The big slope of the line means the short lifetime,which indicates a faster decay process. So we have a comment that the percentage of fast decay process gradually enhances as the increasing with the excitation intensity. As the excitation intensity increases,the percentage of fast decay process gradually enhances,indicating that this fast decay process should be attributed to the exciton-exciton annihilation(EEA)process. The EEA and the subsequent component with a long lifetime in the TA curve at 540 nm are both longer than those at 860 nm. Generally speaking,the organic sensitizers on the nanocrystal film could be divided into two categories. In first situation,the dye is directly anchored on the Al2O3film,and in the second situation,the dye is packing on the sensitizers anchored on the Al2O3film through intermolecular π-π interaction[24]. Comparing with the TA curves at 540 and 860 nm,we consider that the fast one(at 860 nm)could be assigned to the relaxation process of I-SQ02 that is directly anchored on the nanocrystal crystal interface,and the slow one(at 540 nm)may be attributed to the decay of ASQ02 packing on the other dyes. The arrangement of I-SQ02 is compact,but that of A-SQ02 is loose. Their molecular gesture could be responsible for the difference between their relaxation behaviors.

FIG. 5 Excitation intensity-dependent TA curves of SQ02 anchored on Al2O3film at 540 nm(a)and 860 nm(b).

FIG. 6 (a)Time-dependent TA spectra of SQ02 anchored on TiO2film.(b)The TA curves at 520,660,and 740 nm. The excitation intensity is~30µJ/cm2with wavelength of 600 nm.
In order to investigate the interfacial electron transfer process from excited SQ02 to TiO2film,we offer the time-dependent TA spectra of SQ02 anchored on TiO2film as seen in Fig.6(a). The initial TA spectrum is similar to that on Al2O3film,indicating that all transient species on TiO2film after photoexcitation are almost the same as those on Al2O3film. With the delay time prolongs,the peak of GSPB blue shifts from 670 nm to 650 nm. After 36 ps,the negative spectral signal in the blue region gradually disappears,but the other spectral features almost keep their intensity invariable. Multi-transient species may exist after photoexcitation and show different relaxation behaviors. The time-dependent spectral feature at 740 nm contains a transient species,which still exists in 1.9 ns,indicating that it should be assigned to the SQ02+. Meanwhile,the time-dependent negative spectral feature at 520 nm should reflect the electron injection process from the excited SQ02 to the conduction band of TiO2nanocrystal,due to its fast relaxation behavior. In order to obtain the dynamic information in detail,we offer the corresponding TA curves at 520,660,and 740 nm in Fig.6(b)and all of them are fitted with multi-exponential function. The mean lifetime of the TA curve at 520 nm is~2.6 ps,showing that the electron injection time is much faster than those of SQ02 on Al2O3film. We could calculate the yield of electron injection according to the lifetime of interfacial electron transfer and the excited state of I-SQ02 in aggregation as mentioned below:

where ΦYEIis the yield of electron injection,kIETis the rate of interfacial electron transfer,kIERis the relaxation rate of interfacial excited SQ02. Finally,we obtain that the electron injection yield is~89.1%,which almost matches with the IPCE of DSSC based on SQ02. The TA curve at 740 nm consists of two decay behavior with different relaxation rates. The former is fast and relax with a mean lifetime of 61.3 ps. The latter decays so slowly that its lifetime exceeds the window of our detection. Apparently the initial relaxation process has a similar dynamic behavior in comparison with that of A-SQ02 on Al2O3film(as seen from the TA curve at 540 nm in Fig.4(b)),so we expected that this dynamic process may be also attributed to the decay of A-SQ02. Since these A-SQ02s are not directly linked with the TiO2nanocrystal,the electron isn't able to inject from the excited sensitizer to the conduction band of TiO2nanocrystal,and the excited SQ02 may relax to the ground state after photoexcitation.
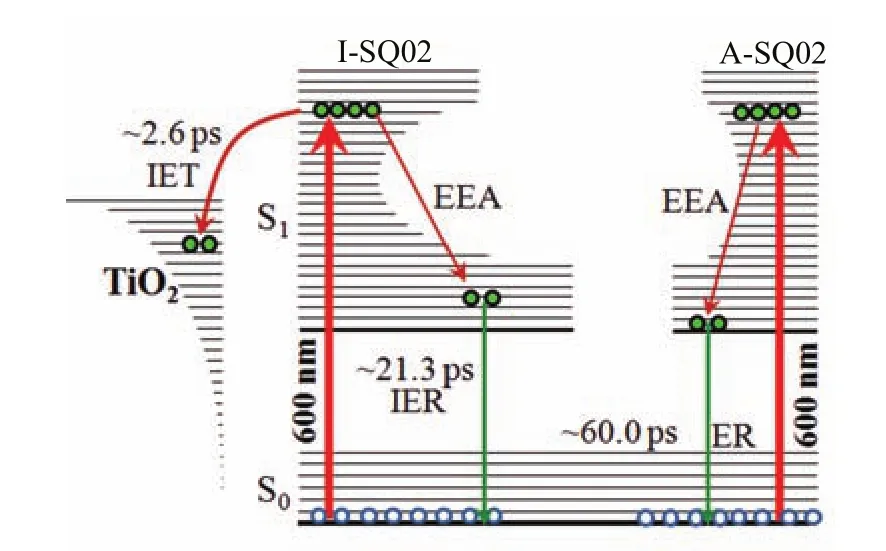
FIG. 7 Sketch of the primary photophysical events occurring upon photoexcitation. S0:ground state,S1:the first excited state,IET:interfacial electron transfer,IER:interfacial excited state relaxation,ER:excited state relaxation,EEA:exciton-exciton annihilation.
Finally,we summarize the dynamic process occurring on SQ02/TiO2film in Fig.7. For the I-SQ02,the interfacial electron transfer and relaxation of excited sensitizer linked to the interface of TiO2nanocrystal could occur at the same time. But the former would be dominant and the corresponding yield of electron injection is estimated about 89.1%. For A-SQ02,they could not participate in the electron injection process,but directly relax to the ground state with time of~60 ps,which is similar to that on Al2O3nanocrystal film. Even though the TiO2active layers have been washed in solvent,the experimental data show that some A-SQ02 could exist on the nanocrystal films,which could affect the performance of the photovoltaic device.
IV. CONCLUSION
We detect the photophysical behavior of SQ02 sensitizers in CB solution,and also anchor SQ02 on Al2O3and TiO2films,so as to obtain the dynamic information of pristine SQ02 in a monodisperse system and the aggregation state,and the corresponding interfacial electron transfer information. The experimental data show that the lifetime of SQ02 can obviously accelerate,when they are aggregated together on the Al2O3film. When the sensitizers are anchored on TiO2nanocrystal,the electron could inject from the excited SQ02 to the conduction band of TiO2film with time of~2.6 ps and the yield of electron injection could reach~89.1%. In addition,some sensitizers are also packing on the other dyes anchored on the TiO2film. They could not participate in the interfacial electron transfer,but directly relax to the ground state.
V. ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China(No.51502109,No.21573094,No.11274142,and No.11474131),the National Found for Fostering Talents of Basic Science (No.J1103202),and the Science & Technology Development Foundation of Tianjin Higher Education Institutions(No.20140904).
[1]B. O'Regan and M. Gr¨atzel,Nature 353,737(1991).
[2]A. Hagfeldt and M. Gr¨atzel,Acc. Chem. Res. 33,269 (2000).
[3]Y. Zhang,Z. H. Wang,Y. J. Hao,Q. P. Wu,M. Liang,and S. Xue,Chin. J. Chem. Phys. 28,91(2015).
[4]H. Cheema,R. Younts,L. Ogbose,B. Gautam,K. Gundogdu,and A. El-Shafei,Phys. Chem. Chem. Phys. 17,2750(2015).
[5]T. Higashino and H. Imahori,Dalton Trans. 44,448 (2015).
[6]M. K. Nazeeruddin,F. De Angelis,S. Fantacci,A. Selloni,G. Viscardi,P. Liska,S. Ito,B. Takeru,and M. Gr¨atzel. J. Am. Chem. Soc. 127,16835(2005).
[7]A. Yella,H. W. Lee,H. N. Tsao,C. Y. Yi,A. K. Chandiran,M. K. Nazeeruddin,E. W. G. Diau,C. Y. Yeh,S. M. Zakeeruddin,and M. Gr¨atzel,Science 334,629 (2011).
[8]C. Y. Lin,C. F. Lo,L. Luo,H. P. Lu,C. S. Hung,and E. W. G. Diau,J. Phys. Chem. C 113,755(2009).
[9]E. M. J. Johansson,T. Edvinsson,M. Odelius,D. P. Hagberg,L. C. Sun,A. Hagfeldt,H. Siegbahn,and H. Rensmo,J. Phys. Chem. C 111,8580(2007).
[10]A. Mishra,M. K. R. Fischer,and P. B¨auerle,Angew. Chem. Int. Ed. 48,2474(2009).
[11]Y. S. Yen,H. H Chou,Y. C. Chen,C. Y. Hsu,and J. T. Lin,J. Mater. Chem. 22,8734(2012).
[12]B. Wenger,M. Gr¨atzel,and J. E. Moser,J. Am. Chem. Soc. 127,12150(2005).
[13]J. B. Asbury,E. Hao,Y. Wang,H. N. Ghosh,and T. Lian,J. Phys. Chem. B 105,4545(2001).
[14]S. E. Koops,B. C. O'Regan,P. R. F. Barnes,and J. R. Durrant,J. Am. Chem. Soc. 131,4808(2009).
[15]V. C. Anitha,A. N. Banerjee,S. W. Joo,and B. K. Min,J. Ind. Eng. Chem. 29,227(2015).
[16]H. P. Lu,C. Y. Tsai,W. N. Yen,C. P. Hsieh,C. W. Lee,C. Y. Yeh,and E. W. G. Diau,J. Phys. Chem. C 113,20990(2009).
[17]H. Tian,X. Yang,R. Chen,R,Zhang,A. Hagfeldt,and L. C. Sun,J. Phys. Chem. C 112,11023(2008).
[18]T. Geiger,S. Kuster,J. H. Yum,S. J. Moon,M. K. Nazeeruddin,M. Gr¨atzel,and F. N¨uesch,Adv. Funt. Mater. 19,2720(2009).
[19]A. Hagfeldt,G. Boschloo,L. C. Sun,L. Kloo,and H. Pettersson,Chem. Rev. 110,6595(2010).
[20]Y. P. Wang,S. Zhang,S. M. Sun,K. Liu,and B. Zhang,Chin. J. Chem. Phys. 26,651(2013).
[21]C. Bauer,G. Boschloo,E. Mukhtar,and A. Hagfeldt,J. Phys. Chem. B 106,12693(2002).
[22]R. Katoh,A. Furube,M. Kasuya,N. Fuke,N. Koide,and L. Han,J. Mat. Chem. 17,3190(2007).
[23]A. El-Zohry,A. Orthaber,and B. Zietz,J. Phys. Chem. C 116,26144(2012).
[24]D. L. Ashford,W. J. Song,J. J. Concepcion,C. R. K. Glasson,M. K. Brennaman,M. R. Norris,Z. Fang,J. L. Templeton,and T. J. Meyer,J. Am. Chem. Soc. 134,19189(2012).
DOI:10.1063/1674-0068/29/cjcp1512251
杂志排行
CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Morphology and Growth Process of Bat-like ZnO Crystals by Thermal Evaporation
- Spin Polarization at Organic-Ferromagnetic Interface:Effect of Contact Configuration
- Comparative Theoretical Studies on Several Energetic Substituted Dioxin-imidazole Derivatives
- Controlled Synthesis of PCL/PVP Copolymer by RAFT Method and Its Hydrophilic Block-Dependent Micellar Behaviors
- Epitaxial Growth and Thermoelectric Measurement of Bi2Te3/Sb Superlattice Nanowires
- Ordered Toroid Structures of Nanoparticles in Self-attractive Semiflexible Polymer/Nanoparticle Composites
