Controlled Synthesis of PCL/PVP Copolymer by RAFT Method and Its Hydrophilic Block-Dependent Micellar Behaviors
2016-07-05RuiLiWenminPngQingrenZhuKngmingNieDeprtmentofBiologilndEnvironmentlEngineeringHefeiUniversityHefei230022ChinAnhuiProvineKeyLbortoryofEnvironmentFriendlyPolymerMterilsCollegeofChemistryndChemilEngineeringAnhuiUniversit
Rui Li,Wen-min Png,Qing-ren Zhu,Kng-ming Nie. Deprtment of Biologil nd Environmentl Engineering,Hefei University,Hefei 230022,Chin b. Anhui Provine Key Lbortory of Environment-Friendly Polymer Mterils,College of Chemistry nd Chemil Engineering,Anhui University,Hefei 230601,Chin . Hefei Ntionl Lbortory for Physil Siene t the Mirosle,University of Siene nd Tehnology of Chin,Hefei 230026,Chin
Controlled Synthesis of PCL/PVP Copolymer by RAFT Method and Its Hydrophilic Block-Dependent Micellar Behaviors
Rui Lia,Wen-min Pangc,Qing-ren Zhuc,Kang-ming Nieb∗
a. Department of Biological and Environmental Engineering,Hefei University,Hefei 230022,China b. Anhui Province Key Laboratory of Environment-Friendly Polymer Materials,College of Chemistry and Chemical Engineering,Anhui University,Hefei 230601,China c. Hefei National Laboratory for Physical Science at the Microscale,University of Science and Technology of China,Hefei 230026,China
(Dated:Received on September 29,2015;Accepted on November 28,2015)
A range of poly(ε-caprolactone)/poly(N-vinyl-2-pyrrolidone)amphiphilic block copolymers with well-defined hydrophilic chain length were synthesized by the living/controlled reversible addition fragmentation chain transfer polymerization method. The composition and structure of the targeted resultants were characterized with1H NMR,13C NMR,FT-IR spectroscopy and gel permeation chromatography. The various block copolymers were successfully employed to fabricate the spherical micelle with core-shell morphological structure. The poly(N-vinyl-2-pyrrolidone)block-dependent characteristics of the copolymeric micelles were investigated by fluorescence spectroscopy,dynamic light scattering,and transmission electron microscopy. The solubilization of the hydrophobic ibuprofen as a model drug in the micelle solution was also explored. It was found that the drug loading contents are related to the micellar morphology structure determined by hydrophilic chain length in the copolymer.
Key words:RAFT polymerization,Poly(N-vinyl-2-pyrrolidone),Core-shell structure,Hydrophilic chain length,Micellar behavior
∗Author to whom correspondence should be addressed. E-mail:niekm@ahu.edu.cn,Tel./FAX:+86-551-63861279
I. INTRODUCTION
Amphiphilic copolymers composed of hydrophilic and hydrophobic blocks have been extensively utilized to fabricate micellar nanocarriers for drug delivery[1-3]. The amphiphilic macromolecular structures designed by using linear block or graft copolymers have the potential to adjust and control the micellar morphology. By manipulating the chemistry of block copolymer,it is possible to control the resulting morphology structure,surface chemistry,and many properties of the corresponding biomaterials[2,4].
For drug delivery applications,spherical polymer micelle with the core-shell structure,in which hydrophobic and hydrophilic segments constitute the cores and outer shells of the micelles,respectively,is the subject of the investigation because of their unique drug loading form as well as their disposition characteristics in the body [5-8]. A hydrophilic shell in combination with a hydrophobic core that can physically entrap hydrophobic drugs is an attractive design for the controlled delivery of drugs[9]. The synthesis method of well-defined diblock copolymers is of great critical for the preparation of novel materials fabricated nanostructured micelle. Therefore,to produce diblock copolymers with well characterized segment lengths,narrow molecular weight distribution,and no homopolymer under appreciate experimental condition,novel model materials need to be synthesized in a well-controlled way[10].
So far,some studies have focused on the synthesis of amphiphilic block copolymers by using atom transfer radical polymerization(ATRP)living polymerization methods[11,12]. Varying systematically hydrophobic chain length of the amphiphilic block copolymer in the molecular design have been found to be crucial to obtain optimal structure,size and hydrophobic drug loading of spherical micelle[13]. However,information on the related influence exerted by hydrophilic chain length in the poly(N-vinyl-2-pyrrolidone)(PVP)based copolymers is relatively lacking,which have unique advantages over PEG-based block copolymers in drug delivery application[14]. The reversible addition fragmentation chain transfer(RAFT)polymerization was recently proposed as a promising controlled radical polymerization technique for the synthesis of well-defined nanostructured polymeric materials[15,16],and also is a particularly versatile synthetic method for building micellar assembles as it is applicable to a wide range of monomer structures with more versatility than ATRP method. Depending on the structure of special RAFT agent,the controlled/living polymerization of a wide range of monomers un-conjugated only by a simple radical mech-anism to form the polymers with high molecular weight,such as N-vinyl-2-pyrrolidone(NVP)monomer[17],can be achieved. Consequently,synthesis of PVP based block copolymers with well-defined hydrophilic chain segment structures by RATF polymerization is of great importance for further investigating fabrication of the suitable morphology used as hydrophobic drug carriers.
In this work,a series of the poly(ε-capro lactone)/poly(N-vinyl-2-pyrrolidone)amphiphilic diblock copolymers(PCL-b-PVP)with the same PCL hydrophobic chain length and the various PVP hydrophilic chain length,which were considered as a self-assembly unit of the nanocarriers for hydrophobic ibuprofen(IBU)drug delivery,were synthesized by using RAFT controlled/living radical polymerization in the presence of PCL-based macromolecular chain transfer agent(macro-CTA). The effects of PVP hydrophilic chain length in the PCL-b-PVP copolymers on the micellar behaviors of nanocarriers as hydrophobic drug delivery were investigated.
II. EXPERIMENTS
A. Materials and measurements
Monomer ε-caprolactone(ε-CL)(Aldrich,99%)was dried over calcium hydride for 48 h at room temperature,followed by distillation under reduced pressure just before use. N-Vinyl pyrrolidone(NVP)(Aldrich)was purified by stirring with MgSO4followed by distillation under reduced pressure. 2,2-Azobis(isobutyronitrile)(AIBN,98%)were purchased from Aldrich and recrystallized from ethanol before use. The dialysis membrane(molecular weight cut-off 3500)was purchased from Shanghai Biological Engineering Co. Ltd.,China. 2-Mercaptoethanol,carbon disulfide(99%),N-isopropylacrylamide(97%),and N,N-dimethylacetamide(99.9%,HPLC grade)were purchased from Aldrich. Benzyl bromide,3,6-dimethyl-1,4-dioxane-2,5-dione,Hexanediol diacrylate(Aldrich),stannous octoate(Sn(Oct)2,Alfa Aesar),pyrene(Acros Organics). All other reagents were of analytical grade and used as received without further purification. Synthesis of 2-(benzylsulfanylthiocarbonylsulfanyl)ethanol RAFT agent was performed according to the previous report[18].
Fourier transform infrared(FTIR)spectra were measured on Nicolet MAGNA-IR 750 spectrometer.1H and13C nuclear magnetic resonance(1H NMR and13C NMR)spectra were recorded on Bruker 400 MHz spectrometer using CDCl3as the solvent. The molecular weight and molecular distributions(Mw/Mn)were determined by Waters 150 C gel permeation chromatograph(GPC)with 410 refractive index detector measurements at 35◦C. DMF was used as eluent at the flow rate of 1 mL/min. Monodispersed polystyrene standards were used to generate the calibration curve. Fluorescent spectra of the PCL-b-PVP block copolymer micelle solution were recorded on a fluorescence spectrophotometer(SPEX Fluorolog 3). The emission wavelength was set at 390 nm and the slit width was 5 nm. The hydrodynamic radius(Rh)of the micelles (0.15 and 0.5 mg/mL)were determined by dynamic laser scattering(DLS)conducted on a laser light scattering spectrometer(ALV/DLS/SLS-5022F)equipped with a cylindrical 22 mW UNIPHASE He-Ne laser at a scattering angle of 15◦at 25◦C. The samples of the micelle solution were filtered by using a 0.45µm membrane filter before measurements. Transmission electron microscopy(TEM)images of the micellar morphology were obtained on a JEM-100SX instrument operating at an acceleration voltage of 100 kV. The samples were prepared by dropping onto a copper grid coated with a carbon membrane and then stained with 2wt% phosphotungstic acid aqueous solution. The samples were dried in air before observation.
B. Synthesis of PCL macro-CTA
A typical synthesis of poly(ε-caprolactone)macro-CTA is as follows:the monomer ε-caprolactone(15 g)and 2-(benzylsulfanylthiocarbonylsulfanyl)ethanol RAFT agent(0.260 or 0.897 g)were mixed and stirred under a vacuum at 120◦C for 12 h. After purging with nitrogen,degassed Sn(Oct)2(55.9 mg,solution in 1 mL of toluene)was added to the yellow mixture. The temperature was increased to 140◦C,and the mixture was left to react for 24 h. The mixture resultant was dissolved in dichloromethane and then precipitated in methanol for purification. The resulting PCL macro-CTA was dried under vacuum at 40◦C for 24 h.
C. RAFT synthesis of PCL-b-PVP block copolymer
The block copolymers were prepared by RAFT polymerization of NVP using PCL macro-CTA as macroinitiator in the presence of AIBN system under inert atmosphere. A typical experiment for synthesis of the PVP-b-PCL was carried out:AIBN(0.0125 mmol,2.0 mg),PCL macro-CTA(Mn=6612 g/mol,0.1 mmol,0.66 mg),NVP(11 mmol,1.21 g),and 4 mL DMF was added to a reaction tube in a glove box under argon. RAFT bulk polymerization was performed in an oil bath at 80◦C for 15 h. After required reaction time,the mixture was cooled and precipitated in anhydrous ether. After the residue was re-dissolved/precipitated several times with DMF/anhydrous ether,the remaining product obtained was dried under vacuum at 40◦C for 24 h.
D. Preparation of PCL-b-PVP block copolymer micelles
The copolymer micelles of PCL-b-PVP with a narrow molecular weight distribution were prepared by dialysismethod[19]. The desired amounts of PCL-b-PVP amphiphilic block copolymer were dissolved into dimethyl formamide(DMF)and stirred overnight at room temperature. The initial concentrations of the corresponding copolymer solutions were fixed at 0.5wt%,1.0wt% and 2.0wt%. Then,the twice-distilled water was slowly added dropwise into the various copolymer solutions. When the content of the water reached 50wt%and 75wt%,the copolymer solution was transferred into a dialysis bags and dialyzed against twice-distilled water for 72 h to remove the solvents.
E. Determination of critical micelle concentration(CMC)
The CMC of the PCL-b-PVP amphiphilic block copolymer were determined by a fluorescence spectroscopic method using pyrene as the hydrophobic fluorescence probe. A given amount of pyrene solution in acetone was added into a series of volumetric flasks and then the acetone was completely removed by evaporating. The various PCL-b-PVP amphiphilic block copolymer solutions with concentrations ranging from 0.1µg/mL to 1.0 mg/mL were added to the bottles in which the concentration of pyrene was kept at 0.6µmol/L. The excitation spectra of the samples were recorded at 20◦C on a fluorescence spectrophotometer.
F. Loading of hydrophobic drug
The hydrophobic IBU drugs were loaded into the micelles in the experiment. Briefly,a given amount of IBU was added into a series of vials containing appropriate amounts of the PCL-b-PVP solutions with different concentrations under magnetic stirring. The mixtures were kept stirring for 48 h at room temperature and filtered through a syringe membrane(2.2µm)to remove insoluble substance. 1 mL of the filtrate was transferred into 50 mL of the volumetric flask and diluted by twice-distilled water,and then,3 mL of the diluted solution was placed into 25 mL of the volumetric flask and diluted by twice-distilled water. The solution was dialyzed against deionized water for 48 h with a dialysis membrane of molecular weight cut-off 3500 and lyophilized for 24 h. Absorbance of the sample at 222 nm in UV-visible spectrum(UV3600)was measured and used to calculate the solubility of IBU in micelle aqueous solution according to the standard curve constructed by concentration-dependent absorbance of IBU at 222 nm in the same solvent.
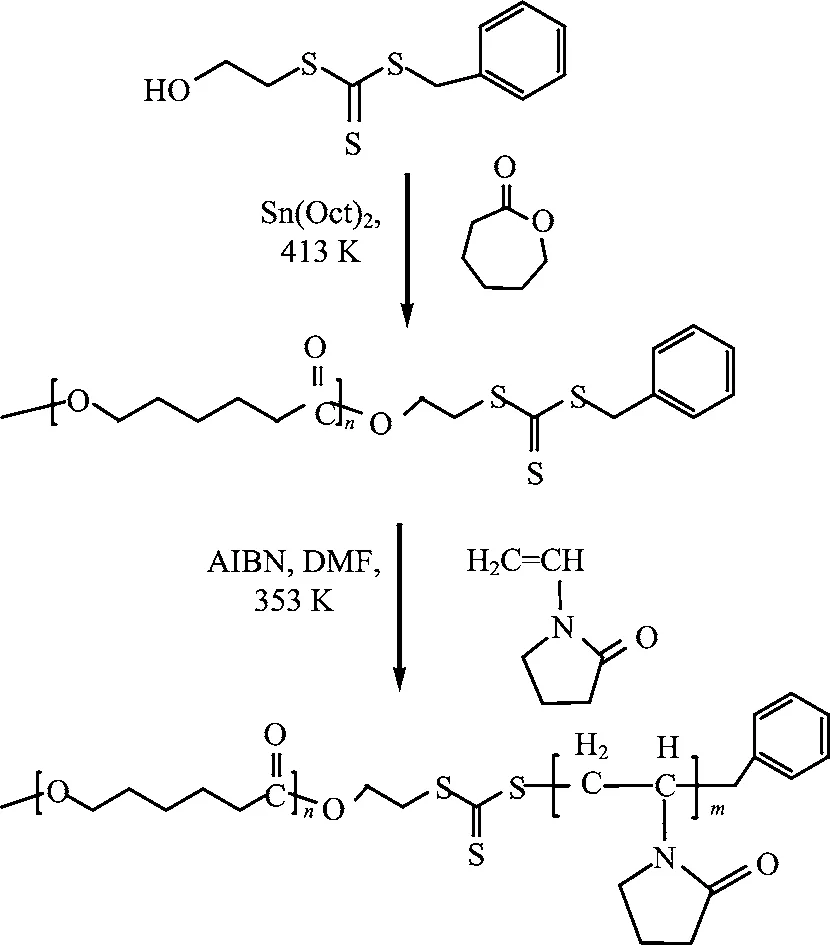
Scheme 1 The synthetic schemes of the PCL-b-PVP amphiphilic block copolymer synthesized by RAFT controlled polymerization.
III. RESULTS AND DISCUSSION
A. Characterization of PCL-b-PVP block copolymer
The PCL-b-PVP block copolymer was synthesized by RAFT according to the steps shown in Scheme 1. In the performed experiment,the molar ratio of CL/Sn(Oct)2was fixed at 500:1. The PCL macro-CTA with desired molecular weight may be obtained by adjusting the molar ratio of ε-CL/OH(-OH in RAFT agent). Compared with Fig.1(A),the various characteristic peaks of the repeat unit[-O-CH2-(CH2)3-CH2-CO-]nfor the PCL chain in the PCL macro-CTA are observed from the1H NMR spectrum shown in Fig.1(B). Peaks a,b,c,and d,which are ascribed to methylene protons in -O-CH2- group,-(CH2)3- protons and methylene protons adjacent to the carbonyl group,respectively. The peak centered at 3.68 ppm(peak e)is attributed to the methylene protons associated with the peripheral hydroxyl group of the PCL chain. According to the integration ratio of peak d to peak centered at 3.68 ppm in the1H NMR spectrum of the PCL macro-CTA,the numbers of repeat unit(i.e DP)of a PCL chain in the typical resultant may be calculated to be 58,which are in line with the value designed.
On the basis of the synthesis of the PCL macro-CTA described above,the PCL-b-PVP block copolymers were obtained by the RAFT polymerization. The1H NMR spectrum of the resultant is illustrated in Fig.1(C). Other signals of the PVP chain segments can also be observed besides the various characteristic peaks of the PCL repeat unit. The signals centered at 2.0 and 3.7 ppm(peak f)are attributed to the methylene and methine backbone protons of N-vinyl-2-pyrrolidone group,respectively. The peak centered at 3.2 ppm(peak i)is ascribed to the methylene protons adjacent to the nitrogen atom. The signals in the range of 2.3-1.7 ppm are attributed to the methylene protonsadjacent to the carbonyl group and the methylene protons of its β site,respectively. The resonance peaks at 1.4 ppm(peak c)are attributed to the methylene protons in the CL monomer units. The number-average degree of polymerization(DPn)for the PVP block can be calculated from the peak intensity ratio(f+i)/c.
The FT-IR spectra of the PCL macro-CTA and the PCL-b-PVP block copolymer are shown in Fig.2. The absorption peaks ascribed to ester carbonyl at 1725 cm-1and hydroxyl at 3441 cm-1in the PCL macro-CTA were respectively observed in Fig.2(a). On the other hand,Fig.2(b)also shows the characteristic absorption peak ascribed to carbonyl at 1672 cm-1in PVP segments of the PCL-b-PVP block copolymers in addition to the absorption peak bands at 1725 cm-1ascribed to ester carbonyl in PCL block of the copolymers. While,the absorption peak centered at 3441 cm-1for the PCL-b-PVP products could be seen. Furthermore,a13C NMR single peak at 173.6 ppm in the carbonyl region shown in Fig.3(A)further indicated the diblock structure of the copolymer[20]. The GPC chromatographs of the PCL macro-CTA and the PCL-b-PVP resultants are shown in Fig.3(B). Each GPC elution profile displays an unimodal peak and a relatively narrow polydispersity. Thus,judging together with analysis by FT-IR,1H NMR and13C NMR,the results showed that a range of PCL-b-PVP block copolymers with the same PCL block(DP=58)and the different PVP block were successfully synthesized by RAFT polymerization. For comparison,the characteristic data determined by GPC and1H NMR spectrum were summarized in Table I. It is found that Mw/Mnvalues of the resultants obtained by RAFT polymerization decrease with increasing the hydrophilic PVP chain length in the copolymer,indicating that the molecular weight distribution tends to the monodispersity.
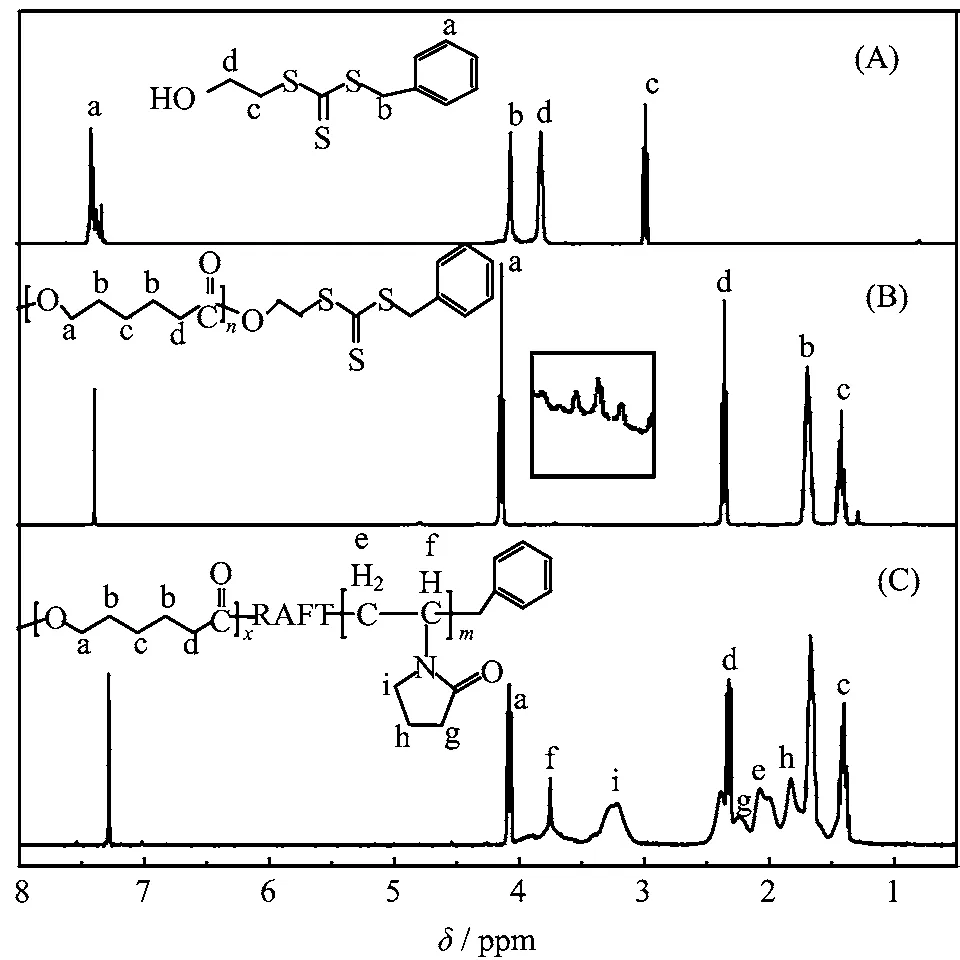
FIG. 1 1H-NMR spectra recorded for(A)RAFT agent,(B)PCL macro-CTA agent,and(C)PCL-b-PVP block copolymer in CDCl3at 20◦C.
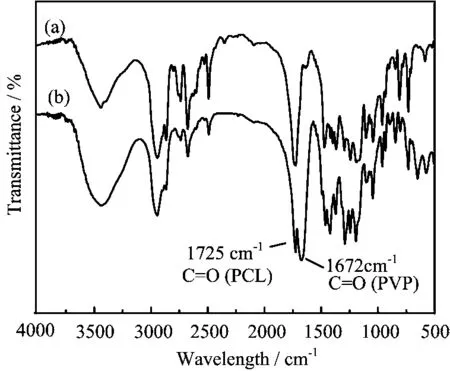
FIG. 2 FT-IR spectra of(a)PCL macro-CTA and(b)PCL-b-PVP block copolymer.
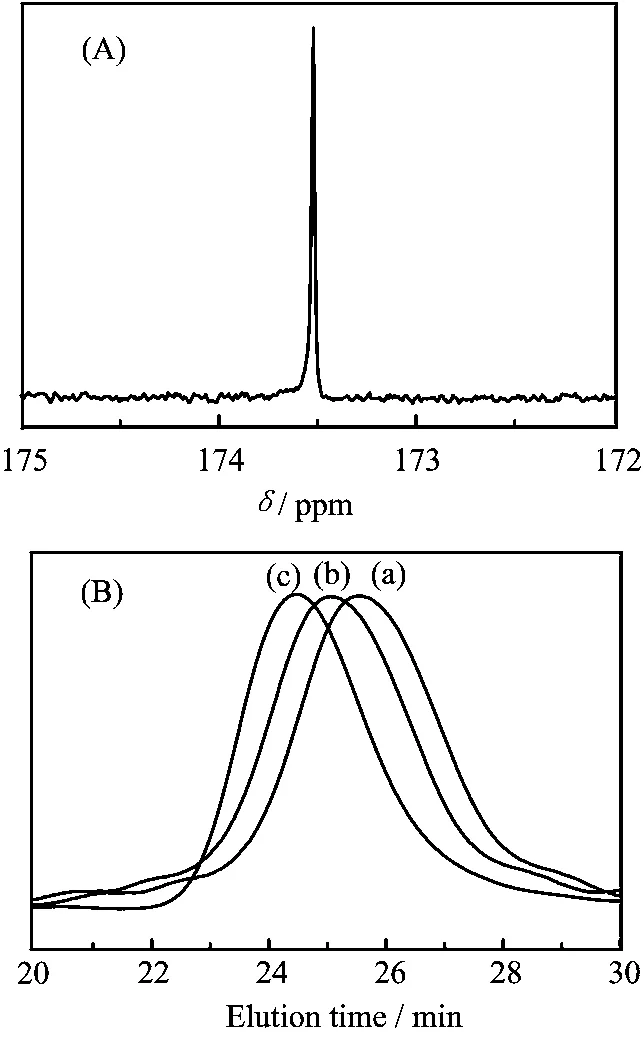
FIG. 3 (A)13C NMR spectrum of carbonyl region of PCL-b-PVP block copolymer in CDCl3.(B)GPC chromatographs of(a)PCL macro-CTA,(b)PCL58-b-PVP29,and(c)PCL58-b-PVP66copolymer.
B. Self-assemble of copolymeric micelle and characterization
To investigate the micellar behaviors in aqueous solution,pyrene with hydrophobicity,whose emission and excitation spectrum are sensitive to the surrounding environment[21],was used as a fluorescence probe inthis experiment to characterize the micellar formation of the amphiphilic copolymers by using the results of its excitation spectra and determining the critical micelle concentration(CMC)referring to the micelle thermodynamic stability. It was found from the excitation spectra of pyrene in the copolymeric solution that the (0,0)absorption band shift from 336 nm to 339 nm as the concentration of the PCL-b-PVP copolymer was increased,indicating that the formation of copolymer micelles in the system[22]. The I339/I336intensity ratios obtained from the excitation spectra were plotted versus the concentration(lgC)as illustrated in Fig.4(A). From the onset value of an increase in the sigmoidal shape curve,the CMC was determined by the corresponding value of the concentration. It is seen from Fig.4(B)that the CMC values of the micelle increase with increasing PVP hydrophilic chain lengths. It is reasonable that the higher contents of the hydrophobic chain segments result in the stronger interactions,leading to a more stable PCL core structure in the micelle and,therefore,to lower CMC value.

TABLE I The characteristic results of PCL macro-CTA and PCL-b-PVP resultants obtained by RAFT polymerization.
C. The morphological structure of micelles aggregates
The micellar mean sizes(Rh)of the various PCL-b-PVP micellar solutions are shown in Fig.5(a)and (b),and Table I,respectively. In the PCL58-b-PVP29copolymer micellar solution of 1.0 mg/mL,the Rhwere determined to be 187 nm,whereas,for the PCL58-b-PVP66(1.0 mg/mL),to be 262 nm. The results show that Rhincrease with increasing the hydrophilic chain length,and the particle size distribution of the micelles shift toward relatively uniform. This kind of the change of micellar structure is corresponding to the morphological results observed by TEM. Upon DMF acting as a common solvent,the aggregates of the PCL58-b-PVP29copolymeric micelles take a spherical morphology with a mean size of 180 nm and the core-shell structure might be seen as shown in Fig.5(c). When the hydrophilic chain length in the copolymer was enhanced(such as PCL58-b-PVP66),the micellar aggregates exhibit the typically spherical morphology with a mean size of 240 nm as shown in Fig.5(d),which were smaller than those of Rhvalue measured by DLS. The phenomenon has been observed in previous report[23]. Moreover,a more integral core-shell structure in which the dark core composed of hydrophobic PCL and gray shell composed of hydrophilic PVP. It is also observed that the shell thickness and size distribution of PCL58-b-PVP66micellar aggregates is apparently thicker and more uniform than that of PCL58-b-PVP29aggregates. The morphological change of the PCL-b-PVP micelles could be mainly attributed to aggregates of the smaller micelles due to hydrophilic interactions between PCL chains and steric stabilization of the micelle provided by the hydrophilic PVP shell part[24]. The morphology of aggregates tend to form the larger hydrophobic core,whereas the long hydrophilic chain length in the copolymers is easy to build an integral and stable shell structure for decreasing the overall free energy of micel-lization.
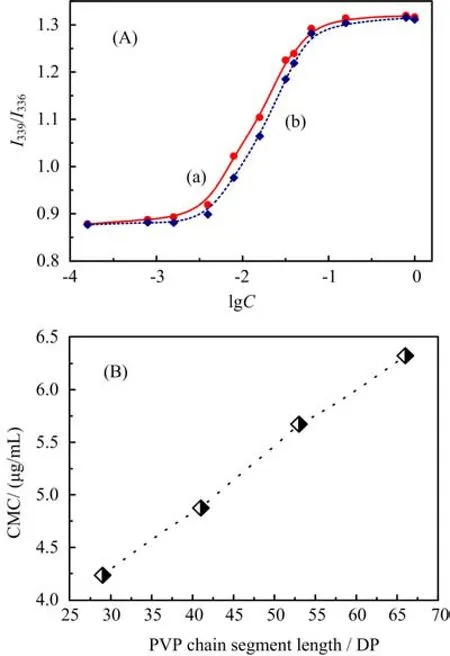
FIG. 4 (A)The plot of the intensity ratio(I339/I336)obtained from pyrene excitation spectra against(a)the PCL58-b-PVP29and(b)PCL58-b-PVP66copolymer solution.(B)the relationship between CMC value and PVP hydrophilic chain length in the copolymer.
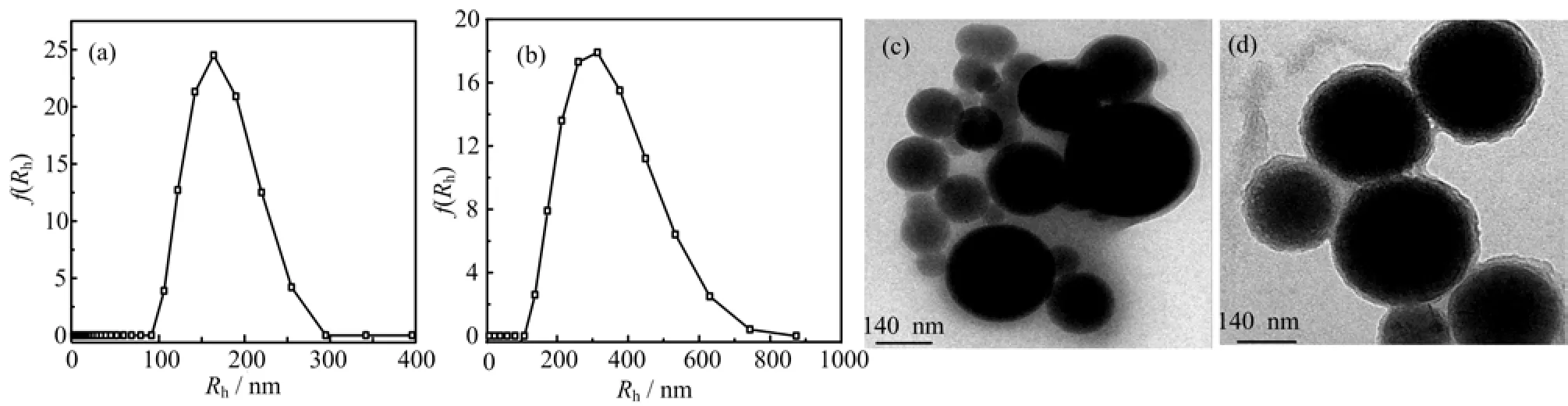
FIG. 5 The hydrodynamic radius(Rh)and particle size distribution of micelles formed by(a)PCL58-b-PVP29and(b)PCL58-b-PVP66copolymer. TEM images of(c)PCL58-PVP29and(d)PCL58-PVP66linear diblock copolymer micelles. DMF was used as the common solvent,the initial copolymer concentration is 1.0wt%. The water content is ca. 20wt%.
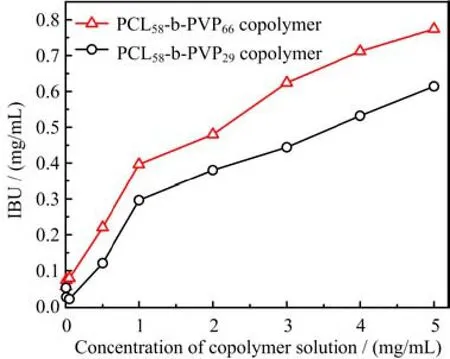
FIG. 6 Plot of solubility capacity of IBU as a function of the concentration for the PCL58-b-PVP29and PCL58-b-PVP66amphiphilic diblock copolymer in the aqueous solution.
D. Loading hydrophobic drug by copolymeric micelles
The specific length changes of the hydrophilic PVP block were also found to have profound influence on the solubilization of the hydrophobic IBU drugs. As the concentration of the PCL58-b-PVP29and PCL58-b-PVP66diblock copolymer in the aqueous solution reach up to 5 mg/mL,it can be seen from Fig.6 that the solubility of IBU increases to 0.61 and 0.74 mg/mL at room temperature,respectively,implying that the diblock copolymer micelles can solubilize 0.524 mg/mL or 0.654 mg/mL IBU in comparison with the solubility(0.086 mg/mL)of IBU in the saturated aqueous solution. The experimental data and the fit curve of IUB loading contents for the micelles composed of the copolymer with various PVP chain lengths are shown in Fig.7. It can be found that IUB loading contents in the micelle increase with increasing of the hydrophilic PVP block in the copolymer. It is reasonable to assume that the loading behaviors should originate from the core-shell structure alteration of the spherical micelle nanocarriers,which morphology is dependent on the hydrophilic PVP chain length,because hydrophobic IBU was physically encapsulated stabilized in the hydrophobic PCL inner core of the copolymer micelles by hydrophobic interaction against water.
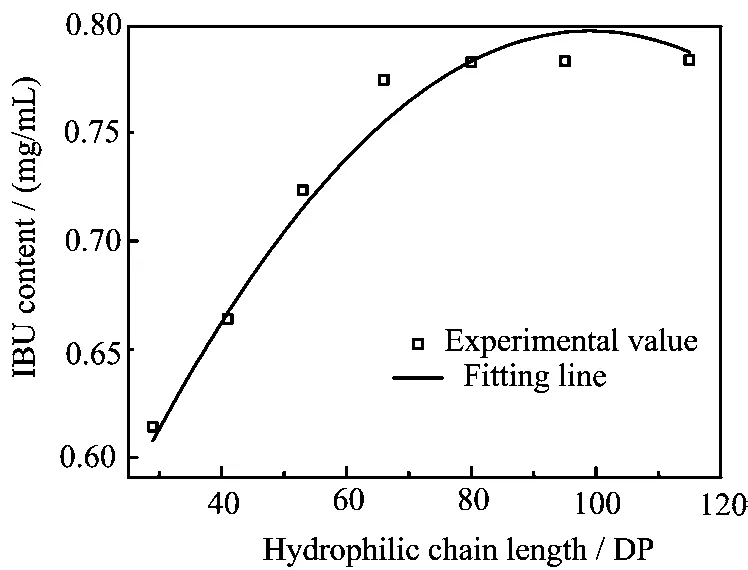
FIG. 7 Drug loading profile of IBU-entrapped micelle composed of the copolymer with various hydrophilic chain lengths.
IV. CONCLUSION
The amphiphilic diblock copolymers composed of the same hydrophobic PCL chain length and the various hydrophilic PVP chain lengths were synthesized by using RAFT controlled/living radical polymerization. The composition and structure of the copolymer were confirmed by1H,13C NMR,FT-IR,and GPC. These copolymers with a narrow molecular weight distribution were successfully employed to fabricate micelle in aqueous media due to their amphiphilic nature. The micellar characteristics explored by fluorescence spectroscopy,DLS and TEM show that CMC,Rhand the spherical morphology with core-shell structure of the copolymer micelles are influenced by the hydrophilic PVP block length in the copolymer. Through the solubilization of the poor water soluble drug in the micelle solution,it is evident that the micellar system basedon the block copolymer can solubilize hydrophobic IBU drug molecules. The IBU loading contents in the micelle increase with increasing of the hydrophilic PVP block in the copolymer. The drug loading behaviors should originate from the core-shell structure alteration of the spherical micelle nanocarriers,which morphology is dependent on the hydrophilic PVP chain length in the copolymer.
V. ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China(No.50973001),the Key Science and Research Project of Anhui Province Education Department,China(No.KJ2012A018),and the Talent Science Research Foundation of Hefei University,China(No.14RC04).
[1]A. Rosler,G. W. Vandermeulen,and H. A. Klok,Adv. Drug Deliv. Rev. 64,270(2012).
[2]J. K. Kim,S. Y. Yang,Y. Lee,and Y. Kim,Prog. Polym. Sci. 35,1325(2010).
[3]J. S. Lee and J. Feijen,J. Control Release 161,473 (2012).
[4]B. Bhushan and S. R. Schricker,J. Biomed. Mater. Res. Part A 102A,2467(2014).
[5]T. G. Park,J. H. Jeong,and S. W. Kim,Adv. Drug Deliv. Rev. 58,467(2006).
[6]R. Savic,A. Eisenberg,and D. Maysinger,J. Drug Target. 14,343(2006).
[7]N. Nishiyama and K. Kataoka,Pharmacol. Ther. 112,630(2006).
[8]L. Qiu and M. Yan,Acta Biomater. 5,2132(2009).
[9]M. H. Stenzel,Macromol. Rapid. Commun. 30,1603 (2009).
[10]T. W. Chunga,K. Y. Choa,H. Chul. Lee,and C. S. Cho,Polymer 45,1591(2004).
[11]S. Shi,Y. Xia,X. Ma,S. Jiao,and X. Li,Adv. Mater. Res. 11/12,469(2006).
[12]J. S. Lee and J. Feijen,J. Control. Release 161,473 (2012).
[13]J. Luo,K. Xiao,Y. Li,J. S. Lee,L Shi,Y. H Tan,L. Xing,R. H. Cheng,G. Y. Liu,and K. S. Lam,Bioconjug. Chem. 21,1216(2010).
[14]H. U. Kang,Y. C. Yu,S. J. Shin,and J. Kim,Macromolecules 46,1291(2013).
[15]G. Moad,E. Rizzardo,and S. H. Thang,Polymer 49,1079(2008).
[16]A. Debuigne,M. Schoumacher,N. Willet,R. Riva,X. M. Zhu,S. R¨utten,C. J´erˆome,and C. Detrembleur,Chem. Commun. 47,12703(2011).
[17]A. N. Zelikin,G. K. Such,and F. Caruso,Biomacromolecules 8,2950(2007).
[18]M. Hales and C. Barner-Kowollik,Langmuir 20,10809 (2004).
[19]S. Q. Liu,Y. W. Tong,and Y. Y. Yang,Biomaterials 26,5064(2005).
[20]P. Vanhoorne,P. Dubois,R. Jerome,and P. Teyssie,Macromolecules 25,37(1992).
[21]M. Wilhelm,C. L. Zhao,Y. C. Wang,R. L. Xu,M. A. Winnik,J. L. Mura,G. Riess,and M. D. Croucher,Macromolecules 24,1033(1991).
[22]C. L. Zhao,M. A. Winnik,G. Riess,and M. D. Croucher,Langmuir 6,514(1990).
[23]J. Zhang,L. Q. Wang,and H. J. Wang,Biomacromolecules 79,2492(2006).
[24]N. Hameed,N. V. Salim,and J. Parameswaranpillai,Mater. Lett. 147,92(2015).
DOI:10.1063/1674-0068/29/cjcp1509203
杂志排行
CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Virtual Screening of Human O-GlcNAc Transferase Inhibitors
- Spin Polarization at Organic-Ferromagnetic Interface:Effect of Contact Configuration
- Comparative Theoretical Studies on Several Energetic Substituted Dioxin-imidazole Derivatives
- Epitaxial Growth and Thermoelectric Measurement of Bi2Te3/Sb Superlattice Nanowires
- Morphology and Growth Process of Bat-like ZnO Crystals by Thermal Evaporation
- Investigation of Ultrafast Electronic Transfer Process on Organic/Inorganic Heterojunction by Femtosecond Transient Absorption
