内皮祖细胞培养上清对高氧暴露下Ⅱ型肺泡上皮细胞增殖和分化的影响*
2016-07-05王传凯陆爱珍祁媛媛钱莉玲
王传凯, 陆爱珍, 祁媛媛, 钱莉玲
(复旦大学附属儿科医院,上海 201102)
内皮祖细胞培养上清对高氧暴露下Ⅱ型肺泡上皮细胞增殖和分化的影响*
王传凯,陆爱珍,祁媛媛,钱莉玲△
(复旦大学附属儿科医院,上海 201102)
[摘要]目的: 研究高氧暴露对内皮祖细胞(EPCs)旁分泌功能的影响,并探索EPCs旁分泌因子对高氧暴露下Ⅱ型肺泡上皮细胞(AECⅡ)功能的影响。方法: 分离大鼠骨髓来源的单个核细胞,EGM-2MV培养基培养7~10 d获取EPCs,并对其进行鉴定。分别在空气条件下及60%的氧浓度条件下培养EPCs,收集空气组EPCs培养上清(E-CM-RA)和高氧组EPCs培养上清(E-CM-O2),ELISA法检测2组上清中VEGF、FGF10、EGF及PDGF-BB的表达水平。分离纯化培养成年大鼠的AECⅡ,常规培养至第2 天,分为4组:(1)RA组:空气条件下培养;(2) O2组:60%的氧浓度条件下培养;(3) O2+E-CM-RA组:在O2组的基础上,在培养基中加入E-CM-RA;(4) O2+E-CM-O2组:在O2组的基础上,在培养基中加入E-CM-O2。分别在干预12 h、24 h、2 d和3 d,用MTT法及细胞计数法检测4组AECⅡ的增殖情况;在24 h、2 d和3 d用real-time PCR法检测4组AECⅡ中SP-C和AQP5的mRNA表达量。结果: E-CM-RA中VEGF及FGF10的表达水平明显高于E-CM-O2(P<0.05)。干预不同时间,各组间AECⅡ的活力及数量均有明显差异(P<0.01)。与RA组相比,O2组AECⅡ各时点的活力明显下降,数量明显减少(P<0.05);与O2组相比,各时点O2+E-CM-RA组AECⅡ的活力明显增高,数量明显增加(P<0.05);但O2+E-CM-O2组与O2组AECⅡ的活力及数量均无显著差异。干预24 h、2 d及3 d,4组间AECⅡ的SP-C与AQP5 mRNA的表达量均具有明显差异(P<0.01)。与RA组相比,O2组AECⅡ中SP-C的mRNA表达量显著降低(P<0.01),AQP5的mRNA表达量显著升高(P<0.01)。与O2组相比,在24 h、2 d及3 d时, O2+E-CM-RA组和O2+E-CM-O2组AECⅡ中SP-C的mRNA表达量显著增高(P<0.05),而在2 d及3 d时,AQP5的mRNA表达量显著降低(P<0.01)。结论: EPCs可分泌肺发育相关生长因子VEGF和FGF10,高氧暴露抑制EPCs表达 VEGF和FGF10。高氧暴露抑制AECⅡ的增殖,降低AECⅡ表达SP-C mRNA,促进AECⅡ表达AQP5 mRNA。EPCs培养上清能够改善高氧暴露对AECⅡ增殖的抑制作用,维持AECⅡ本身特性,抑制AECⅡ向AECⅠ分化。而高氧暴露部分削弱了EPCs培养上清的这种作用,这可能与高氧暴露抑制EPCs表达肺发育相关因子VEGF和FGF10有关。
[关键词]内皮祖细胞; 旁分泌功能; 肺泡Ⅱ型上皮细胞; 高氧损伤
支气管肺发育不良(bronchopulmonary dysplasia,BPD)在早产儿表现为肺发育停滞,病理表现为肺血管异常和肺泡简化[1]。研究显示肺内血管是肺泡化的“源动力”,血管生成机制的破坏削弱了肺泡化[2]。内皮祖细胞(epithelial progenitor cells,EPCs)是影响血管发育的关键细胞之一,具有很强的自我更新和高度增殖能力,并能分化为血管内皮细胞参与血管生成[3]。临床研究发现早产儿循环EPCs的数量和功能与BPD发生相关[4]。动物实验也发现,高氧暴露降低了新生小鼠骨髓、循环和肺组织EPCs数目,破坏了肺血管发育和肺泡化,而给予外源性EPCs可促进肺部血管的形成,增加肺泡数量[5]。然而,单纯增加移植EPCs的数目对高氧肺损伤的疗效有限[6]。近来研究发现EPCs具有旁分泌功能[7],旁分泌因子包括血管内内皮细胞生长因子(vascular endothelial growth factor,VEGF)、成纤维细胞生长因子(fibroblast growth factor,FGF)、血小板源性生长因子BB(platelet-derived growth factor-BB,PDGF-BB)、表皮细胞生长因子(epidermal growth factor,EGF)、转化生长因子(transforming growth factor,TGF)、角化细胞生长因子(keratinocyte growth factor,KGF)、基质金属蛋白酶9(matrix metalloproteinase 9,MMP9)等。EPCs旁分泌因子能促进血管形成,维持血管内皮结构的完整[8-9];在促进血管生成方面,EPCs旁分泌因子与EPCs移植疗效相当[10]。
Ⅱ型肺泡上皮细胞(type Ⅱ alveolar epithelial cells,AECⅡ)是肺组织中的干细胞,能分化为Ⅰ型肺泡上皮细胞(type Ⅰ alveolar epithelial cells,AECⅠ),参与肺损伤修复,并分泌肺泡表面活性物质(pulmonary surfactant, PS)维持肺泡稳定。动物研究发现切除小鼠单侧肺可诱导EPCs分泌MMP14,使EGF样配体增加,刺激AEC Ⅱ增殖,促进再生性肺泡化[11]。在高氧肺损伤的研究方面,目前鲜有研究EPCs旁分泌功能对AECⅡ作用的报道。大量研究表明高浓度氧气抑制血管内皮细胞和肺泡上皮细胞的增殖,诱导凋亡[12]。本研究设想在体外建立高氧环境,研究高氧暴露对EPCs旁分泌功能的影响,以及EPCs旁分泌因子对高氧暴露下AECⅡ的增殖分化的影响。
材料和方法
1EPCs原代培养及免疫荧光染色鉴定
分离大鼠骨髓来源的单个核细胞,用EGM-2MV培养基(LONZA)重悬,以每孔2×106的密度接种于纤连蛋白(Sigma)包被过夜的6孔板中。培养3 d后弃去悬液,已贴壁细胞换新鲜培养液继续培养,每3 d换液,观察细胞生长形态变化。培养7~10 d,消化收获细胞进行EPCs鉴定。培养所得的爬片细胞分别加入DiI-acLDL(Biomedical Technologies)37 ℃孵育4 h和FITC-UEA-1(Sigma)37 ℃孵育1 h,经4%多聚甲醛固定,0.3% Triton Χ-100破膜,DAPI染核后,免疫荧光显微镜下计数双阳性细胞比例。
2AECⅡ原代培养及细胞鉴定
取大鼠肺组织,采用弹力蛋白酶/胰酶混合消化获得混合细胞,进一步采用免疫黏附法纯化AECⅡ。纯化后细胞用含10% 胎牛血清的DMEM培养基重悬,以每孔4×106的密度接种于6孔板内,每天换液。纯化培养2 d的细胞,采用BCIP/NBT碱性磷酸酯酶显色试剂盒(江苏碧云天生物技术研究所)染色,普通显微镜下计数蓝紫色深染细胞的比例,计算细胞纯度。纯化培养2 d的细胞离心后,加2.5%戊二醛固定细胞沉淀过夜,依次进行固定、脱水、包埋及超薄切片后,在透射电镜下观察AECⅡ特征性结构板层小体和微绒毛。
3实验分组
3.1EPCs分组培养7~10 d的EPCs,以4×105的密度接种于6 cm培养皿,待细胞生长达到80%融合时开始干预,分为空气组和高氧组干预EPCs。空气组直接置于普通CO2培养箱中培养,高氧组则置于自制培养器皿中(10 cm×15 cm×25 cm),持续通入含有60% O2和5% CO2的混合气体。培养2 d后2组均更换培养基为3 mL无血清基础培养基,置于普通CO2培养箱中培养1 d后收集上清,空气组EPCs培养上清(EPC-conditioned medium-room air,E-CM-RA)和高氧组EPCs培养上清(EPC-conditioned medium-O2,E-CM-O2),-80 ℃保存备用[13]。
3.2AECⅡ分组置于6孔板中原代培养第2 天的AECⅡ,分为正常室内空气(room air, RA)组、O2组、O2+E-CM-RA组和O2+E-CM-O2组。RA组置于普通CO2培养箱中培养,培养液为1 mL完全培养基+1 mL无血清培养基; O2组置于自制培养器皿中,持续通入含有60% O2和5% CO2的混合气体,培养液为1 mL完全培养基+1 mL无血清培养基; O2+E-CM-RA组高氧干预同O2组,培养液为1 mL完全培养基+1 mL E-CM-RA;O2+E-CM-O2组高氧干预同O2组,培养液为1 mL完全培养基+1 mL E-CM-O2。
4方法
4.1ELISA检测EPCs培养上清中的细胞因子ELISA法检测2组上清中VEGF、EGF(Maibio)、HGF10(Mybiosource)及PDGF-BB(R&D)的表达水平,实验严格按照说明书进行。
4.2MTT法测量AECⅡ的细胞活力在各组AECⅡ培养12 h、24 h、2 d和3 d时加入MTS工作液(Biovision),2 h后492 nm处检测各孔的吸光度(A)值。
4.3血球计数板计数AECⅡ细胞数量的变化在各组AECⅡ培养12 h、24 h、2 d、3 d时,弃去培养基,0.25%的胰酶完全消化、重悬后,取2 μL加入血球计数板,计数各组AECⅡ的细胞数目。
4.4Real-time PCR实验分别在培养24 h、2 d和3 d,收集ACEⅡ,采用TRIzol试剂提取总RNA,将1 μg的RNA用PrimeScript® RT试剂盒(TaKaRa)逆转录成cDNA。每个生物学指标用2 μL 的cDNA 采用SYBR® Premix Ex TaqTMⅡ 试剂盒(TaKaRa)进行实时荧光定量PCR分析。反应条件为95 ℃ 5 min;95 ℃ 5 s,60 ℃ 30 s,40循环。GAPDH 作为内参照。各生物学指标的引物见表1。采用2-ΔΔCt方法进行半定量分析。

表1 各基因的引物序列及扩增片段长度
F: forword; R: reverse.
5统计学处理
采用SPSS 16软件进行统计学分析。数据以均数±标准差(mean±SD)表示。正态分布的两组计量资料采用t检验,多组比较采用单因素方差分析(one-way ANOVA),并用Bonferroni方法进行组间两两比较。以P<0.05 为差异有统计学意义。
结果
1EPCs培养与鉴定
倒置显微镜下观察发现,培养3 d,EPCs未贴壁,细胞多为小圆形;培养4 d,细胞开始贴壁,并逐渐由小圆形细胞向梭形细胞转化;培养至7~10 d,梭形细胞不断增多,呈典型的鹅卵石铺路样改变。在荧光显微镜下,胞膜结合FITC-UEA-1显示为绿色,胞浆摄取DiI-acLDL显示为红色,双染色阳性细胞为橙色,为正在分化的EPCs,约占总细胞数的80%,见图1。
Figure 1.Characterization and identification by fluorescent staining of isolated EPCs. A: bone marrow-derived mononuclear cells cultured for 7~10 d, under inverted microscope; B: EPCs-uptaking DiI-acLDL was red; C: EPC-binding FITC-UEA-1 was green; D: double DiI-acLDL/FITC-UEA-1 positive cells were EPCs.
图1EPCs培养的形态学特征及荧光染色鉴定
2AECⅡ的培养与鉴定
倒置显微镜观察发现,培养24 h,AECⅡ贴壁,呈圆形、多角形,岛状分布。培养2 d,细胞岛状分布典型,胞浆内有大量反差明显的小颗粒,细胞核明显。碱性磷酸酶染色,蓝紫色阳性细胞达75%。透射电镜下观察到AEC Ⅱ特征性结构微绒毛及板层小体结构,见图2。
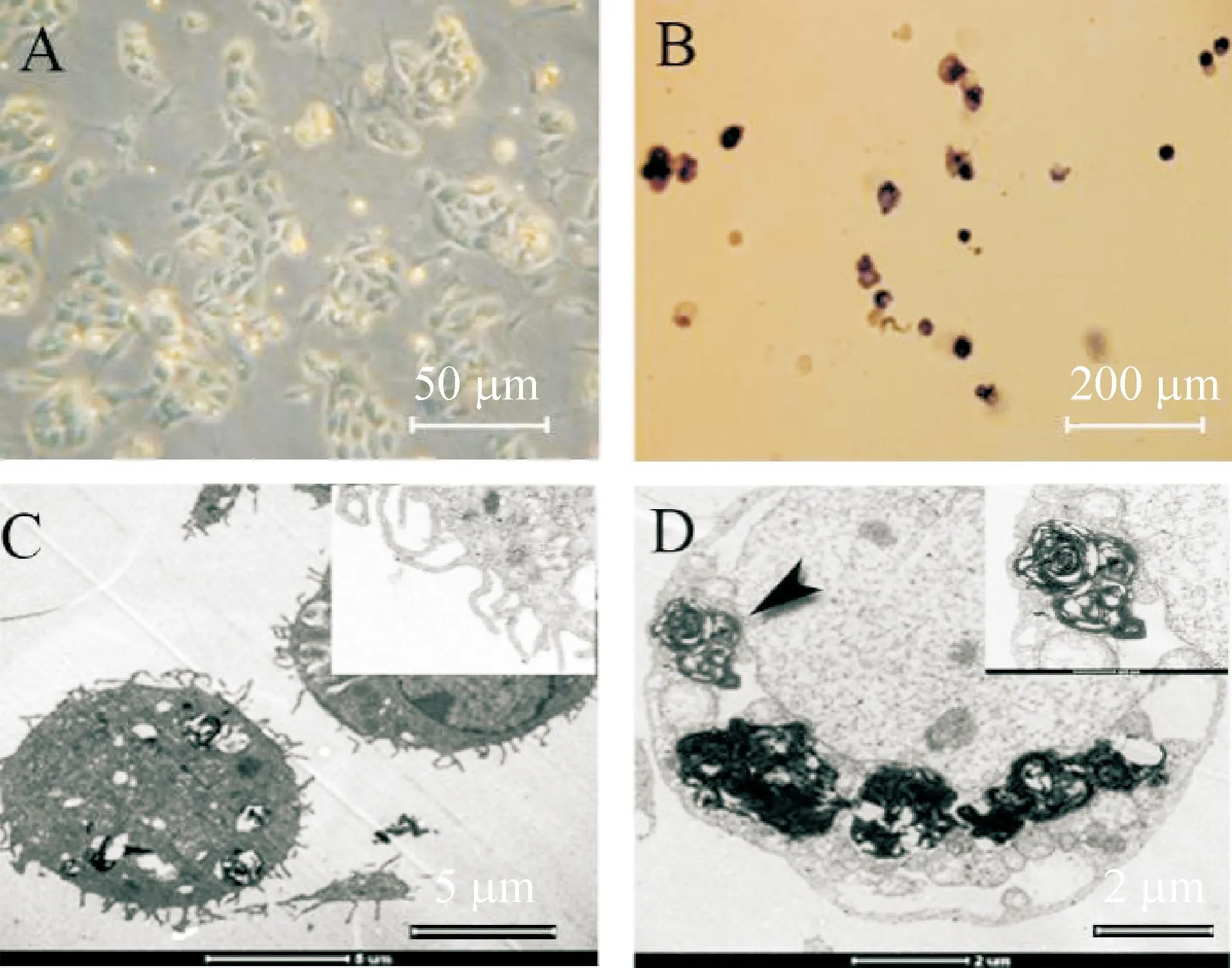
Figure 2.Characterization and identification of isolated AECⅡ. A: AECⅡ cultured for 2 d was observed under inver-ted microscope; B: AKP staining; C~D: microvilli and lamellar body were observed under transmission electron microscope.
图2AECⅡ培养的形态学特征及细胞鉴定
3高氧暴露对EPCs旁分泌功能的影响
ELISA法检测显示空气组EPCs培养上清中VEGF及FGF10的水平显著高于高氧组。ELISA法在两组EPCs培养上清中均未检测到PDGF-bb及EGF的表达,见表2。
4EPCs培养上清对高氧暴露下AECⅡ功能的影响
4.1EPCs培养上清对高氧暴露下AECⅡ细胞增殖的影响在培养12 h、24 h、2 d及3 d时,4组AECⅡ的细胞活力及细胞计数的差异均有统计学意义(P<0.01);与RA组相比, O2组AECⅡ的细胞活力显著降低,细胞数显著减少(P<0.05);与O2组相比,O2+E-CM-RA组AECⅡ的细胞活力增高,细胞数增多(P<0.05)。与O2+E-CM-O2组相比,在培养24 h、2 d及3 d时,O2+E-CM-RA组AECⅡ的细胞活力显著增高,细胞数增多(P<0.05)。O2+E-CM-O2组与O2组间AECⅡ的细胞活力和细胞数无显著差异,见图3及表3。
表2ELISA检测EPCs培养上清中VEGF、FGF10的表达水平
Table 2.The levels of VEGF and FGF10 in EPCs CM measured by ELISA (ng/L. Mean±SD.n=3)

TreatmentVEGFFGF10Air293.62±53.74161.01±17.98Hyperoxia168.51±15.05*87.07±13.95**
*P<0.05,**P<0.01vsair group.

Figure 3.Effects of EPC-conditioned medium on the viability of AECⅡdetected by MTT assay. Mean±SD.n=3.△△P<0.01vsRA group;*P<0.05vsO2group;#P<0.05,##P<0.01vsO2+E-CM-O2group.
图3MTT实验检测EPCs培养上清对AECⅡ细胞活力的影响
表3细胞计数检测EPCs培养上清对AECⅡ细胞增殖的影响
Table 3.Effects of EPC-conditioned medium on the proliferation of AECⅡ determined by cell counting (108cells/L. Mean±SD.n=3)
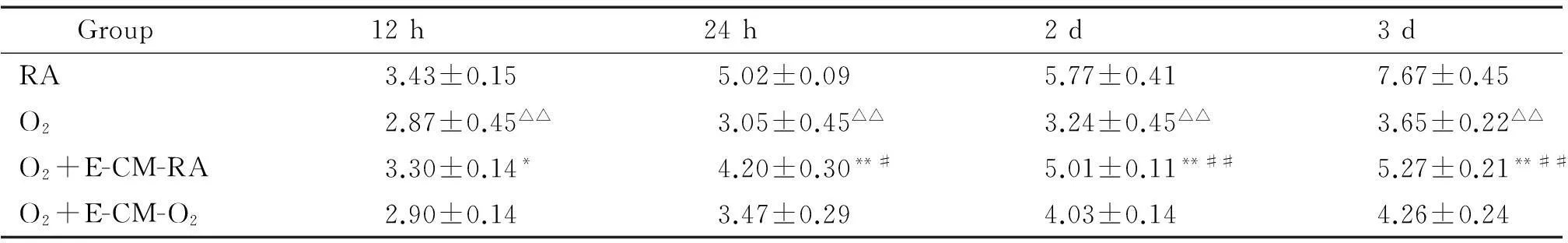
Group12h24h2d3dRA3.43±0.155.02±0.095.77±0.417.67±0.45O22.87±0.45△△3.05±0.45△△3.24±0.45△△3.65±0.22△△O2+E-CM-RA3.30±0.14*4.20±0.30**#5.01±0.11**##5.27±0.21**##O2+E-CM-O22.90±0.143.47±0.294.03±0.144.26±0.24
△△P<0.01vsRA group;*P<0.05,**P<0.01vsO2group;#P<0.05,##P<0.01vsO2+E-CM-O2group.
4.24组AECⅡ中SP-C及AQP5 的mRNA表达情况在培养24 h、2 d及3 d时,4组AECⅡ的SP-C mRNA表达量均有显著差异(P<0.01)。与RA组相比,O2组在培养24 h、2 d及3 d时,SP-C的mRNA表达显著降低(P<0.01)。与O2组相比,在培养24 h、2 d及3 d时,O2+E-CM-RA组和O2+E-CM-O2组AECⅡ的SP-C mRNA显著增高(P<0.05)。 与O2+E-CM-RA组相比,在培养24 h和2 d时,O2+E-CM-O2组AECⅡ的SP-C mRNA表达显著降低(P<0.05),见图4。

Figure 4.Effects of EPC-conditioned mdeium on the mRNA expression of SP-C in AECⅡ. Mean±SD.n=3.△△P<0.01vsRA group;*P<0.05,**P<0.01vsO2group;#P<0.05vsO2+E-CM-O2group.
图4EPCs培养上清对AECⅡ的SP-C mRNA表达的影响
在培养24 h、2 d及3 d时,4组AECⅡ中AQP5的mRNA表达量的差异均有统计学意义(P<0.01)。与RA组相比,在培养24 h、2 d及3 d时,O2组AQP5的mRNA显著增高(P<0.01)。与O2组相比,在培养2 d及3 d时,O2+E-CM-RA组和O2+E-CM-O2组AECⅡ的AQP5 mRNA显著降低(P<0.01)。O2+E-CM-RA组和O2+E-CM-O2组间AQP5的mRNA表达无显著差异,见图5。
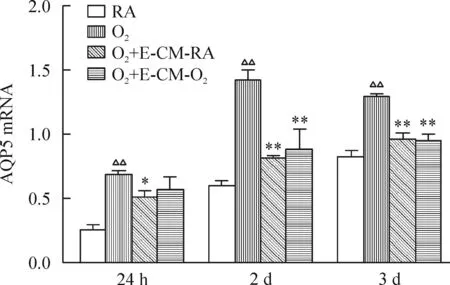
Figure 5.Effects of EPC-conditioned mdeium on the mRNA expression of AQP5 in AECⅡ. Mean±SD.n=3.△△P<0.01vsRA group;*P<0.05,**P<0.01vsO2group.
图5EPCs培养上清对AECⅡ的AQP5 mRNA表达的影响
讨论
早在2003年,Rehman等[7]就发现EPCs具有旁分泌功能,其旁分泌因子不仅能够促进内皮细胞增殖,促进血管形成;同时也可作用于组织细胞,加速组织的损伤修复[ 8, 11, 14]。然而,迄今为止,鲜有研究报道EPCs旁分泌功能在高氧肺损伤修复方面的作用。本研究拟在体外条件下,探讨高氧对EPCs旁分泌功能的影响以及EPCs旁分泌因子对高氧暴露下AECⅡ的影响。
本研究检测了EPCs培养上清中促肺发育和肺损伤修复的几种重要的生长因子VEGF、EGF、HGF10及PDGF-BB。我们发现大鼠骨髓来源的EPCs可分泌VEGF及FGF10,这与Pula等[15]的研究结果一致。VEGF是内皮细胞最有效的有丝分裂原,通过促血管生成作用从而促进肺组织的发育[16]。FGF10表达于肺发育的整个过程中,可促进肺泡上皮细胞的增殖及血管的发育[17]。有研究表明FGF10可改善高氧暴露对肺发育的阻滞[18]。本研究采用ELISA方法未检测到EGF及PDGF-BB的表达,而Pula等[15]采用ELISA法检测发现人外周血来源EPCs高表达PDGF-BB;Lee等[19]采用Milliplex液相芯片检测技术发现人脐带血EPCs可分泌EGF、PDGF-AA、GM-CSF、IL-6等因子。这些研究结果不同可能与EPCs来源不同以及检测技术精密性相关。本研究发现高氧暴露抑制了EPCs分泌VEGF和FGF10, Baker等[9]也发现高氧暴露可抑制脐带血来源EPCs的旁分泌功能,高氧暴露下,EPCs旁分泌因子对内皮细胞的促增殖作用明显下降。
以往的研究表明AEC Ⅱ在原代培养24~96 h处于最佳状态,此阶段适合做体外实验研究[20]。故在实验中,我们采用原代培养第2天的细胞作为研究对象,进行干预。我们发现在空气条件下,随着培养时间的延长,AECⅡ增殖趋缓,SP-C的mRNA表达下降,而AQP5的mRNA表达逐渐增高。AECⅡ在长时间高氧暴露下增殖能力下降[12, 21],本研究发现60%的高氧暴露下AECⅡ的增殖能力明显降低;EPCs培养上清能改善高氧损伤,促进AECⅡ增殖;而高氧暴露下的EPCs培养上清促AECⅡ增殖作用明显降低。这可能与EPCs培养上清中VEGF及FGF10等肺组织发育相关的生长因子浓度相关[22-23]。Baker等[9]也发现脐带血来源EPCs的旁分泌因子可促进高氧暴露下AECⅡ的增殖。
体外培养的AECⅡ具有向AECⅠ转分化的特性[24],高表达的SP-C是AECⅡ的特异性表型,高表达AQP5是AECⅠ的特异性表型[25-26],SP-C和AQP5的检测可以作为这2种细胞分化的标志。我们的研究发现高氧暴露使AECⅡ的 SP-C mRNA表达量下降,失去AECⅡ表型,而AQP5 mRNA表达量均大幅上升,逐渐向AECⅠ转分化。这与Lu等[27]的研究结果相似,Lu等发现90%的氧浓度条件可下调AECⅡ SP-C的mRNA表达量,上调AQP5的mRNA表达量,促进AECⅡ分化。我们的研究还发现EPCs培养上清可维持高氧暴露下AECⅡ SP-C的mRNA高表达,抑制AQP5的mRNA表达,抑制高氧暴露下AECⅡ向AECⅠ分化;而高氧暴露部分削弱了EPCs培养上清的这种作用。这可能是高氧暴露抑制了EPCs分泌VEGF及FGF10的能力,从而削弱了EPCs培养上清维持ACEⅡ本身特性的能力。
综上所述,我们的研究发现体外培养的大鼠骨髓来源的EPCs可分泌VEGF、FGF10等肺发育相关生长因子,而高氧暴露损害了EPCs的旁分泌功能,抑制VEGF和FGF10表达。高氧暴露可抑制AECⅡ的增殖,促进AECⅡ向AECⅠ分化。EPCs培养上清能够改善高氧暴露下AECⅡ增殖,维持AECⅡ本身特性,抑制AECⅡ向AECⅠ分化。而高氧暴露部分性削弱了EPCs培养上清的这种作用,这可能与高氧暴露损害EPCs的旁分泌功能,抑制肺发育相关因子VEGF和FGF10的表达相关。本研究存在以下不足之处,由于不同来源EPCs旁分泌功能存在差异加之EPCs旁分泌成分复杂,因此骨髓来源EPCs的旁分泌功能还有待蛋白芯片技术的进一步检测。
[参考文献]
[1]Merritt TA, Deming D, Boynton BR. The ′new′ bronchopulmonary dysplasia: challenges and commentary[J]. Semin Fetal Neonatal Med, 2009, 14(6):345-357.
[2]Thebaud B. Angiogenesis in lung development, injury and repair: implications for chronic lung disease of prematurity[J]. Neonatology, 2007, 91(4):291-297.
[3]崔斌,黄岚,武晓静,等. 内皮祖细胞移植对血管内膜修复的影响[J]. 中国病理生理杂志, 2007, 23(4):625-628.
[4]Baker CD, Ryan SL, Ingram DA, et al. Endothelial co-lony-forming cells from preterm infants are increased and more susceptible to hyperoxia[J]. Am J Respir Crit Care Med, 2009, 180(5):454-461.
[5]Borghesi A, Massa M, Campanelli R, et al. Circulating endothelial progenitor cells in preterm infants with bronchopulmonary dysplasia[J]. Am J Respir Crit Care Med, 2009, 180(6):540-546.
[6]Yang Z, Di Santo S, Kalka C. Current developments in the use of stem cell for therapeutic neovascularisation: is the future therapy "cell-free"?[J]. Swiss Med Wkly, 2010, 140:w13130.
[7]Rehman J, Li J, Orschell CM, et al. Peripheral blood "endothelial progenitor cells" are derived from monocyte/macrophages and secrete angiogenic growth factors[J]. Circulation, 2003, 107(8):1164-1169.
[8]Zhao YD, Ohkawara H, Rehman J, et al. Bone marrow progenitor cells induce endothelial adherens junction integrity by sphingosine-1-phosphate-mediated Rac1 and Cdc42 signaling[J]. Circ Res, 2009, 105(7):696-704.
[9]Baker CD, Seedorf GJ, Wisniewski BL, et al. Endothelial colony-forming cell conditioned media promote angiogenesisinvitroand prevent pulmonary hypertension in experimental bronchopulmonary dysplasia[J]. Am J Physiol Lung Cell Mol Physiol, 2013, 305(1):L73-L81.
[10]Kim JY, Song SH, Kim KL, et al. Human cord blood-derived endothelial progenitor cells and their conditioned media exhibit therapeutic equivalence for diabetic wound healing[J]. Cell Transplant, 2010, 19(12):1635-1644.
[11]Ding BS, Nolan DJ, Guo P, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization[J]. Cell, 2011, 147(3):539-553.
[12]Kallet RH, Matthay MA. Hyperoxic acute lung injury[J]. Respir Care, 2013, 58(1):123-141.
[13]Urbich C, De Souza AI, Rossig L, et al. Proteomic cha-racterization of human early pro-angiogenic cells[J]. J Mol Cell Cardiol, 2011, 50(2):333-336.
[14]Urbich C, Aicher A, Heeschen C, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells[J]. J Mol Cell Cardiol, 2005, 39(5):733-742.
[15]Pula G, Mayr U, Evans C, et al. Proteomics identifies thymidine phosphorylase as a key regulator of the angiogenic potential of colony-forming units and endothelial progenitor cell cultures[J]. Circ Res, 2009, 104(1):32-40.
[16]Jakkula M, Le Cras TD, Gebb S, et al. Inhibition of angiogenesis decreases alveolarization in the developing rat lung[J]. Am J Physiol Lung Cell Mol Physiol, 2000, 279(3):L600-L607.
[17]Ramasamy SK, Mailleux AA, Gupte VV, et al. Fgf10 dosage is critical for the amplification of epithelial cell progenitors and for the formation of multiple mesenchymal lineages during lung development[J]. Dev Biol, 2007, 307(2):237-247.
[18]Jankov RP, Keith TA. Growth factors, postnatal lung growth and bronchopulmonary dysplasia[J]. Paediatr Respir Rev, 2004, 5(Suppl A):S265-S275.
[19]Lee MJ, Kim J, Lee KI, et al. Enhancement of wound healing by secretory factors of endothelial precursor cells derived from human embryonic stem cells[J]. Cytotherapy, 2011, 13(2):165-178.
[20]樊燕蓉,姚汝琳. 肺泡Ⅱ型上皮细胞的分离培养及其在矽肺研究中的应用[J]. 国外医学:卫生学分册, 1996,23(4):212-214,218.
[21]周海,潘晓军,杨建平,等. 一氧化氮及一氧化氮合酶在急性吸入高浓度氧大鼠肺泡上皮细胞凋亡中的作用[J]. 中国病理生理杂志, 2012,28(1):109-113.
[22]Raoul W, Chailley-Heu B, Barlier-Mur AM, et al. Effects of vascular endothelial growth factor on isolated fetal alveolar type Ⅱ cells[J]. Am J Physiol Lung Cell Mol Physiol, 2004, 286(6):L1293-L1301.
[23]Mondrinos MJ, Jones PL, Finck CM, et al. Engineering de novo assembly of fetal pulmonary organoids[J]. Tissue Eng Part A, 2014, 20(21-22):2892-2907.
[24]Dobbs LG, Williams MC, Brandt AE. Changes in biochemical characteristics and pattern of lectin binding of alveolar type Ⅱ cells with time in culture[J]. Biochim Biophys Acta, 1985, 846(1):155-166.
[25]Nielsen S, King LS, Christensen BM, et al. Aquaporins in complex tissues. Ⅱ. Subcellular distribution in respi-ratory and glandular tissues of rat[J]. Am J Physiol, 1997, 273(5 Pt 1):C1549-C1561.
[26]Mason RJ, Kalina M, Nielsen LD, et al. Surfactant protein C expression in urethane-induced murine pulmonary tumors[J]. Am J Pathol, 2000, 156(1):175-182.
[27]Lu HY, Shao GB, Li WB, et al. Effects of hyperoxia on transdifferentiation of primary cultured typeⅡ alveolar epithelial cells from premature rats[J]. In Vitro Cell Dev Biol Anim, 2011, 47(1):64-72.
(责任编辑: 卢萍, 罗森)
Effects of endothelial progenitor cell-conditioned medium on proliferation and differentiation of type Ⅱ alveolar epithelial cells exposed to hyperoxia
WANG Chuan-kai, LU Ai-zhen, QI Yuan-yuan, QIAN Li-ling
(Children’sHospitalofFudanUniversity,Shanghai201102,China.E-mail:llqian@126.com)
[ABSTRACT]AIM: To investigate the effect of hyperoxia exposure on the paracrine function of endothelial progenitor cells (EPCs), and to explore the effects of paracrine factors of EPCs on the proliferation and differentiation of type Ⅱ alveolar epithelial cells (AECⅡ) exposed to hyperoxia. METHODS: Bone marrow-derived mononuclear cells were isolated and cultured in EGM-2MV medium for 7~10 d to obtain and identify EPCs. EPCs were cultured in room air (RA) or 60% O2. The normoxia EPC-conditioned medium (E-CM-RA) and hyperoxia EPC-conditioned medium (E-CM-O2) were collected. The levels of VEGF, FGF10, PDGF-BB and EGF in E-CM-RA and E-CM-O2 were detected by ELISA. AECⅡ from adult rats were isolated, purified and cultured for 2 d, then divided into RA group, O2 group, O2+E-CM-RA group and O2+E-CM-O2 group. The proliferation of AECⅡ was detected by MTT assay and cell counting. The mRNA expression of SP-C and AQP5 was quantified by real-time PCR. RESULTS: The expression of VEGF and FGF10 in E-CM-O2 group decreased significantly compared with E-CM-RA group (P<0.01). There were significant differences in AECⅡ viability and number among the 4 groups at 12 h, 24 h, 2 d and 3 d (P<0.01). Compared with RA group, AECⅡ viability and number in O2 group decreased significantly at 12 h, 24 h, 2 d and 3 d (P<0.05). The AECⅡ viability and number in O2+E-CM-RA group were significantly higher than those in O2 group at 12 h, 24 h, 2 d and 3 d (P<0.05). However, no significant difference in AECⅡ viability and number between O2+E-CM-O2 group and O2 group at 12 h, 2 d and 3 d was observed. There were significant differences in the mRNA expression of SP-C and AQP5 in the 4 groups at 24 h, 2 d and 3 d (P<0.01). Compared with RA group, the mRNA expression of SP-C in O2 group was significantly inhibited (P<0.01), but the mRNA expression of AQP5 was promoted (P<0.01) at 24 h, 2 d and 3 d. Compared with O2 group, the mRNA level of SP-C in O2+E-CM-RA group and O2+E-CM-O2 group (P<0.05) at 24 h, 2 d and 3 d was increased, and the mRNA expression of AQP5 (P<0.01) at 2 d and 3 d was inhibited.CONCLUSION: EPCs secrete VEGF and FGF10, and hyperoxia impairs this paracrine function. Hyperoxia exposure inhibits AECⅡ proliferation and the mRNA expression of SP-C, but promotes the mRNA expression of AQP5. EPC-conditioned medium improves the proliferation of hyperoxia-exposed AECⅡ, and inhibits the transformation of AECⅡ. Hyperoxia exposure impairs the paracrine function of EPCs, and weakened the effects of E-CM-O2 on AECⅡ.
[KEY WORDS]Endothelial progenitor cells; Paracrine; Type Ⅱ alveolar epithelial cells; Hyperoxia injury
[文章编号]1000- 4718(2016)01- 0008- 07
[收稿日期]2015- 08- 18[修回日期] 2015- 12- 04
*[基金项目]国家自然科学基金资助项目(No. 81270727)
通讯作者△Tel: 021-64931916; E-mail: llqian@126.com
[中图分类号]R363
[文献标志码]A
doi:10.3969/j.issn.1000- 4718.2016.01.002
