不同剂量双氯芬酸钠预防内镜逆行胰胆管造影术后胰腺炎的疗效观察
2016-06-15陈泽宇曹文瑜
陈泽宇, 曹文瑜, 林 晖
福建医科大学 附属福州市第一医院内镜室,福州 350009
不同剂量双氯芬酸钠预防内镜逆行胰胆管造影术后胰腺炎的疗效观察
陈泽宇, 曹文瑜, 林晖
福建医科大学 附属福州市第一医院内镜室,福州350009
摘要:目的探讨不同剂量双氯芬酸钠对内镜逆行胰胆管造影术(ERCP)术后胰腺炎(PEP)及高淀粉酶血症的预防效果。方法收集行ERCP取石术的患者244例,随机分为4组,每组61例,其中低剂量组、中剂量组、高剂量组于术前30~60 min分别予双氯芬酸钠栓剂50,100,150 mg塞肛,空白组术前未予处理,观察其术前及术后3,24 h血清淀粉酶水平,评估ERCP术后PEP及高淀粉酶血症发生率及严重程度。结果4组在年龄、性别、ERCP手术方式及操作时间等方面差别均无统计学意义。PEP发生率低剂量组为16.4%(10/61),中剂量组为6.6%(4/61),高剂量组为4.9%(3/61),空白组为18%(11/61),4组术后PEP总体发病率差别有统计学意义(χ2=8.07,P=0.045),其中,空白组与低剂量组、中剂量组与高剂量组比较差别均无统计学意义(χ2=0.058,P=0.810;χ2=0.152,P=0.697),而低剂量组与高剂量组比较差别有统计学意义(χ2=4.219,P=0.040)。术后高淀粉酶血症总体发病率差别无统计学意义(χ2=2.83,P=0.419)。空白组发生重度PEP 1例,各实验组均未发生;空白组发生中度PEP 3例,低剂量组发生2例;中、高剂量组发生的PEP均为轻度。结论50 mg双氯芬酸钠栓剂未能有效预防PEP的发生,100及150 mg双氯芬酸钠栓剂均能有效降低PEP的发生率,且效果相当,故推荐使用100 mg双氯芬酸钠栓剂预防PEP发生。
关键词:胰胆管造影术,内窥镜逆行; 胰腺炎; 高淀粉酶血症; 双氯酚酸
目前内镜逆行胰胆管造影术(endoscopic retrograde cholangiopancreatography, ERCP)已成为诊断和治疗胰胆系统疾病的重要手段,但其系创伤性手术,术中及术后并发症不可避免,尤其ERCP术后胰腺炎(post-ERCP pancreatitis, PEP)和高淀粉酶血症最为常见,国内外报道,PEP总发生率约1.3%~24.4%[1-2]。磷脂酶A2(phospholipase A2,PLA2)在急性胰腺炎早期炎症的瀑布效应中起重要作用,而非甾体类抗炎药(non-steroidal antiinflammatory drug,NSAID)作为有效的PLA2抑制剂,阻断急性胰腺炎早期的炎症反应,可降低PEP的发生率[3-5]。研究表明,直肠应用NSAID可以作为适宜的预防PEP的药物,尤其是对于高风险患者,欧洲胃肠内窥镜学会建议,对于ERCP术前或者术后的患者,直肠应用吲哚美辛或者双氯芬酸预防PEP,推荐等级A级[6]。大多数学者采用吲哚美辛或双氯芬酸钠100 mg一次性给药,Otsuka等在一项随机对照研究中认为,低剂量(25或50 mg)双氯芬酸钠经直肠给药同样有效,但未与100 mg给药组进行对比研究[7]。目前临床上用药剂量尚无明确的统一标准,故本研究通过比较不同剂量双氯芬酸钠对PEP及高淀粉酶血症的预防效果,探讨适宜剂量,为临床应用提供依据。
1对象与方法
1.1对象选取2013年1月-2015年6月行ERCP诊疗的患者244例,男性112例,女性132例,年龄(60.32±15.68)岁(18~90岁)。纳入标准:(1)年龄>18岁;(2)术前血清淀粉酶正常;(3)术前影像学提示胰腺组织正常;(4)胆管结石。排除标准:(1)非胆管结石疾病;(2)近期使用NSAID药物;(3)术前血清淀粉酶异常或急慢性胰腺炎;(4)心肺功能不全;(5)<18岁患者、孕妇、泛影葡胺过敏者;(6)凝血机制异常;(7)近期出现消化道出血;(8)贫血(血红蛋白<9.0 g/L);(9)对双氯芬酸钠或其他NSAID药物过敏;(10)严重胆管感染。本研究已报笔者医院伦理委员会审批通过,入选患者术前均签署知情同意书。
1.2方法按随机数字表将患者随机分为4组,每组61例,各组间性别、年龄、手术方式及操作时间差别均无统计学意义(P>0.05),基线资料具有可比性(表1)。低剂量组、中剂量组、高剂量组于ERCP术前30~60 min分别予双氯芬酸钠栓剂50,100,150 mg塞肛,空白组术前未予处理。患者均在全麻下完成手术。根据术中情况,选用乳头括约肌切开术或乳头括约肌气囊扩张取石、碎石后取石或气囊取石、放置塑料胆管支架等相关的治疗性操作方法。4组患者术后均常规禁食24 h,若发现PEP,可适当延长禁食时间,并常规应用抑酸、抗炎、补液等治疗。分别于术前及术后3,24 h监测血清淀粉酶以及患者腹痛、发热、恶心、呕吐等情况,观察4组PEP发生例数和严重程度以及其他不良反应情况。
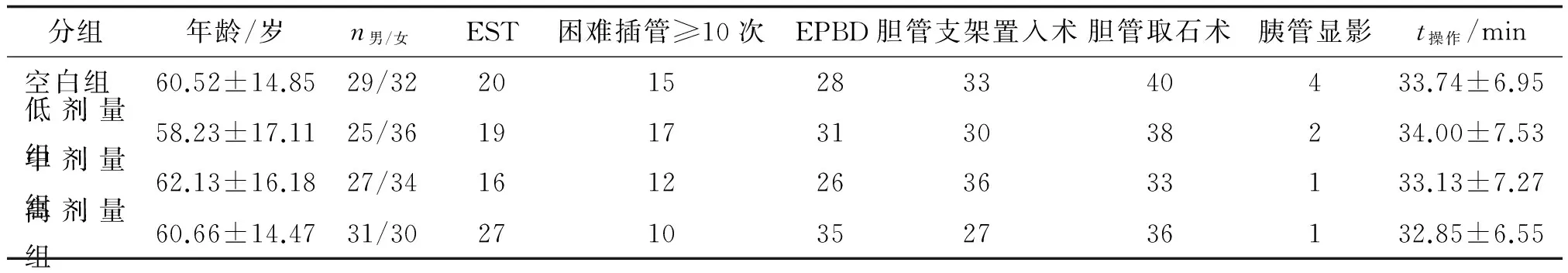
表1 各组间一般资料及手术方式
EPBD:内镜下十二指肠乳头球囊扩张术;EST:内镜下乳头括约肌切开取石术.表中数据除年龄、操作时间,余为n.
1.3高淀粉酶血症和PEP诊断标准根据1991年Cotton等专家组制定的标准[8]:高淀粉酶血症可定义为ERCP术后血淀粉酶高于正常上限值(笔者医院为96 U/L)。ERCP术后血清淀粉酶高于正常上限的3倍,且出现腹痛持续时间超过24 h,可定义为PEP。PEP严重程度分级:轻度,需入院治疗或延长原计划入院时间至2~3 d;中度,需入院治疗4~10 d;重度,需入院治疗10 d以上,出现局部或全身并发症,需住ICU治疗或行侵入性治疗(如经皮引流或外科手术)。
2结果
2.1各组术后血清淀粉酶比较术后3h血清淀粉酶F=3.52,P=0.016,可认为4组术后3h血清淀粉酶的均数不全相同,即至少有2个不同。术后24h血清淀粉酶F=4.50,P=0.004,可认为4组术后24h血清淀粉酶的均数不全相同,即至少有2个不同(表2)。各组间两两比较,结果提示,术后3h,血清淀粉酶低、中剂量组及空白组均高于高剂量组,差别均有统计学意义(P<0.05);低、中剂量组与空白组间相互比较差别均无统计学意义(P>0.05)。术后24h,血清淀粉酶空白组与低剂量组均高于中、高剂量组,差别均有统计学意义(P<0.05);空白组与低剂量组比较差别无统计学意义(P>0.05,表3)。
表2各组间术后3,24h血清淀粉酶比较
Tab2Comparisonofserumamylase3hor24hafterERCPineachgroupU/L
与术后3 h比较,P<0.05.
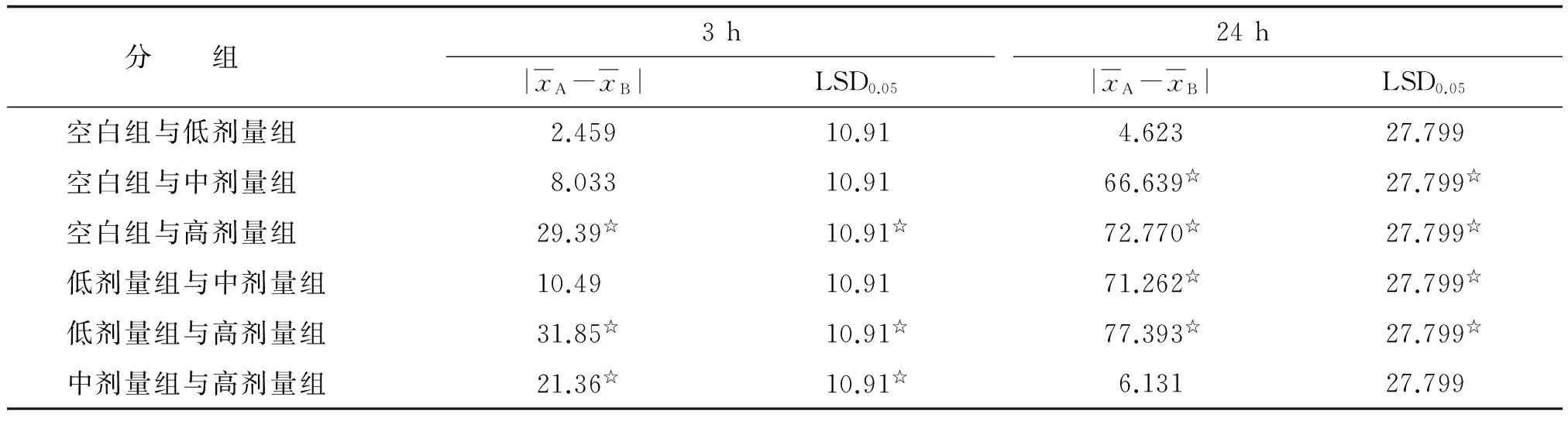
表3 各组间术后3,24 h血清淀粉酶的两两比较
组间比较,☆:P<0.05.
2.2PEP及术后高淀粉酶血症发生情况各组术后PEP发生率见表4。按α=0.05检验水准,认为4组术后PEP总体发病率有差别(χ2=8.07,P=0.045),其中,空白组与低剂量组比较、中剂量组与高剂量组比较差别均无统计学意义(χ2=0.058,P=0.810;χ2=0.152,P=0.697),低剂量组与高剂量组比较差别有统计学意义(χ2=4.219,P=0.040)。各组术后高淀粉酶血症发生率见表4,各组总体发病率差别无统计学意义(χ2=2.83,P=0.419)。
表4各组间术后胰腺炎及高淀粉酶血症的发生情况
Tab 4Comparison of incidence of PEP and hyperamylasemia in each group
表中数据为n(%).☆:P<0.05.
2.3PEP分级及不良反应空白组发生重度PEP 1例、中度PEP 3例;3个实验组均未发生重度PEP,其中低剂量组发生中度PEP 2例,中、高剂量组发生的PEP均为轻度。使用双氯芬酸钠栓剂过程未发生消化道出血、恶心、呕吐、胃不适、烧灼感、返酸等不适。
3讨论
PEP是ERCP术后最为常见的并发症之一,其影响因素与括约肌功能障碍、既往有PEP病史、女性患者、乳头括约肌预切开、胰管注射造影剂、多次插管困难及操作者的技术娴熟程度等有关[9]。目前预防及减少PEP发生的措施包括药物干预和内镜干预,其中药物干预包括:(1)减少炎症反应的药物如抗氧化剂、抗生素、类固醇激素及非甾体类抗炎药的使用;(2)降低Oddi’s括约肌压力的药物如硝酸甘油等;(3)抑制胰酶活性的药物如加贝酯、乌司他丁;(4)抑制胰酶分泌的药物如生长抑素及其衍生物奥曲肽。目前只有直肠给予双氯芬酸或吲哚美辛预防PEP疗效确切,其余药物的疗效尚存争议[6]。
近年来,关于NSAID预防PEP的临床研究显示出良好的应用前景。NSAID预防PEP的机制尚不完全清楚,可能是通过抑制前列腺素的合成和阻断胰腺炎的炎症级联反应来预防PEP的发生[10]。Makela等研究发现,NSAID可有效抑制重症胰腺炎患者血清磷脂酶A2的活性及中性粒细胞/内皮细胞的粘附,减轻急性胰腺炎的炎症反应[11]。Simon等研究表明,NSAID可通过抑制中性粒细胞活化,减轻PEP的炎症程度[12]。Murray等研究发现,220例ERCP患者术后立即经直肠给予100 mg双氯芬酸钠,与对照组相比,PEP发生率显著降低(7/110vs17/110,P=0.049)[13]。
NSAID抑制前列腺素的效果与剂量呈正相关性,双倍剂量的双氯芬酸钠能够使抑制前列腺素的效果增加60%~65%[14]。大部分随机对照试验采用100 mg双氯芬酸钠栓剂预防PEP,但也有报道,低剂量(25 mg或50 mg)双氯芬酸钠经直肠给药同样有效[3-5,7]。该研究随机纳入104例行ERCP的患者,低剂量组术前30 min给予50 mg双氯芬酸钠栓剂,体质量不足50 kg者剂量减至25 mg,空白组未予处理,结果显示PEP发生率低剂量组显著低于空白组(2/51vs10/53,P=0.017)。本研究采用50,100,150 mg的双氯芬酸钠观察PEP的预防效果,结果提示,50 mg双氯芬酸钠栓剂未能有效预防PEP的发生,100 mg及150 mg双氯芬酸钠栓剂均能有效降低PEP发生率,高剂量组PEP发生率低于中剂量组(4.9%vs6.6%),但两者之间比较差别无统计学意义(P>0.05)。提示增加剂量未能降低PEP的发生率,其原因可能与以下因素有关:(1)虽增加双氯芬酸钠的剂量能够加强对前列腺素的抑制作用,但前列腺素仅是PEP的发病因素之一,未能完全影响PEP预后效果;(2)血药浓度与药效为非线性相关,血药浓度上升,药效上升,达到最大值后,血药浓度再上升,药效持平甚至回落,故可考虑双氯芬酸钠栓剂预防PEP最大药效剂量为100 mg。
目前双氯芬酸钠预防PEP采用直肠给药有效,而通过口服、肌内注射或静滴效果不佳或无效[15-16]。其原因可能与药物首关消除有关,双氯芬酸钠通过口服、肌内注射或静滴,大约50%在肝脏代谢,减少进入体循环量,而直肠给药可避免首关消除,且吸收迅速。双氯芬酸和吲哚美辛经直肠给药达药物峰浓度需30~90 min,其生物利用度100%,半衰期2 h。经口途径,达药物峰浓度需2 h,生物利用度50%~60%,半衰期1.2~2 h[17]。因此ERCP术前即刻直肠给药起效可能更快,效果可能更好,且经直肠给药可避免关于NSAID可能引发上消化道出血不良反应的担忧,较口服更加安全。本研究在使用双氯芬酸钠栓剂过程中未发现任何不良反应。
总之,本研究结果表明,50 mg双氯芬酸钠栓剂未能有效预防PEP的发生,100及150 mg双氯芬酸钠栓剂均能有效降低PEP的发生率,且效果相当,故推荐使用100 mg双氯芬酸钠栓剂预防PEP发生。当然,鉴于本研究为单中心小样本研究,亟待多中心、大样本的研究证实。
参考文献:
[1]Andriulli A, Loperfido S, Napolitano G,etal. Incidence rates of post-ERCP complications: a systematic survey of prospective studies[J].AmJGastroenterol, 2007,102(8):1781-1788.
[2]Freeman M L, Guda N M. Prevention of post-ERCP pancreatitis: a comprehensive review[J].GastrointestEndosc, 2004,59(7):845-864.
[3]Yuhara H, Ogawa M, Kawaguchi Y,etal. Pharmacologic prophylaxis of post-endoscopic retrograde cholangiopancreatography pancreatitis: protease inhibitors and NSAIDs in a meta-analysis[J].JGastroenterol, 2014,49(3):388-399.
[4]Ding X, Chen M, Huang S,etal. Nonsteroidal anti-inflammatory drugs for prevention of post-ERCP pancreatitis: a meta-analysis[J].GastrointestEndosc, 2012,76(6):1152-1159.
[5]Yuhara H, Ogawa M, Kawaguchi Y,etal. Pharmacologic prophylaxis of post-endoscopic retrograde cholangiopancreatography pancreatitis: protease inhibitors and NSAIDs in a meta-analysis[J].JGastroenterol, 2014,49(3):388-399.
[6]Dumonceau J M, Andriulli A, Elmunzer B J,etal. Prophylaxis of post-ERCP pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) guideline updated June 2014[J].Endosc, 2014,46(9):799-815.
[7]Otsuka T, Kawazoe S, Nakashita S,etal. Low-dose rectal diclofenac for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: a randomized controlled trial[J].JGastroenterol, 2012,47:912-917.
[8]Cotton P B, Lehman G, Vennes J,etal. Endoscopic sphincterotomy complications and their management: an attempt at consensus[J].GastrointestEndosc, 1991,37(3):383-393.
[9]Cotton P B, Garrow D A, Joseph G,etal. Risk factors for complications after ERCP: a multivariate analysis of 11,497 procedures over 12 years[J].GastrointestEndosc, 2009,70(1):80-88.
[10]Santos-Antunes J, Pereira P, Vilas-Boas F,etal. Efficacy of post-ERCP pancreatitis prophylaxis[J].Pancreatology, 2015,15(3):S124-S124.
[11]Makela A, Kuusi T, Schroder T. Inhibition of serum phospholipase-A2in acute pancreatitis by pharmacological agentsinvitro[J].ScandJClin, 1997,57(5):401-407.
[12]Simon L S. Actions and toxicity of nonsteroidal anti-inflammatory drugs[J].CurrOpinRheumatol, 1995,7(3):169-175.
[13]Murray B, Carter R, Imrie C,etal. Diclofenac reduces the incidence of acute pancreatitis after endoscopic retrograde cholangiopancreatography.[J].Gastroenterol, 2003, 124(7):1786-1791.
[14]George G, Markantonis S L. Relationships between the concentrations of prostaglandins and the nonsteroidal antiinflammatory drugs indomethacin, diclofenac, and ibuprofen[J].PharmacotherapytheJournalofHumanPharmacology&DrugTherapy, 2005,25(1):18-25.
[15]Se Woo P, Moon Jae C, Tak Geun O,etal. Intramuscular diclofenac for the prevention of post-ERCP pancreatitis: a randomized trial[J].Endoscopy, 2015,47(1):33-39.
[16]Cheon Y K, Cho K B, Watkins J L,etal. Efficacy of diclofenac in the prevention of post-ERCP pancreatitis in predominantly high-risk patients: a randomized double-blind prospective trial[J].GastrointestEndosc, 2007,66(6):1126-1132.
[17]Dai H F. Role of nonsteroidal anti-inflammatory drugs in the prevention of post-ERCP pancreatitis: a meta-analysis[J].Hepatobiliary&PancreaticDiseasesInternational, 2009,8(1):11-16.
(编辑:何佳凤)
Efficacy of Different Doses of Diclofenac for Prevention of Post-endoscopic Retrograde Cholangiopancreatography Pancreatitis
CHEN Zeyu, CAO Wenyu, LIN Hui
Department of Endoscopy, Fuzhou First Hospital Affiliated to Fujian Medical University, Fuzhou 350009, China
ABSTRACT:ObjectiveTo investigate the preventive effect of diclofenac at different doses on post-ERCP pancreatitis and hyperamylasemia.MethodsA total of 244 patients with choledocholithiasis from January 2013 to June 2015 were enrolled to receive ERCP and randomly divided into four groups to receive low dose diclofenac group (50 mg, n=61), moderate dose diclofenac group (100 mg, n=61), high dose diclofenac group (150 mg, n=61) or no diclofenac, at half an hour to one hour, before ERCP.The level of serum amylase before and 3 h or 24 h after ERCP were measured, Incidence of post-ERCP pancreatitis and hyperamylasemia were assessed in four groups.ResultsThere were no significant differences among four groups in age, gender, surgery method and time of ERCP.The incidences of post-ERCP pancreatitis were 16.4%(10/61), 6.6%(4/61) and 4.9%(3/61), and 18%(11/61)in diclofenac dose groups and in blank group respectively.The total incidences of post-ERCP pancreatitis in four groups were significantly different (χ2=8.07, P=0.045).There was no significant difference either between low dose group and blank group (χ2=0.058, P=0.810), or between moderate dose group and high dose group (χ2=0.152, P=0.697).There was significant difference between low dose group and high dose group (χ2=4.219, P=0.040).The incidences of hyperamylasemia had no significant difference among four groups (χ2=2.83, P=0.419).There was one case of severe PEP in blank group, while none in experimental groups.Three moderat PEP was observed in blank group and two in low dose group.The PEP that was observed in moderate and high dose groups were mild.ConclusionThe prevention of PEP may not be achieved with diclofenac suppository at 50 mg, however at 100 mg or 150 mg, diclofenac suppository can have preventive effect on post-ERCP pancreatitis and the effect is similar for the two doses.Therefore, we recommend that 100 mg diclofenac suppository should be used for preventing PEP.
KEY WORDS:cholangiopancreatography, endoscopic retrograde; pancreatitis; hyperamylasemia; diclofenac
收稿日期:2015-11-05
作者简介:陈泽宇(1977-),男,主治医师. Email: 37430578@qq.com
中图分类号:R576; R619.2; R971.1
文献标志码:A
文章编号:1672-4194(2016)01-0043-05
