The effi cacy of TKIs in treatment of human primary small cell lung cancer xenograft model in vivo
2016-06-05Yu-huaZHANG,LiangSUN,BinLIU等
The effi cacy of TKIs in treatment of human primary small cell lung cancer xenograft model in vivo
Objective:To study the treatmaient of non-small cell lung cancer, we established the HU-Prim allograft transplantati on tumor model.Methods:The fresh tumor samples were transplanted in the right scapular subcutaneous layer of the severe combined immunodefi cient Non-obese diabeti c/severe combined immunodefi cient (NOD/SCID) mice. The pathological features of the tumors were observed. Nonnecroti c ti ssue was inoculated subcutaneously into the right axillary. When the tumor in burdened rat grew approximately 100 mm3, according to the tumor size all the animals were divided into the following four groups, eight rats in each group: solvent control group, gefitinib group (100 mg/kg), erlotinib group (50 mg/kg), afati nib group (20 mg/kg). Aniamals were treated with drugs by intragastric (i.g.) administrated, once daily, for consecuti vely 14 days. Measure the tumor size 2-3 ti mes every week.Results:HuPrime1-NSCLC mutant sensiti ve xenograft model research data showed that reversible tyrosine kinase inhibitors gefi ti nib, erloti nib and irreversible tyrosine kinase inhibitor afati nib could eff ecti vely inhibit tumor growth in EGFR positi ve NSCLC allograft s model. The pharmacodynamic acti vity of irreversible inhibitor was bett er than that of the reversible inhibitor. Specimens from clinical anthropogenic tumor retain characteristi cs of the human primary malignancy, histopathology, biological characteristi cs, and tumor markers, etc., which can more accurately refl ect the characteristi cs of the tumor and the impact of interventi ons.Conclusion:The model is not only a good anti tumor drug experimental platf orm, but also a new evaluati on tool of individualized medicati on.
non-small-cell lung cancer (NSCLC); Non-obese diabetic/severe combined immunodeficient (NOD/SCID) mice; effi cacy; tyrosine kinase inhibitors (TKIs); disease model
Introduction
Currently, lung cancer has gradually become the highest incidence and mortality of malignant tumors in the world [1]. According to the structure characteristics, lung cancer is divided into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). NSCLC accounts for more than 80% of lung cancer deaths [2]. Surgical resection, radiotherapy, chemotherapy and the above two or three combined are the traditional therapy for lung cancer. Due to the limitations of surgical resection and the clinical benefit of radiotherapy
and chemotherapy maximized, recently molecular targeted drugs tyrosine kinase inhibitors (TKIs) has become a hot topic. Preclinical evaluation of targeted drugs largely depends on the animal tumor models [3, 4]. To establish a reliable animal model is extremely important for screening and preclinical trials. Animal models of lung cancer play an important role in etiology, diagnosis, treatment and so on. We used the fresh tissue complete tumor samples to establish the HU-Prim allograft transplantation tumor model. The model is not only a good antitumor drug experimental platform, but also a new evaluation tool of individualized medication.
Materials and Methods
Patient samples
Th irty-four NSCLC patient specimens were obtained from the Tumor Surgery Department of the Affi liated Hospital of the Armed Police Logistics College. 12 patients confirmed as positive by EGFR test,including 4 patients relapsed aft er gefi tinib treatment. Eight cases f EGFR test results was L858R sensitive mutant (HuPrime1-NSCLC), 4 recurrent cases of EGFR test results was T790M resistant mutant (HuPrime2-NSCLC). Written informed consent was signed with each patient. Th e study was approved by the hospital ethics committee. Specimens were from initial lung cancer resection, instant access to sterile surgery. Eight patient specimens with EGFR positive and 4 recurrent tumor patient specimens were established xenograft tumor model.
Method of animal model
The fresh tumor samples were transplanted in the right scapular subcutaneous layer of the severe combined immunodefi cient NOD/SCIDNOD/SCID mice under sterile conditions 24 hours before 200 cGY cesium source irradiation. When the xenograft tumors grow to 200-500 mm3, the xenograft tumors were passaged in sterile conditions. By detecting pathological tumor xenograft observe whether to retain the pathological features of clinical specimens. If there is no difference between the pathological tumor xenograft and the primary pathology clinical specimens, the allograft transplantation tumor was used as a follow-up experiments source for subsequent pharmacodynamic evaluation.
Grouping and administration
When the tumor in burdened rat grew about to 500 mm3, it was removed under sterile and cut into 2 mm × 2 mm × 2 mm fragment. Nonnecrotic tissue was inoculated subcutaneously into the right axillary. When the tumor in burdened rat grew approximately 100 mm3, according to the tumor size all the animals were divided into the following four groups: solvent control group, gefi tinib group (100 mg/kg), erlotinib group (50 mg/kg), and afatinib group (20 mg/kg), Eight rats in each group. The animals were treated with drugs by intragastric (i.g.) administrated, once daily, for consecutively 14 days. Th e tumor size was measured 2-3 times every week during the treatment period. When the mean tumor volume of the solvent control group reached about 500 mm3, dislocated neck to put the animal death, according to the tumor volume evaluated the effi cacy of drug.
Index detection and calculation
The major endpoint was to evaluate if the tumor growth can be delayed or mice can be cured. Tumor size was measured twice weekly in two dimensions using a caliper, and the volume was expressed in mm3using the formula: V = 0.5a×b where a and b are the long and short diameters of the tumor, respectively. Th e tumor size was then used for calculations of T/C (tumor volume of the treated groups/tumor volume of the control groups) values. The T/C value (in percent) was an indication of antitumor eff ectiveness.Statistical analysis
To evaluate the statistical signifi cance in anti-tumor effi cacy study, the experimental data were expressed in terms of mean ± standard deviation (x±s) and analyzed by soft ware SPSS17.0. Depending on result of Levene test on variance homogeneity, one-way ANOVA analysis or Nonparametric Text was used. LSD method was applied to analyze either two sets of data. It’s considered signifi cant if P < 0.05.
Results
Figure1-A and table1 data show that the tumor of the solvent control group grew well; indicating that xenograft model of HuPrime1-NSCLC was established successfully. The relative tumor growth rate T/C ratio of gefitinib and erlotinib group is 28.8% and 24.3%, respectively. Afatinib could significantly inhibit the growth of allograft transplantation tumor model, reduce the volume of the tumor; even some experimental animal tumors disappeared. Especially aft er the withdrawal (14 days to 20 days), afatinib still had a continuous growth inhibition of xenograft tumor, suggesting that the effect of afatinib was obviously superior to gefitinib and erlotinib.
Figure1-B and table2 data show that the tumor of the solvent control group grew well; indicating that xenograft model of HuPrime2-NSCLC was established successfully. The relative tumor growth rate T/C ratio of gefi tinib and erlotinib group is 57.3% and 45.2%, respectively. Afatinib could signifi cantly inhibit the growth of allograft transplantation tumor model, almost stop tumor growth. Especially after the withdrawal (14 days to 20 days), afatinib still had a continuous growth inhibition of xenograft tumor, suggesting that afatinib was still eff ective in patients with gefi tinib resistance.
Discussion
HuPrime1-NSCLC mutant sensitive xenograft model research data showed that reversible tyrosine kinase inhibitors gefi tinib, erlotinib and irreversible tyrosine kinase inhibitor afatinib could effectively inhibit EGFR positive NSCLC allografts model of tumor growth. The pharmacodynamic activity ofirreversible inhibitor was better than that of the reversible inhibitor. HuPrime2-NSCLC mutant resistant xenograft model research data showed that tumor growth accelerated significantly after administrated 12 days. Th is result was consistent with clinical application. Patients applied the reversible tyrosine kinase inhibitors gefitinib and erlotinib gradually developed resistance with the extension of the delivery time. It gradually led to tumor recurrence. In HuPrimel-NSCLC xenograft tumor model, 12 days aft er administration the tumor grew significantly faster. Application irreversible tyrosine kinase inhibitor Afatinib could eff ectively inhibit the tumor which developed resistance growing.
Th e disadvantage of human tumor cell lines xenograft tumor model is that the tumor cell lines in vitro cultivated and acclimatizated, leading to the biological behavior and natural properties changed. Synergies among the tumor cells reduced, thereby aff ecting the growth and metastasis of tumor cells [5]. Good animal model should be able to maximize the simulation of the human body environment. Researchers have begun to recognize the histological complete tumor compared with tumor cell suspension is a more ideal transplant material. Perez-Soler et al [6] subcutaneously implanted 100 cases of fresh lung tissue blocks into nude mice in 2000; modeling success rate was 34% (34/100). Iduna, etc. [7] subcutaneously transplanted 102 cases of lung cancer specimens into NOD/SCID mice in 2008; modeling success rate was 25%. This way of transplant to maximize the preservation of the tumor microenvironment, including tumor cells, infiltrating lymphocytes, fibroblasts, extrace-llular matrix and vascular systems [8, 9]. Compared with modeling approach, tumor cell line modeling method could be more in-depth study of the complex relationship and interaction between tumor and tumor stroma, such as tumor cells and tumor-associated cells is how to activate and promote mutual invasion [8-13], and slightly graft versus host reaction (GVHR).
HU-Prim tumor xenograft model can more accurately predict patients in drug sensitivity, which can take advantage of this model to screen large quantities of anticancer drugs. Simultaneously detection of EGFR mutations is sensitive or resistant can effectively predict the patients for drug sensitivity, which can serve as a routine test for medicine.From page 498
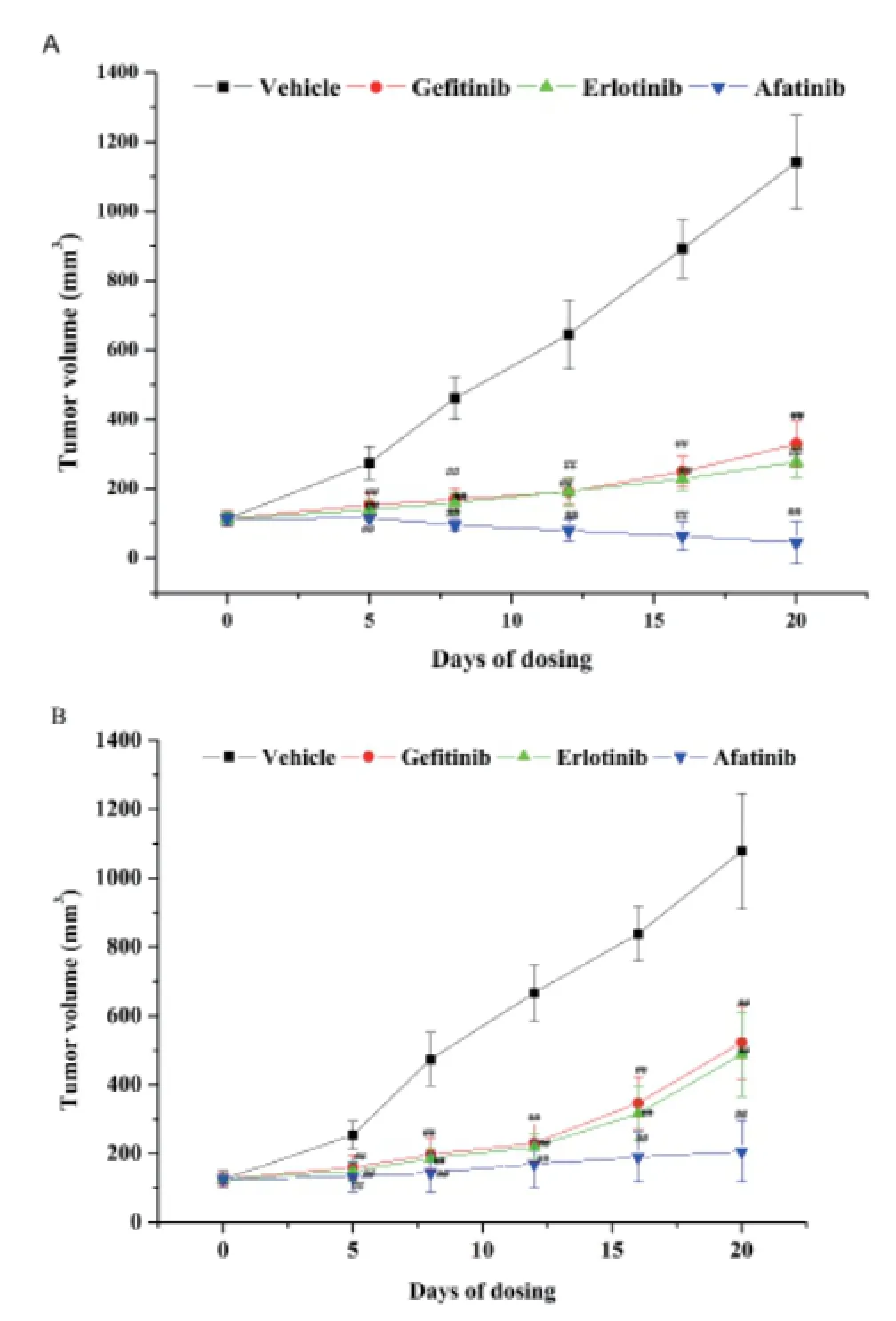
Fig.1 The inhibition of TKIs on xenograft model of HuPrime1-NSCLC and the inhibition of TKIs on xenograft model of HuPrime2-NSCLC. (A) Daily variation of tumor volume in xenograft model of HuPrime1-NSCLC. (B) Daily variation of tumor volume in xenograft model of HuPrime2-NSCLC.
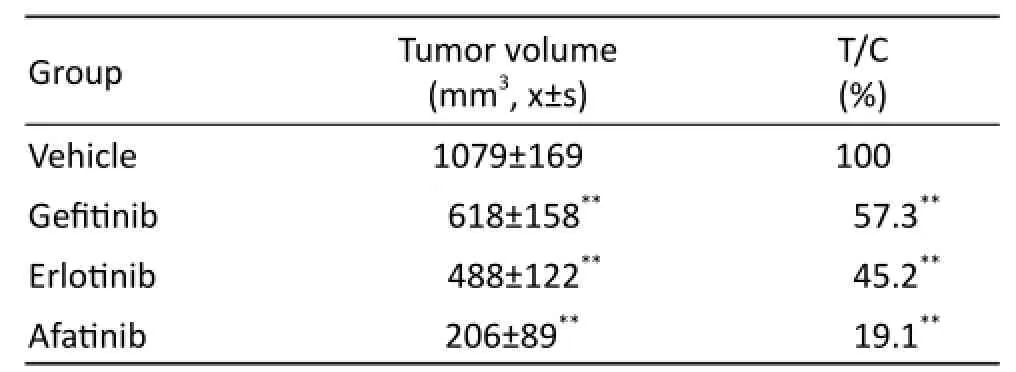
Tab. 1 Tumor volume and T/C value of each group at the end of the experiment on xenograft model of HuPrime1-NSCLC.
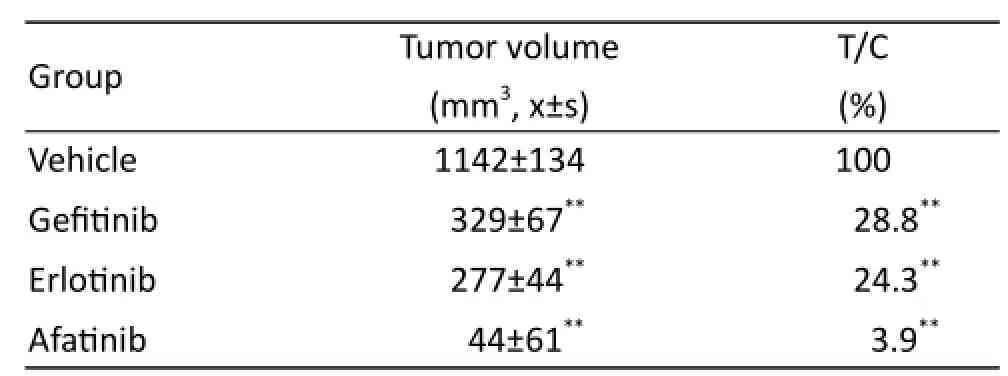
Tab. 2 Tumor volume and T/C value of each group at the end of the experiment on xenograft model of HuPrime2-NSCLC.
22. Passino C, Bernardi L, Spadacini G, et al. Autonomic regulation of heart rate and peripheral circulation: comparison of high altitude and sea level residents[J]. Clin Sci(Lond), 1996, 91(suppl): 81-83.
23. Luo X, Wang L, Yang L. Effects of rapid ascent to high altitude on vascular tone[C]//2015 7th International Conference on Information Technology in Medicine and Education (ITME). IEEE, 2015: 103-107.
24. Luo X, Wang L, Yang L. Infl uence of induced altitude acclimatization on development of acute mountain sickness associated with a subsequent rapid ascent to high altitude [C]//2016 16th International Conference on BioInformatics and BioEngineering (BIBE). IEEE, 2016: 289-292.
25. Droma Y, Hayano T, Takabayashi Y, et al. Endothelin-1 and interleukin-8 in high altitude pulmonary edema[J]. Eur Respir J, 1996, 9(9): 1947-1949.
26. Blitzer ML, Loh E, Roddy MA, et al. Endothelium-derived nitric oxide regulates systemic and pulmonary vascular resistance during acute hypoxia in humans[J]. J Am Coll Cardiol, 1996, 28(3): 591-596.
27. Carroll D, Ring C, Shrimpton J, et al. Secretory immunoglobulin A and cardiovascular responses to acute psychological challenge[J]. Inte J Behav Med, 1996, 3(3): 266-279.
28. Othman M, Kawar B, El Nahas AM. Infl uence of obesity on progression of non-diabetic chronic kidney disease: a retrospective cohort study[J]. Nephron Clin Pract, 2009, 113(1): c16-c23.
29. Shah AM, Mebazaa A, Yang ZK, et al. Inhibition of myocardial crossbridge cycling by hypoxic endothelial cells a potential mechanism for matching oxygen supply and demand[J]? Circ Res, 1997, 80(5): 688-698.
30. Su F, Li Z, Sun X, et al. Th e pulse wave analysis of normal pregnancy: investigating the gestational effects on photoplethysmographic signals[J]. Biomed Mater Eng, 2014, 24(1): 209-219.
31. Han N, Luo X, Su F. A quantitative investigation of hemodynamic adaptation to pregnancy using uterine artery Doppler ultrasonography and finger photoplethysmography[J]. Hypertens Pregnancy, 2014, 33(4): 498-507.
32. Lyu Y, Luo X, Zhou J, et al. Measuring photoplethysmogram-based stress-induced vascular response index to assess cognitive load and stress[C]//Proceedings of the 33rd Annual ACM Conference on Human Factors in Computing Systems. ACM, 2015: 857-866. 33. Liu Z, Zhou Y, Yi R, et al. Quantitative research into the deconditioning of hemodynamic to disorder of consciousness carried out using transcranial Doppler ultrasonography and photoplethysmography obtained via finger-transmissive absorption[J]. Neurol Sci, 2016, 37(4): 547-555.
34. Scholze A, Burkert A, Mardanzai K, et al. Increased arterial vascular tone during the night in patients with essential hypertension[J]. J Hum Hypertens, 2007, 21(1): 60-67.
Acknowledgements
National Natural Science Foundation of Tianjin, China (16JCYBJC27500).
1. Jemal A, Bray F, Center MM, et al. Global cancer statistics[J]. CA Cancer J Clin, 2011, 61(2): 69-90.
2. Favaretto AG, Pasello G, Magro C. Second and third line treatment in advanced non-small cell lung cancer[J]. Discov Med, 2009, 8(43): 204-209.
3. Fichtner I, Rolff J, Soong R, et al. Establishment of patient-derived Non-small cell lung cancer xenograft s as models for the identifi cation of predictive biomarkers[J]. Clin Cancer Res, 2008, 14(20): 6456-6468.
4. Fiebig HH, Dengler WA, Roth T. Human tumor xenograft s: predictivity, and discovery of new anticancer agents[J]. Contrib Oncol.Basel Karger, 1999, 54: 29-50.
5. Zhang XC. Primary lung cancer animal model of progress[J]. Evidence-Based Med, 2009, 9(4): 195.
6. Perez-Soler R, Kemp B, Wu QP, et al. Response and determinants of sensitivity to paclitaxel in human non-small cell lung cancer tumors heterotransplanted in nude mice[J]. Clin Cancer Res, 2000, 6(12): 4932-4938.
7. Fichtner I, Rolff J, Soong R, et al. Establishment of patient-derived non-small cell lung cancer xenograft s as models for the identifi cation of predictive biomarkers[J]. Clin Cancer Res, 2008, 14(20): 6456-6468.
8. Bankert RB, Egilmez NK, Hess SD. Human-SCID mouse chimeric models for the evaluation of anti-cancer therapies[J]. Trends Immunol, 2001, 22(7): 386-393.
9. Bankert RB, Hess SD, Egilmez NK. SCID mouse models to study human cancer pathogenesis and approaches to therapy: potential, limitations, and future directions[J]. Front Biosci, 2002, 7: c44-c62.
10. Dustin ML, de Fougerolles AR. Reprogramming T cells: the role of extracellular matrix in coordination of T cell activation and migration[J]. Curr Opin Immunol, 2001, 13(3): 286-290.
11. Ibe S, Qin Z, Schuler T, et al. Tumor rejection by disturbing tumor stroma cell interactions [J]. J Exp Med, 2001, 194(11): 1549-1559.
12. McCawley LJ, Matrisian LM. Tumor progression: defi ning the soil round the tumor seed[J]. Curr Biol, 2001, 11(1): R25-R27.
13. Tlsty TD. Stromal cell can contribute oncogenic signals[J]. Semin Cancer Biol, 2001, 11(2): 97-104.
Yu-hua ZHANG, Liang SUN, Bin LIU, Guo-qiang LI
Department of Respiratory and Intensive Care Unit, Affi liated Hospital of Logistics College of Chinese People’s Armed Police Forces, Tianjin 300162, China
doi 10.13459/j.cnki.cjap.2016.06.009
Guo-qiang LI, MD, PhD, Department of Respiratory and Intensive Care Unit, Affiliated Hospital of Logistics College of Chinese People’s Armed Police Forces, 220 Chenglin Street, Hedong District, Tianjin 300162, China. Tel: 86-22-60578941; E-mail: tjzyh1@126.com.
2016-08-20; accepted 2016-11-21
杂志排行
中国应用生理学杂志的其它文章
- 5-HT1B受体亚型对小脑顶核介导的运动行为的影响*
- Association study between the angiotensin converting enzyme gene insertion/deletion polymorphism and Qinghai Han Chinese with congenital heart disease
- The infl uence of heterogeneity on the analysis of sleep-wake architecture in the single-prolonged stress rats
- Effect of creatine phosphate sodium on miRNA378, miRNA378* and calumenin mRNA in adriamycin-injured cardiomyocytes
- Changes of microcirculation in healthy volunteers and patients with septic shock in Xining
- 当归黄芪提取物对慢性腹膜功能衰竭大鼠腹膜功能、结构及TGF-β1表达的影响*
