The infl uence of heterogeneity on the analysis of sleep-wake architecture in the single-prolonged stress rats
2016-06-05YingWANGHongshengBIANHangZHAOTingliLI
Ying WANG, Hong-sheng BIAN, Hang ZHAO, Ting-li LI
The infl uence of heterogeneity on the analysis of sleep-wake architecture in the single-prolonged stress rats
Ying WANG1,2, Hong-sheng BIAN1, Hang ZHAO1, Ting-li LI1
1. Heilongjiang University of Chinese Medicine, Harbin 150040, China; 2. Da Li University, Dali 671000, China
Objective:To observe the influence of heterogeneity on sleep-wake architecture in single-prolonged stress (SPS) animal model.Methods:SPS rats were subdivided into low responders (LR) and high responders (HR) based on their freezing responses to a novel environment. Sleeping ti me (ST), awakening numbers (AN), brief awakening numbers (bAN) and frequency distributi on of sleep bouts were used as observing indicators, single factor variance analysis combined with Dunnett t test were used to compare the diff erences between control, exposure, LR and HR groups.Results:We found sleeping ti me was increased only in HR group. Moreover, awakening numbers and brief awakening number increased in exposure group and HR group during the light phase, but not in LR group. The number of sleep bouts for the ranges of 40-80s increased obviously in HR group, but not in exposure and LR group. In additi on, there were signifi cant correlati on between sleep-related parameters and freezing in HR group, but not in LR group.Conclusion:Heterogeneity existed in SPS model in view of different sleep-wake architectures of SPS rats. Rats in HR group exactly mimicked the freezing response and sleep disorders of PTSD. So HR rats were more appropriate to be used as PTSD-like models, especially when studying sleep disorder in PTSD.
sleep-wake architecture; heterogeneity; single prolonged stress
Introduction
Hyperarousal and sleep disturbances are common symptoms of post-traumatic stress disorder (PTSD), and sleep complaints and disturbances are continuing symptoms in patients[1]. Some researcher consider sleep mechanisms have been linked to the development and maintenance of PTSD and sleep disorder may independently exacerbate daytime symptoms, and contribute to poor clinical outcomes in PTSD, such as increased severity of depression[2]. So there is an urgent need to apply animal experiment to bridge the apparent gap between the clinical significance of sleep disturbances in PTSD and the limited understanding of their neurobiological underpinnings [3]. Various animal paradigms have been developed in an attempt to model PTSD. Among them the single-prolonged stress (SPS) is a rodent model of traumatic stress that has been shown to induce multiple PTSD-like physiological and behavioral abnormalities[4−6]. But unfortunately, chronic alterations in sleep−wake architecture in SPS model have not be seen in some research [7-8]. We think one underlying factors is the heterogeneity among samples. In fact, Clinical observation had revealed the existence of heterogeneity in population. For example, Breslau et al reported only about 20% to 30% of individuals exposed to severe stressors will develop PTSD[9]. Considering the individual differences in response to traumatic events clinical studies employ stringent inclusion/ exclusion criteria[10]. Because diagnosis criteria in human patients relies heavily on personal reports of thoughts, dreams, and images which cannot be studied in rodents, most animal studies have to relate to“global”groups, i.e., the entire exposed population versus control populations without distinction[11]. Whereas in practice, stress-exposed animals display a diverse range of responses. Xiaoyu Chen ect found shock rats displayed a more wide range of freezingresponses to a novel tone and rats with high level of fear (HR) to a novel tone showed more avoidance of open spaces compared with rats with low level of fear (LR)[7]. In previous experiments we found SPS-exposed rats also have various fear response to novel circumstance, so we used the same classification method to divided rats into subgroups and observed the influence of heterogeneity on analyzing sleep−wake architecture in SPS model.
Materials and Methods
Animals and housing
Subjects were adult male Sprague-Dawley rats (n = 60) weighing 270 ± 30 g. They were housed in the plastic cages in a colony room on a 12 h/12 h light/dark cycle (lights on 08:00 h) with controlled temperature (22–25°C) and humidity (45–60%). Animal had free access to food and water in their home cages. All of the experimental procedures were approved by Research Ethics Review Board of Heilongjiang University of Chinese medicine.
Experimental schedules
Rats were divided into control group (n=20) and exposure groups (n=40) randomly. Exposure group received SPS procedure. Seven days after SPS treatment, freezing response of two groups were observed in fear response to novel environment test (on day 1), and then divided rats in exposure group into LR and HR group based on their freezing response. Sleep disorder were studied at day 2. Sleeping time, awakening numbers and brief awakening number in the light phase (rat’s sleep phase) were recorded by infrared activity monitors. In the control group, the procedures were similar except that SPS exposure was not performed.
Single Prolonged Stress (SPS)
According to the method by Liberzon et al [12], SPS was conducted in three stages: restraint for 2 h, forced swim for 20 min, and ether anesthesia. Control rats were left in a novel room for the duration of the SPS procedure. Following the SPS or control procedure, rats were allowed a post-stress incubation period of seven days, during which they were left undisturbed in their home cages.
Fear response to novel environment

Fig. 1 Experimental schedules.
Fear sensitization was assessed on Day 1 by measuring the amount of freezing expressed in rats placed in a novel chamber with a light intensity of 10 lux. Th e duration of the test was 3 min which was composed of a 1 min period with background noise and the second 2 min period with a novel auditory tone present (9 kHz, 75 dB against background noise levels of 45–50 dB). Th e percentage of freezing (the freezing duration/3 min) was scored. Freezing was defined as the completely lack of body movement except breathing movements as previously described [13]. The novel test chamber, as well as other test chambers, was cleaned with ethanol (30.0%) after each rat.
Behavioral criteria for subdivision of HR and LR
In order to distinguish between high-response versus and low-response of study subjects, SPS rats were exposed to novel environment and the percentage of freezing were recorded. Based on the freezing response SPS rats were divided into LR (freezing < 40%) and HR (freezing > 60%) groups, the sizeable proportion of study subjects in-between them were excluded in order to maximize the resolution of diff erent subgroups.
Infrared beams assessment of sleep and wakefulness
Considering EEG surgery is a strong stress factor for animals, Columbus Instruments’ Comprehensive Lab Animal Monitoring System (CLAMS) equipped with infrared activity monitors (Columbus Instruments, Columbus) were used to analyze sleep behavior based on locomotion. For sleep analysis, every ten seconds was referred to as an “epoch”. The subject was understood to be sleeping if it fails to break a particular number beams over four consecutive epochs [14]. The sleeping bout continues until the animal breaks that activity threshold. We used the software programed by myself to calculate sleeping time and awakening numbers during sleep phase.
Statistical analysis
All results were expressed as the means ± SD (standard error of mean). Comparisons between two groups use Unpaired Student’s t-test. One-way ANOVA followed by Tukey’s test was used when comparing more than two groups.
Results
Freezing responses
Rats in exposure group displayed a wide range of freezing responses as shown in Figure 2A. Based on the freezing response we subdivide exposure rats into LR (freezing < 40%) and HR (freezing > 60%) groups (Fig. 2B). Th e result revealed that rats exposed to SPS showed more freezing to a novel environment [t (58) =6.83, P<0.01] than control group (Fig. 2C). Oneway ANOVA analysis indicated there was significant difference among three groups in freezing response [F (2, 36) =116, P<0.01; Fig. 2D]. Compared with control group, freezing response in HR were increasing obviously and P values less than 0.01, but the diff erences had not be found in LR group.
Sleep related parameters
Then we compared sleep-related parameters including sleeping time, awakening numbers and brief awakening number between control and exposure groups, as well as between control, LR and HR groups. Compared with control group exposure rats show decreasing sleeping time [t (58) =1.756, P=0.0844; Fig. 3A], increasing awakening number [t (58) =2.878, P=0.0056; Fig. 3C] and brief awakening number [t (58) =2.520, P=0.0145; Fig. 3E]. Oneway ANOVA analysis showed there was significant difference among three groups in sleeping time [F (2, 39) =5.855, P=0.0060; Fig. 3B], awakening number [F (2, 39) =7.589, P=0.0016; Fig. 3D] and brief awakening number [F (2, 39) =17.44, P<0.01; Fig. 3F]. Tukey’s test revealed exposure decreased sleeping time in HR group (P<0.01), but not in LR group compared with control (Fig. 3B). Moreover increased awakening number (P<0.01) and brief awakening number (P<0.01) in HR group had been observed when compared with control (Fig. 3D and 3F). Significant diff erences on sleeping time (P<0.01), awakening numbers (P<0.05) and brief awakening number (P<0.01) have also been found between LR and HR groups. As shown in Table 1, comparison with control group the number of sleep bouts for the range of 40-80 s increased in HR group [t (58) =2.793, P=0.009], but not in exposure [t (58) =1.942, P=0.0570] and LR group [t (58) =0.8357, P=0.4104]. However, the number of sleep bouts for the other ranges showed no statistical diff erence.
Correlation of sleep related parameters with freezingThe Pearson correlation analysis indicated that the sleeping time was positively correlated with freezing in HR group [r2= 0.6483, P=0.0016; Fig. 4B]. While it was not present in LR group [r2= 0.086, P=0.4646; Fig. 4A]. Similarly a correlation was found between awakening number and freezing in HR group [r2= 0.6424, P=0.0017; Fig. 4D] which was not present in LR group [r2= 0.0166, P=0.7225; Fig. 4C]. The correlation between brief awakening number and freezing reach signifi cance in HR group [r2= 0.6975, P=0.0007; Fig. 4F], but not in LR group [r2= 0.1360, P=0.2943; Fig. 4E].

Fig. 2 Individuals in exposure group displayed a more wide range of freezing responses to a novel environment as shown in plots (A). Rats in exposure group were subdivided into low responders (freezing < 40%) and high responders (freezing > 60%) based on their freezing responses to the novel environment (B). Freezing response to a novel environment 7 days after SPS in control and exposure groups (C). Freezing response to a novel environment in control, LR and HR groups (D). HR: High response; LR: Low response. The values in the histograms are mean ± SD.**P< 0.01.
Discussion
In animals these sleep disorder of PTSD, such as longer sleep latency, disrupted sleep maintenance and an increased number of awakenings[15], are not easy to observe by polysomnography at a later time after SPS exposure. It could be interpreted as that sleep disorder in SPS rats can’t maintain a long time, or maybe some factors influence the results. If the latter exist, one possible reason may be the surgery performed before polysomnography, thatgives additional stress on animals and complicates the stress conditions. So in our present experiment we used infrared activity monitors which was noninvasive record method to observe the sleep−related parameters. Th e other more important reason is the heterogeneity between individuals. The exist of heterogeneity have been confirmed in clinic for a long time. As human beings animals display a range of responses to stimuli, as do the humans they are intended to model. Though researchers who work with animals have long been aware that, this heterogeneity in responses was just accepted for many years and regarded as unavoidable. Any heterogeneity in animal responses might be regarded as confirming the validity of animal studies, rather than as a problem[11]. Obviously the inclusion of all exposed animals in data analysis overlooks the individual variability in their behavioral response to the stressor, and represents a source for potential bias. In our present experiment we distinguish LR and HR rats based on freezing response to novel environment. We found HR rats had decreased sleeping time, increased awakening number, brief awakening number and increased sleep bouts in the range of 40-80 s in sleep phase compared with control and LR rats. But diff erence did not be found between LR and control groups. These results indicated heterogeneity exist in SPS rats and that influenced the analyses on sleep-wake architecture in singleprolonged stress (SPS) animal model. Th e decreased sleeping time and increased awakening number in HR rats are consistent with that symptoms in PTSD patients who usually complained disrupted sleep maintenance and increased number of awakenings. Because HR rats exactly mimic the behavior and sleep-wake profi le of PTSD, they can be used as the ideal animal model to study the neurobiological mechanisms of sleep disorders and new therapeutic drug.

Fig. 3 Total sleeping time during the light phase (sleep phase) in control and exposure groups (A). Total sleeping time in control, LR and HR groups (B). The numbers of awakening expressed relative to the amount of sleeping time (ST) in control and exposure groups (C). The numbers of awakening relative to the amount of sleeping time in control, LR and HR groups (D). The numbers of brief awakening relative to the amount of sleeping time in control and exposure groups (E). The numbers of brief awakening relative to the amount of sleeping time in control, LR and HR groups (F). HR: High response; LR: Low response. The values in the histograms are mean ± SD.*P< 0.05,**P< 0.01.
Acknowledgements
This research is supported by the Key Project of Science Research Foundation of Yunnan Provincial Department of Education (The study on the sleepimproving eff ect of Rhizoma, 2015Z151).
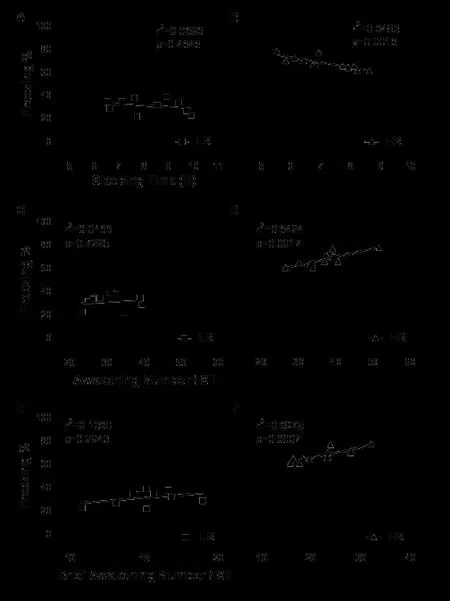
Fig. 4 Correlation between sleep related parameters and freezing in LR (n = 10) and HR rats (n = 12). There is not correlation between sleeping time and freezing in LR rats (A) exposed to a novel environment, but there is a positive correlation in HR rats (B). There is not correlation between awakening number and freezing in LR rats (C). While there is a correlation between them in HR rats (D). There is positive correlation between brief awakening number and freezing in HR rats (F), but not in LR rats (E).
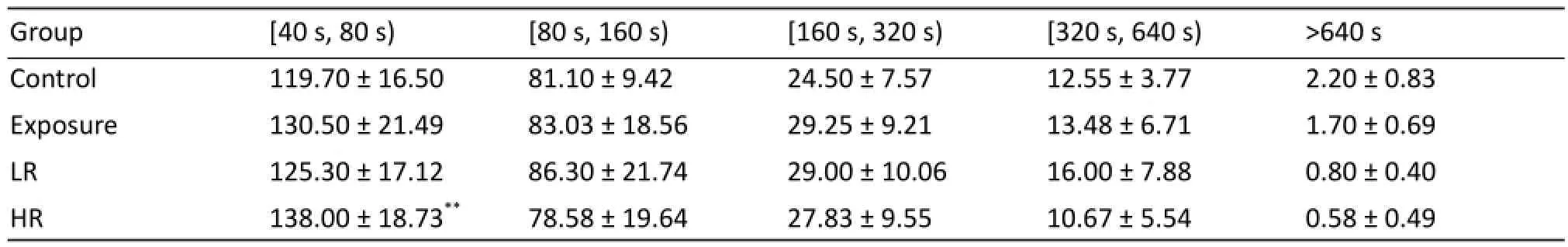
Tab. 1 The numbers of sleep bouts on light phase.
1. Harvey, AG, Jones C, Schmidt DA. Sleep and posttraumatic stress disorder: a review [J]. Clin Psychol Rev, 2003, 23(3): 377-407.
2. Brownlow JA, Harb GC, Ross RJ. Treatment of sleep disturbances in post-traumatic stress disorder: a review of the literature[J]. Curr Psychiatry Rep, 2015, 17(6): 41.
3. Germain A, Buysse DJ, Nofzinger E. Sleep-specifi c mechanisms underlying posttraumatic stress disorder: integrative review and neurobiological hypotheses [J]. Sleep Med Rev, 2008, 12(3): 185-195.
4. Liberzon I, Krstov M, Young EA. Stress−restress: eff ects on ACTH and fast feedback [J]. Psychoneuroendocrinology, 1997, 22(6): 443−453.
5. Liberzon I, Lopez JF, Flagel SB, et al. Diff erential regulation of hippocampal glucocorticoid receptors mRNA and fast feedback: relevance to post-traumatic stress disorder [J]. J Neuroendocrinol, 1999, 11(1): 11−17.
6. Yamamoto S, Morinobu S, Takei S, et al. Single prolonged stress: toward an animal model of posttraumatic stress disorder [J]. Depress Anxiety, 2009, 26(12): 1110−1117.
7. Nedelcovych MT, Gould RW, Zhan X, et al. A Rodent Model of Traumatic Stress Induces Lasting Sleep and Quantitative Electroencephalographic Disturbances [J]. ACS Chem Neurosci, 2015, 6(3): 485-493.
8. Vanderheyden WM, George SA, Urpa L, et al. Sleep alterations following exposure to stress predict fear-associated memory impairments in a rodent model of PTSD [J]. Exp Brain Res, 2015, 233(8): 2335-2346.
9. Breslau N, Davis GC, Andreski P, et al. Traumatic events and posttraumatic stress disorder in an urban population of young adults[J]. Arch Gen Psychiatry, 1991, 48(3): 216-222.
10. Cohen H, Zohar J. An animal model of posttraumatic stress disorder: the use of cut-off behavioral criteria [J]. Ann N Y Acad Sci, 2004, 1032: 167-178.
11. Cohen H, Matar MA, Joseph Z. Animal Models of Post-Traumatic Stress Disorder [J]. Curr Protoc Neurosci, 2013, 77: 9.54.1-9.54.10.
12. Liberzon I, Lopez JF, Flagel SB, et al. Differential regulation of hippocampal glucocorticoid receptors mRNA and fast feedback: relevance to post-traumatic stress disorder [J]. J Neuroendocrinol, 1999, 11(1): 11–17.
13. Blanchard RJ, Blanchard DC. Passive and active reactions to fear-eliciting stimuli [J]. J Comp Physiol Psychol, 1969, 68(1): 129–135.
14. Pack AI, Galante RJ, Maislin G, et al. Novel method for high-throughput phenotyping of sleep in mice [J]. Physiol Genomics, 2007, 28(2): 232-238.
15. Herbst E, Metzler TJ, Lenoci M, et al. Adaptation eff ects to sleep studies in participants with and without chronic posttraumatic stress disorder[J]. Psychophysiology, 2010, 47(6): 1127–1133.
doi 10.13459/j.cnki.cjap.2016.06.005
Ting-li LI, MD, Professor, Heilongjiang University of Chinese Medicine, 24 He Ping Street, Harbin 150040, China. Tel: 86-451-87266874; E-mail: lilhlj@126.com .
2016-07-24; accepted 2016-10-24
杂志排行
中国应用生理学杂志的其它文章
- 5-HT1B受体亚型对小脑顶核介导的运动行为的影响*
- Iptakalim ameliorates relaxation to acetylcholine in thoracic aortic rings impaired by microvesicles derived from hypoxia/ reoxygenation-treated HUVECs
- Association study between the angiotensin converting enzyme gene insertion/deletion polymorphism and Qinghai Han Chinese with congenital heart disease
- Effect of creatine phosphate sodium on miRNA378, miRNA378* and calumenin mRNA in adriamycin-injured cardiomyocytes
- Changes of microcirculation in healthy volunteers and patients with septic shock in Xining
- 当归黄芪提取物对慢性腹膜功能衰竭大鼠腹膜功能、结构及TGF-β1表达的影响*
