Changes in oxygen saturation can not help diagnose acute mountain sickness (AMS): ascending to Lhasa on theQinghai-Tibet train
2016-06-05JunboANHaoranGUYuWUYongjunLUO
Jun-bo AN, Hao-ran GU, Yu WU, Yong-jun LUO
1. Department of Military Medical Geography, College of High Altitude Military Medicine, Third Military Medical University, Chongqing 400038, China; 2. Battalion 5 of Cadet Brigade, Third Military Medical University, Chongqing 400038, China; 3. Key Laboratory of High Altitude Medicine (Ministry of Education), Third Military Medical University, Chongqing 400038, China; 4. The12th hospital of PLA, Kashi 844200, China
Changes in oxygen saturation can not help diagnose acute mountain sickness (AMS): ascending to Lhasa on the
Qinghai-Tibet train
Jun-bo AN1,2,3, Hao-ran GU4, Yu WU1,3, Yong-jun LUO1,3
1. Department of Military Medical Geography, College of High Altitude Military Medicine, Third Military Medical University, Chongqing 400038, China; 2. Battalion 5 of Cadet Brigade, Third Military Medical University, Chongqing 400038, China; 3. Key Laboratory of High Altitude Medicine (Ministry of Education), Third Military Medical University, Chongqing 400038, China; 4. The12th hospital of PLA, Kashi 844200, China
Objective:Acute mountain sickness (AMS) is a common condition in individuals who ascend to alti tudes over 2 500 m. There is no measurements that can reliably predict or diagnose this conditi on. We therefore determined whether pulse oximetry data are associated with the development of AMS and can help diagnose AMS.Methods:We studied 58 young male undergraduates who traveled from Chongqing (300 m) to Lhasa (3 658 m) by train. We collected data on the ascent profi les and AMS symptoms based on the Lake Louise Score (LLS). The resti ng arterial oxygen saturati on (R-SpO2) and pulse rate were then measured using fi nger pulse oximetry.Results:In Golmud(2 800 m) and Tanggula(5 200 m), R-SpO2was signifi cantly lower in the AMS group than in the group without AMS (P<0.05). However, upon arrival in Lhasa (3 658 m), the R-SpO2was higher in the AMS group than in the non-AMS group (P<0.05). In Tanggula, the change in the SpO2(CR-SpO2) in the AMS group was higher than that in the non-AMS group (P<0.05). But in Lhasa, the CR-SpO2in the AMS group was lower than that in the non-AMS group (P<0.05). We also monitored heart rate (HR) throughout the study. In Xining(2 200 m) and Golmud, the HRs in the AMS group were higher than those in the non-AMS group. However, the HRs in the AMS group were lower than those in the non-AMS group in Tanggula and Lhasa.Conclusion:Based on the results of this study, the R-SpO2graph was not consistent. We can thus conclude that the uti lity of SpO2remains limited in the diagnosis of AMS. The results suggest that using pulse oximetry to diagnose AMS is not valuable in people ascending to Lhasa on the Qinghai-Tibet train.
acute mountain sickness; oxygen saturati on; heart rate; alti tude
Introduction
Acute mountain sickness (AMS) is a common condition in individuals who ascend to altitudes over 2 500 m without prior acclimation[1]. The most common characteristic of AMS is headache, and it’s always accompanied with at least one other symptom such as nausea, vomiting, anorexia, dizziness, lethargy, fatigue, or sleep disturbance[2, 3]. If person with AMS does not receive timely treatment, AMS may proceed to high altitude cerebral edema (HACE) and other high altitude-related disorders[4], and AMS has been suggested as a mild form or precursor of HACE[5].
When individuals rapidly ascend from low altitudes to high altitudes of more than 2 500 m above sea level, AMS can be diagnosed based on a Lake Louise Score (LLS)[3, 5-7]. AMS typically develops within 48 h after ascending. The incidence of AMS diff ers among studies, ranging from 10% to 93%, and it depends on the rate of ascent to the high altitude, the height reached, and the mode of transportation used to ascend[8-11]. Among these factors, pulse oximetry is commonly used as a noninvasive method to assess subjects’ oxygenation and determine whether supplemental oxygen is needed. In some studies, the oxygen saturation at rest (R-SpO2) and heart rate (HR), as monitored by pulse oximetry, has been propounded as indicators of an acclimatization and closed to AMS at high altitude[12-14, 25]. It has showed that development of clinical AMS is aff ected by resting arterial hypoxemia [27] and R-SpO2correlates with the LLS at diff erent high altitudes[3, 15],whereas other reports have shown that R-SpO2had no correlation with AMS[4, 16-19].
In fact, a SpO2below the normal reference range is often observed in individuals with AMS. A depressed SpO2may not only be an important feature of AMS, but it can also refl ect impaired gas exchange concomitant with the presence of HACE and high altitude pulmonary edema (HAPE), and subclinical HAPE may be a normal finding in healthy individuals at high altitudes. However, whether the R-SpO2can help clinically diagnose AMS is not clear. Some studies have shown that it’s reliable while others show not. Therefore, we aim to determine whether pulse oximetry is a safe, simple, and effective method for clinically diagnosing AMS among those ascending to Lhasa on the Qinghai-Tibet train.
Subjects and Methods
Subjects
In August 2012, 58 young, healthy males traveled from Chongqing (300 m) to Lhasa (3 658 m) by train. Th e train route is illustrated in Figure 1. All of these males were a tightly controlled group of recruits, who were graduated from the Th ird Military Medical University. A physician with experience in high-altitude medicine administered the demographic survey. During the train ride, medicines and supplemental oxygen (except that available to all passengers) were not allowed to prevent AMS without physician’s permission. Written consent was obtained from all subjects in agreement with the guidelines of the ethical committee of the Th ird Military Medical University.
Methods
In this survey, the subjects left Chongqing (300 m) by train and reached Lhasa, Tibet (3 658 m) 44 hours later. As the Qinghai-Tibet train moved toward Lhasa, each subject was required to complete the questionnaire aft er arrival in Xining (2 200 m), Golmud (2 800 m), and Tanggula (5 200 m) and immediately aft er the subjects got off the train in Lhasa (3 658 m). All subjects completed the AMS questionnaire within the fi rst hour aft er reaching each destination site. Th e questionnaire included two sections. Th e fi rst section comprised demographic information about the subjects, such as height, weight, plateau experience and tobacco use. Th e second section comprised the Lake Louise AMS score system. Th is measurement system of AMS is related to the following self-assessments: headache, gastrointestinal symptoms, fatigue and/ or weakness, dizziness/lightheadedness, difficulty sleeping. Th e scale ranges from 0 to 3: 0 is not at all, 1 is mild reduction, 2 is moderate reduction and 3 is severe reduction (bed rest). AMS was diagnosed if an individual had an LLS score greater than 3 and had a headache plus one of the other symptoms[3]. Additionally, HR and R-SpO2were recorded using a TuffSat Handheld Pulse Oximeter (GE, USA). To eliminate variability in this experiment, the SpO2probe was placed on the right index fi nger. When the subjects were on the Qinghai-Tibet train, the R-SpO2levels were measured when the train was at a railway station. During the 30 min before the measurements, all subjects were told to rest without eating or exercising. Th e pulse oximetry data were based on measurements taken over an average of 30 s with a steady value.
Statistical analysis
All data were collected by investigators. Data were expressed as the mean±SD and analyzed using independent two-sample Student’s t test for continuous variables. Th e incidence of AMS between the smokers and the non-smokers was analyzed by one-sample t test. During ascent, the subjects who suff ered AMS among the 58 subjects was calculated based on LLS scores and severity of symptoms. The relations between LLS scores and R-SpO2(and HR) at various altitudes was calculated using Spearman’s correlations coeffi cients. We used the PASW Statistics 18 soft ware (IBM, Chicago, IL) to analyze the data. Th e level of statistical signifi cance was set at <0.05.
Results
None of the subjects who participated in the study (n=58) had severe medical disorders such as heart disease, hypertension or absence of a patent foramen ovale [24]. Their demographic data are shown in Table 1. Th e study participants were healthy men between 21 and 27 years of age. No one had plateau experience one month lately. There was no significant difference between smokers and non-smokers in AMS subjects based on one-sample t test. Others demographic data also hadn’t statistical signifi cance. Th e LLS at various altitudes and the Spearman's correlation coeffi cients of HR and R-SpO2are illustrated in Table 2. As the altitude increased, the prevalence of AMS increased, except in Lhasa. Th e number of AMS cases was 2 (3%) in Xining, 10 (17%) in Golmud, 27 (46%) in Lhasa, and 18 (31%) in Tanggula. Th e total prevalence of AMS from Xining to Lhasa was 58% (34 of the 58 subjects). Among the AMS subjects, 11 suff ered AMS at two altitudes, 4 at three altitudesand 1 at four altitudes. At four altitudes, the mean LLS scores in the AMS group were very similar, the diff erence among the altitudes was 0.6, and only one subject's LLS scores reached six.
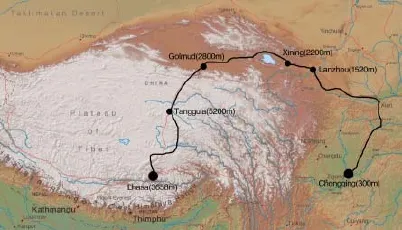
Fig. 1 The train route from Chongqing to Lhasa.

Tab. 1A 58 young male’s demographic characteristics.

Tab. 1B AMS difference in smokers and non-smokers.
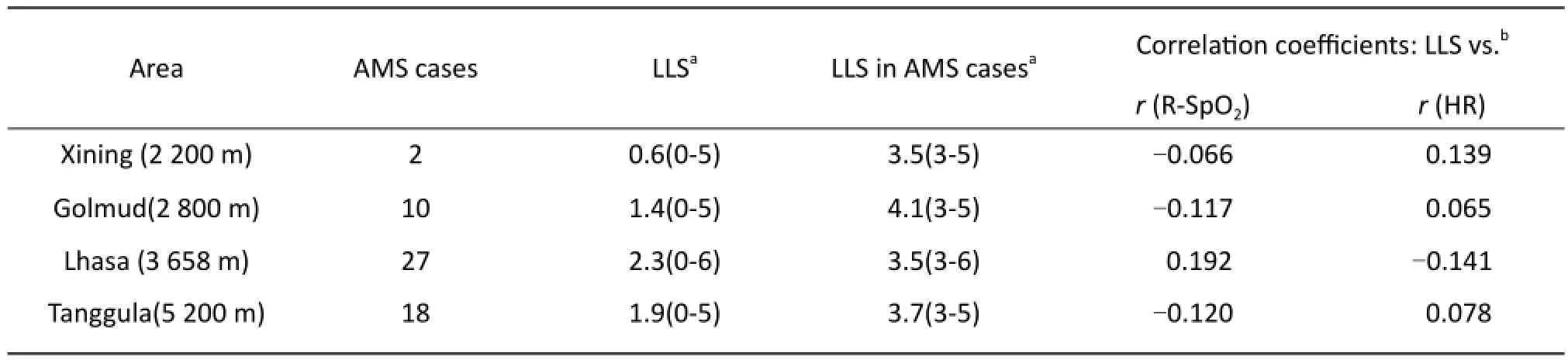
Tab. 2 Lake Louise Scores (LLS) at various altitudes and Spearman’s correlation coefficients.
In Xining (2 200 m), the R-SpO2of AMS and non-AMS subjects did not significantly differ (90.50±0.71% & 89.68±2.54%, P=0.65). As the altitude increased over 2 500 m, the R-SpO2of subjects decreased quickly. In Golmud (2 800 m), the mean R-SpO2of the AMS and non-AMS groups was 82.60±3.24% and 83.17±3.51 (P=0.64), respectively. As the train ascend, the concentration of oxygen were maintained at 24 to 25% inside the aircraft of Qinghai-Tibet train. Because of this, the effective altitude reduces about 1 200 m in Tanggula[26]. Th e difference between the AMS and non-AMS groups was not statistically significant too. As the train ascended to Tanggula and then arrived in Lhasa, the result of R-SpO2in AMS and non-AMS groups was similar to that observed in Golmud. In Tanggula, the mean R-SpO2of subjects with AMS and without AMS were 83.06±3.25% and 83.51±3.95% (P=0.68), respectively. In Lhasa, these values were 84.81±2.86% and 83.29±4.29% (P=0.32). Additionally, in Golmud and Tanggula, the mean R-SpO2of the AMS group was significantly lower than in the group without AMS. However, in Xining and upon arrival in Lhasa, the R-SpO2was higher in the AMS group than in the non-AMS group. The R-SpO2in Xining was totally higher than the other three higher altitudes (Fig. 2).
We also measured HR throughout the study. Resting HR remained stable at different altitudes throughout the study, and the differences between AMS and non-AMS subjects were also not signifi cant at any altitude (Tab. 3). Th e change in the SpO2(CRSpO2) ([SpO2at Chongqing–SpO2at the test location]/ SpO2at Chongqing) ×100)[4] was evaluated inthe AMS and non-AMS groups (Tab. 3). There was no signifi cant diff erence in the CR-SpO2between the AMS and non-AMS groups.
Discussion
There was no subject bias in our study because all subjects were asked to participate in the subsequent survey. We ensured that all subjects had adequately rested (30 min) before the measurements were taken and that all of the data were recorded while the subjects were seated without eating or exercising. All subjects were close in age and had a similar diet and physical background. Th e data were acquired in a standardized fashion from all subjects. Aft er completion of the Lake Louise Consensus Questionnaire, the pulse oximetry data were acquired immediately. As reported, the recommendations are only 300 to 600 m one day to ascend and gain an 600 to 1200 m altitude acclimatization [5, 18]. In this study, the train ran 3641 km in 44 hours from Chongqing to Lhasa and average ascending speed was 174.75 km/ h besides Tanggula(5 200 m) to Lhasa(3 658 m). Th e detailed running information is showed in Table 4. All subjects have been stayed in Chongqing over 30 days before this trip. So we can determined there was no acclimatization the whole trip and creating a standard method (oxygen saturation) to diagnose AMS. Based on the statistical analysis, the correlation between SpO2and AMS was not signifi cant. In examining the same altitude, the mean R-SpO2of AMS was similar to that of the non-AMS group, and the diff erences were not significant. In various altitudes, the SpO2in Golmud (2 800 m) was lower than in Lhasa (3 658 m), regardless of AMS status. All these results differed from those of many other publications[20, 21]. Therefore, the association between the SpO2and AMS may be aff ected by the ascent profi le of the population studied in this investigation. No association between SpO2and AMS was found in our study. Ventilatory changes reflected by the SpO2may follow a diff erent timescale than the cerebral response to hypoxia, as refl ected by the LLS. In such a study, the AMS incidence would not be associated with the SpO2(as observed in this study). Alternatively,the degree of hypoxia may play an important role in the relationship between the SpO2and AMS. Th e decreased SpO2observed at higher-altitude locations may refl ect SpO2changes due to the development of subclinical HAPE. Another measure in this study, CR-SpO2, was evaluated in the AMS and non-AMS groups, and no signifi cant diff erence was observed. The train from Golmud to Lhasa, the highest point being 5 200 m in Tanggula, travelers are exposed to severe hypoxia. So through oxygen generators to increase the oxygen concentration in each traveler capsule of train from 21% to 25% approximately and this causes about 1 200 m altitude reduced [26]. Therefore, the practical experiment altitude is about 4,000 m in Tanggula. In Golmud, we monitored R-SpO2before using oxygen generators and the pulse oximeter was used after subjects leaving train in Lhasa, there is no alter in these two altitudes. Th e aff ect that we have described in Result.

Tab. 3 Resting heart rates (HR) (beats/min) and change in the resting oxygen saturation (CR-SpO2) in subjects with and without AMS.
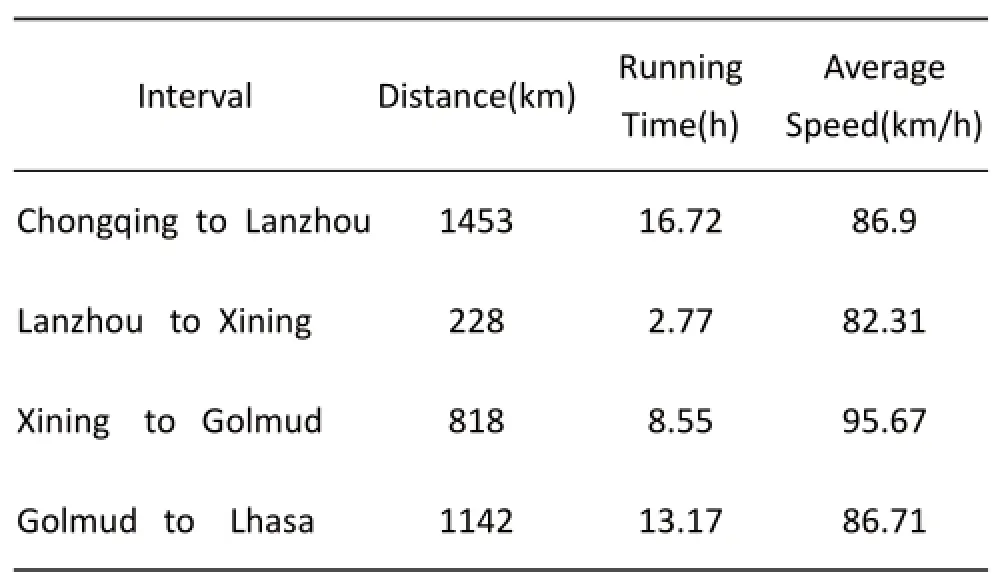
Tab. 4 The train route, distance of each interval and ascending speed of the train.

Fig. 2 Resting oxygen saturation at various altitudes( Altitude value spots are 2 200 m, 2 800 m, 3 658 m, 5 200 m, respectively, n=58 ).
Additionally, we attempted to determine whether the HR could be used to diagnose AMS. As others studies, measuring HR is useful to predict AMS[22, 23]. There was a significant difference in the HR between the AMS and non-AMS groups. However, from the subjects investigated in this study, there was no highly significant relationship between the HR and the presence of AMS. Th e “r” value were so small, the P value were so high. At sea level, there are typically wide variations in the HR among individuals, and HR variations may exist during exposure to high altitudes. Thus, the HR alone could not effectively discriminate between individuals with AMS and those without AMS.
SpO2is a common factor which have used to diagnose AMS and there are many articles focused on it to study high mountain disease. Th e subjects ascend to high altitude by foot/climbing mostly[3,16,19]. In our study, the capsule of train providing a peaceful surrounding benefited to monitor SpO2in resting. The statistic of R-SpO2would be more practical in subjects without exercise just like climbing. In O’Connor’s article, the writer monitored a 3 080 m altitude and AMS[19], while Karinen recorded three different ascending altitudes to make a comparison[20]. Both of them have the benefi ts and we recorded like Karinen. When study AMS, the LLS and pulse oximeter are common methods that every research will depend on.
Th e limitations of our study includes its small sample size, the lack of precise estimates of the alcohol and we ignore the aff ection of the train opening and closing the door in station. Th ese factors may lead to a slight deviation in prevalence of AMS. Additionally, all subjects were young males aged 21-27, study’s conclusion may be limited to this group.
Based on the results of this study, we can conclude that the utility of the SpO2remains limited to diagnosis of AMS on the Qinghai-Tibet train. We recommend further studies to determine the possible utility of heart rate in the prediction and diagnosis of AMS.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81372125).
Disclosures
Th e authors state that there are no confl icts of interest regarding the publication of this article or fi nancial ties to disclose.
Contributions
Junbo An and Haoran Gu collected the data and completed the manuscript. Yongjun Luo reviewed results and provided guidelines for presentation and interpretation.
1. Imray C, Wright A, Subudhi A, et al. Acute mountain sickness: pathophysiology, prevention, and treatment[J]. Prog Cardiovasc Dis, 2010, 52(6): 467-484.
2. Luo YJ, Chen Y, Zhou Q, et al. A survey of acute mountain sickness and vital signs in subjects ascending to Lhasa via the Qinghai-Tibet train[J]. SJR, 2011, 6(13): 2639-2645.
3. Roach RC, Bärtsch P, Oelz O, et al. The Lake Louise acute mountain sickness scoring system[J]. Hypoxia Mol Med, 1993: 272-274.
4. Chen HC, Lin WL, Wu JY, et al. Change in oxygen saturation does not predict acute mountain sickness on Jade Mountain[J]. Wilderness Environ Med, 2012, 23(2): 122-127.
5. Hackett PH, Roach RC. High-altitude illness[J]. N Engl J Med, 2001, 345(2): 107-114.
6. Maggiorini M, Muller A, Hofstetter D, et al. Assessment of acute mountain sickness by diff erent score protocols in the Swiss Alps[J]. Aviat Space Environ Med, 1998, 69(12): 1186-1192.
7. Savourey G, Guinet A, Besnard Y, et al. Evaluation of the Lake Louise acute mountain sickness scoring system in a hypobaric chamber[J]. Aviat Space Environ Med, 1995, 66(10): 963-967.
8. Gertsch JH, Seto TB, Mor J, et al. Ginkgo biloba for the prevention of severe acute mountainsickness (AMS) starting one day before rapid ascent[J]. High Alt Med Biol, 2002, 3(1): 29-37.
9. Honigman B, Th eis MK, Koziol-McLain J, et al. Acute mountain sickness in a general tourist population at moderate altitudes[J]. Ann Intern Med, 1993, 118(8): 587-592.
10. Pradhan S, Yadav S, Neupane P, et al. Acute Mountain Sickness in Children at 4 380 Meters in the Himalayas[J]. Wilderness Environ Med, 2009, 20(4): 359-363.
11. Basnyat B, Subedi D, Sleggs J, et al. Disoriented and ataxic pilgrims: an epidemiological study of acute mountain sickness and high-altitude cerebral edema at a sacred lake at 4 300 m in the Nepal Himalayas[J]. Wilderness Environ Med, 2000, 11(2): 89-93.
12. Burtscher M, Flatz M, Faulhaber M. Prediction of susceptibility to acute mountain sickness by SaO2values during short-term exposure to hypoxia[J]. High Alt Med Biol, 2004, 5(3): 335-340.
13. Fagenholz PJ, Gutman JA, Murray AF, et al. Optic nerve sheath diameter correlates with the presence and severity of acute mountain sickness: evidence for increased intracranial pressure[J]. J Appl Physiol, 2009, 106(4): 1207-1211.
14. Koehle MS, Guenette JA, Warburton DE. Oximetry, heart rate variability, and the diagnosis of mild-to-moderate acute mountain sickness[J]. Eur J Emerg Med , 2010, 17(2): 119-122.
15. Kao WF, Kuo CC, Hsu TF, et al. Acute mountain sickness in jade mountain climbers of Taiwan. Aviat Space Environ Med, 2002, 73(4): 359–362.
16. Wagner DR, Knott JR, Fry JP. Oximetry fails to predict acute mountain sickness or summit success during a rapid ascent to 5 640 meters[J]. Wilderness Environ Med, 2012, 23(2): 114-121.
17. Saito S, Tanobe K, Yamada M, et al. Relationship between arterial oxygen saturation and heart rate variability at high altitudes[J]. Am J Emerg Med, 2005, 23(1): 8-12.
18. Gallagher SA, Hackett PH. High-altitude illness[J]. Emerg Med Clin North Am, 2004, 22(2): 329-355, viii.
19. O’Connor T, Dubowitz G, Bickler PE. Pulse oximetry in the diagnosis of acute mountain sickness[J]. High Alt Med Biol, 2004, 5(3): 341-348.
20. Karinen HM, Peltonen JE, Kahonen M, et al. Prediction of Acute Mountain Sickness by Monitoring Arterial Oxygen Saturation During Ascent[J]. High Alt Med Biol, 2010, 11(4): 325-332.
21. Guo GN, Zhu GY, Sun W, et al. Association of Arterial Oxygen Saturation and Acute Mountain Sickness Susceptibility: A Meta-analysis[J]. Cell Biochem Biophys, 2014, 70(2): 1427-1432.
22. Maggiorini M, Buhler B, Walter M, et al. Prevalence of acute mountain sickness in the Swiss Alps[J]. BMJ, 1990, 301(6756): 853-855.
23. Roach RC, Houston CS, Honigman B, et al. How well do older persons tolerate moderate altitude[J]? West J Med, 1995, 162(1): 32-36.
24. Elliott JE, Laurie SS, Kern JP, et al. AltitudeOmics: Impaired pulmonary gas exchange effi ciency and blunted ventilatory acclimatization in humans with patent foramen ovale aft er 16 days at 5 260 m[J]. J Appl Physiol, 2015, 118(9): 1100-1112.
25. Loeppky JA. Hypoxemia and Acute Mountain Sickness: Which Comes First[J]? High Alt Med Biol, 2008, 9(4): 271-279.
26. West JB. A New Approach to Very-High-Altitude Land Travel: The Train to Lhasa, Tibet[J]. Ann Intern Med, 2008, 149(12): 898-900.
27. Hackett PH, Rennie D, Hofmeister SE, et al. Fluid retention and relative hypoventilation in acute mountain sickness[J]. Respiration, 1982, 43(5): 321–329.
doi 10.13459/j.cnki.cjap.2016.06.008
Yong-jun LUO, College of High Altitude Military Medicine, Th ird Military Medical University, Chongqing 400038, China; Tel: 86 23 6875 2396; Fax: 86 23 6875 2396; E-mail: luoyongjun2011@gmail.com.
2016-09-20; accepted 2016-10-19
杂志排行
中国应用生理学杂志的其它文章
- 5-HT1B受体亚型对小脑顶核介导的运动行为的影响*
- Association study between the angiotensin converting enzyme gene insertion/deletion polymorphism and Qinghai Han Chinese with congenital heart disease
- The infl uence of heterogeneity on the analysis of sleep-wake architecture in the single-prolonged stress rats
- Effect of creatine phosphate sodium on miRNA378, miRNA378* and calumenin mRNA in adriamycin-injured cardiomyocytes
- Changes of microcirculation in healthy volunteers and patients with septic shock in Xining
- 当归黄芪提取物对慢性腹膜功能衰竭大鼠腹膜功能、结构及TGF-β1表达的影响*
