Iptakalim ameliorates relaxation to acetylcholine in thoracic aortic rings impaired by microvesicles derived from hypoxia/ reoxygenation-treated HUVECs
2016-06-05KunweiZHANGShaoxunWANGYeyiLISuWEIManSHANGChaoLIUMiaoLIUYiluWANGQianZHUYannaWUJunqiuSONGYanxiaLIU
Kun-wei ZHANG, Shao-xun WANG, Ye-yi LI, Su WEI, Man SHANG, Chao LIU, Miao LIU, Yi-lu WANG, Qian ZHU, Yan-na WU, Jun-qiu SONG, Yan-xia LIU
Iptakalim ameliorates relaxation to acetylcholine in thoracic aortic rings impaired by microvesicles derived from hypoxia/ reoxygenation-treated HUVECs
Kun-wei ZHANG, Shao-xun WANG, Ye-yi LI, Su WEI, Man SHANG, Chao LIU, Miao LIU, Yi-lu WANG, Qian ZHU, Yan-na WU, Jun-qiu SONG, Yan-xia LIU
Department of Pharmacology, School of Basic Medical Sciences, Tianjin Medical University, Tianjin 300070, China
Objecti ve:To investi gate the eff ect of Iptakalim (Ipt) preventi ng injury of endothelial microvesicles (EMVs) derived from hypoxia/reoxygenati on (H/R)-treated HUVECs on the relaxati on of rat thoracic aorti c rings and explore the underlying mechanism.Methods:H/R injury model was established to release H/ R-EMVs from HUVECs. H/R-EMVs from HUVECs were isolated by ultracentrifugati on from the conditi oned culture medium. H/R-EMVs were characterized by using Transmission Electron Microscope (TEM). Thoracic aorti c rings of rats were incubated with 10-7-10-3mol/L Ipt and co-cultured with 10 μg/ml H/R-EMVs for 4 hours, and their endothelium- dependent relaxati on in response to acetylcholine (ACh) was recorded in vitro. The nitric oxide (NO) producti on of ACh-treated rat thoracic aorti c rings was measured by using Griess reagent. The expression of endothelial NO synthase (eNOS), phosphorylated eNOS (p-eNOS, Ser-1177), serine/threonine kinas (Akt) and phosphorylated Akt (p-Akt, Ser-473) in the thoracic aorti c rings of rats was detected by Western blotting.Results:H/R-EMVs were induced by H/R-treated HUVECs and isolated by ultracentrifugati on. The isolated H/R-EMVs subjected to TEM revealed small, rounded vesicles (100–1 000 nm) surrounded by a membrane. H/R-EMVs impaired relaxati on induced by ACh of rat thoracic aorti c rings signifi cantly. Compared with H/R-EMVs treatment individually, relaxati on and NO producti on of rat thoracic aorti c rings were increased by Ipt treatment in a concentrati on-dependent manner (P<0.05, P<0.01). The expression of total eNOS (t-eNOS) and total Akt (t-Akt) was not aff ected by Ipt or H/R-EMVs. However, the expression of p-eNOS and p-Akt increased aft er treated with Ipt (P<0.01).Conclusion:Based on H/R-EMVs treatment, ACh induced endothelium-dependent relaxati on of rat thoracic aorti c rings was ameliorated by Ipt in a concentrati on-dependent manner. The mechanisms involved the increase in NO producti on, p-eNOS and p-Akt expression.
Iptakalim; hypoxia/reoxygenati on; endothelial microvesicles; rat aorti c rings; NO; eNOS; Akt
Introduction
Microvesicles (MVs, also known as microparticles) are small vesicles of 0.1~1μm diameters released from cells membranes under various pathological conditions. An increased level of MVs occurred in patients with cardiovascular diseases, diabetes and cancer. Th e membrane proteins of MVs that derived from metrocyte allow them to play an important role in different biological processes. Moreover, MVs carry multifarious bioactive molecules, such as cytokines, mRNA and DNA which mediate cluster of biological effects after taken into target cells [1-2]. Its role in cardiovascular disease has been widely concerned [3-4].
Cardiovascular diseases, such as acute myocardial ischemia, diabetes and atherosclerosis are all leading risk factors of endothelial dysfunction. MVs participate in the process of endothelial dysfunction both as a marker and functional vesicle [5-7]. Hypertension, a primary risk factor for endothelial dysfunction exhibits impairment of vascular relaxation due to theinterruption of the integrity of endothelium and decrease in nitric oxide (NO) production [8]. Iptakalim (Ipt) is a novel drug which works effectively in the treatment of pulmonary arterial hypertension. Ipt promotes the function of endothelial cells through improving the release of nitric oxide (NO), which is the major vasodilatation material, and inhibiting the secretion of vasoconstrictor substance endothelin (ET) [9]. In our previous studies, hypoxia/ reoxygenation (H/R) injury model in vitro to mimic ischemia/reperfusion (I/R) injury model in vivo was established to induce HUVECs to release H/R-EMVs. H/R-EMVs can impair the endothelium dependent relaxation of rat thoracic aortic rings by down-regulating the expression of eNOS.
In this study, Ipt was used to alleviate the impairment of endothelium dependent relaxation of rats thoracic aortic rings aft er incubation of H/R-EMVs, the mechanism may involve in eNOS/Akt pathway.
Materials and Methods
Materials
HUVECs EA.hy926 were purchased from the Cell Bank of Chinese Academy of Sciences. High glucose Dulbecco’s Modified Eagle Medium (DMEM) and fetal bovine serum (FBS) were purchased from Hyclone. Ipt was gift ed from the Academy of Military Medical Sciences, 20131126. Acetylcholine (ACh) and phenylephrine (PE) were purchased from Sigma. NO kits were purchased from Beyotime Institute of Biotechnology. Anti-eNOS, anti-p-eNOS and anti-Akt antibodies were obtained from Cell Signaling Technology. Anti-p-Akt antibody was obtained from Abcam. The Hypoxic Modular Incubator was purchased from Billups-Rothenberg.
Preparation of hypoxia/reoxygenation injury model
HUVECs 1×105were seeded and cultured in 6 wells for 24 h. Normal medium was changed with 2 ml hypoxic buffer of following composition (mmol/L): NaH2PO40.9, NaHCO36.0, CaCl21.0, MgSO41.2, HEPES 20.0, NaCl 98.5, KCl 10.0, sodium lactate 40.0, pH 6.2. Plates were instated into a hypoxic chamber. The gas mixture containing 95% N2and 5% CO2were fi lled into a chamber at a rate of 20 L/ min. The hypoxic chamber was closed after 15 min and was placed into the incubator in 95% N2and 5% CO2condition in 37 °C for 12 h. HUVECs were reoxygenated under standard cell-culture (95% O2and 5% CO2) condition in 37 °C for 4 h.
Preparation of H/R-EMVs
Hypoxic buffer was collected in 15 ml centrifuge tubes and centrifuged at 2 700 g, 4 °C for 20 min to remove cell debris. Most supernatants were collected in 13.2 ml ultracentrifuge tubes and centrifuged at 33 000 r/min for 148 min. The pellet was resuspended in 100 μl PBS and kept at -20 °C for subsequent experiments.
Characterization by transmission electron microscope and protein quantifi cation of H/R-EMVs
40 μl H/R-EMVs samples were dropped on the copper net for 2 min, absorbed excess liquid from the side. And then added 40 μl 2% phosphotungstic acid dyeing for 2 min. After drying under the incandescent light, the net was observed under transmission electron microscope (TEM, HT 7700, HITACHI). Protein quantitative of H/R-EMVs was performed by using a BAC protein assay.
Preparation of thoracic aortic rings of rats treated with Ipt and H/R-EMVs
All animal procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was approved by the Laboratory Animal Care and Use Committee of Tianjin Medical University, Tianjin, People’s Republic of China. Rat thoracic aorta obtained from male Wistar rats (body wt 250±10 g) were carefully removed and placed in Krebs solution of following compositions (mmol/L): NaCl 118; KCl 4.7; KH2PO41.2; MgSO4·7H2O 1.2; CaCl2·2H2O 2.5; NaHCO325 and glucose 10. Aorta was cleared from periadventitial tissue and cut into 3-4 mm rings. Control aortic rings were incubated in DMEM with 10% FBS, while Ipt treated aortic rings were incubated with 10-7-10-3mol/L Ipt respectively and 10 μg/ml H/R-EMVs. On the contrary, H/ R-EMVs treated aortic rings was incubated with 10 μg/ml H/R-EMVs individually. Each group was cultured in the same medium at 37 °C in a 95% O2and 5% CO2atmosphere cell culture incubator for 4 h. Then, aortic rings were placed in 10 ml tissue chambers filled with Krebs solution. Tissue baths were gassed with 95% O2-5% CO2and maintained at 37 °C, pH 7.4. Rings were suspended by two parallel stainless steel wires, and tension was recorded isometrically with a force transducer connected to a computer-based data acquisition system.
Relaxations of thoracic aortic rings of rats induced by ACh
Aortic rings were allowed to equilibrate for 60 min under a basal resting tension of 2.0 g. After the equilibration, aortic rings were treated with phenylephrine (PE, 10-6mol/L) to test their contractility, and then washed for several times with Krebs solution until the tension was restored to a stable level around the baseline. Th e rings were treated with PE again. When they arrived at the stable maximal contractile response which usually took 15 min [10], the relaxations of aortic rings response to ACh at the concentrations of 10-9-10-6mol/L were examined to detect the endothelium- dependent or independent relaxation.
Measurement of NO in thoracic aortic rings of rats
Rat aortic rings stored at -80 °C were put into 4 °C refrigerator to keep freeze in thawing condition and washed by normal saline. Aortic rings in normal saline (W:V=1 g: 9 ml) were homogenated on the ice and then centrifuged at 3 000 r/min for 5 min. Supernatant was collected into Eppendorf tubes for subsequent experiments. Concentrations of NO were measured by the method of Griess Reagent. The final concentrations of NO were calculated by the equations supplied by the kit producer.
Western blot for detecting the expressions of t-eNOS, p-eNOS, t-Akt and p-Akt
Proteins from aortic rings homogenate were extracted with RIPA lysis buff er. Protein lysates were then centrifuged at 12 000 g at 4 °C for 10 min, and the supernatant was collected. Th e harvested proteins were used to perform Western blot analysis. In brief, the samples were electrophoresed on 8% SDSPAGE gel and transferred onto PVDF membranes. The membranes were blocked with 5% skim milk powder solution for 2 h at room temperature and incubated with primary rabbit anti-eNOS (1: 500), anti-phospho-eNOS (Ser-1177) (1: 500), anti-Akt (1:1 000) or anti-phospho-Akt (Ser-473) (1: 1 000) antibodies at 4 °C overnight respectively. After washing for 3 times, membranes were incubated with horseradish peroxidase (HRP) conjugated IgG (1: 1 000) for 2 h at room temperature. Blottings were then developed with enhanced chemiluminescence reagents, and quantification of protein bands was performed using the densitometry with software Quantity One.
Statistical analysis
Data were expressed as mean ± SD. One-way analysis of variance (ANOVA) followed by Dunnett or Bonferroni post hoc test was used. P value less than 0.05 was regarded as statistically significant. Statistical evaluation was performed by using SPSS 18.0 soft ware.
Results
Characterization of H/R-EMVs
The morphology of the H/R-EMVs was observed directly by using TEM, the particles were revealed as round-shaped vesicles with double layer membrane structures, which is diameters from 100 nm to 1 000 nm, without cell debris and other organellar structures (Fig.1).
Effects of Ipt on ACh-induced vasorelaxation
H/R-EMVs decreased the relaxation of rat thoracic aortic rings signifi cantly. Compared with H/R-EMVs group, the relaxation of aortic rings induced by a cumulative increments of ACh was increased by Ipt at the concentrations of 10-6, 10-5, 10-4and 10-3mol/L (Emax: 74.9% ± 1.9%, 81.1% ± 2.7%, 86.2% ± 2.3%, 90.5% ± 1.2% and 66.8% ± 3.6% for 10-6, 10-5, 10-4, 10-3mol/L Ipt groups and H/R-EMVs group, respectively, P<0.05, P<0.01) significantly in a concentrationdependent manner. There was no significant difference between 10-7mol/L Ipt and H/R-EMVs group (Fig.2).
Effects of Ipt on NO production
H/R-EMVs reduced NO production in aortic rings significantly. Compared with H/R-EMVs group, Ipt at the concentrations of 10-6, 10-5, 10-4and 10-3mol/L increased the NO production in aortic rings significantly in a concentration-dependent manner (P<0.01). Th ere was no signifi cant diff erence between 10-7mol/L Ipt and H/R-EMVs group (Fig.3).
Effects of Ipt on p-Akt, t-Akt, p-eNOS and t-eNOS expression
H/R-EMVs decreased the expressions of p-Akt-Ser-473 and p–eNOS-Ser-1177 and did not affect the expression of t-eNOS of aortic rings. Compared with H/R-EMVs group, there was no significant difference between 10-4mol/L Ipt group and H/ R-EMVs group on the expressions of t-Akt and t-eNOS, but the expressions of p-Akt-Ser-473 andp-eNOS-Ser-1177 were increased in the 10-4mol/L Ipt group signifi cantly (P<0.01). Th e ratio of p-Akt/ t-Akt (Fig.4A, 4B) and p-eNOS/t-eNOS (Fig.4C, 4D) were also increased (P<0.01).
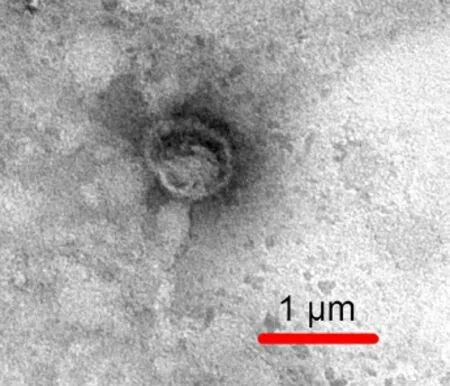
Fig. 1 Characterization of H/R-EMVs. H/R-EMVs observed by transmission electron microscope.

Fig. 2 Effects of Ipt and H/R-EMVs on endothelium-dependent relaxation of thoracic aortic rings of rats. ACh-induced relaxation of thoracic aortic rings of rats (n = 5) after incubated with Ipt and H/R-EMVs jointly or H/R-EMVs individually for 4 h. Values are means ± SD,**P<0.01 vs control;&&P<0.01 vs 10 μg/ml H/R-EMVs;##P<0.05 vs 10-7mol/L Ipt;$P<0.05,$$P<0.01 vs 10-6mol/L Ipt;@@P<0.01 vs 10-5mol/L Ipt.
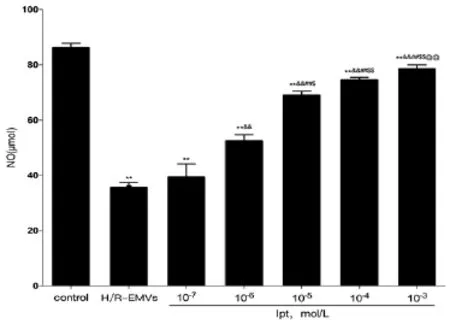
Fig. 3 Nitric oxide (NO) production in thoracic aortic rings of rats (n = 5) after incubated with Ipt based on H/R-EMVs or H/R-EMVs individually for 4 hours. Values are means ± SD,**P<0.01 vs control;&&P<0.01 vs 10 μg/ml H/R-EMVs;##P<0.05 vs 10-7mol/L Ipt;$P<0.05,$$P<0.01 vs 10-6mol/L Ipt;@@P<0.01 vs 10-5mol/L Ipt.
Discussion
EMVs involve vital function on the pathogenesis of vascular endothelial dysfunction, which closely linked to angiocardiopathy. Ipt has been identified as a new anti-hypertensive drug to improve vascular endothelial function. However, whether Ipt can alleviate the injury of vascular endothelial function caused by H/R-EMV has not been elucidated. The results in our study indicated that Ipt could ameliorate the relaxation of thoracic aorta impaired by H/R-EMVs in a concentration-dependent manner. The underlying mechanism involved an increased NO production as well as activation of Akt/eNOS pathway accompanying with p-AKT, p-eNOS upregulation.
EMVs, which are shed from endothelial cell aft er oxidative stress, infl ammation, hypoxia, could infl uence the endothelial function conversely. Endothelial dependent vasorelaxation is the most intuitionistic index of tissue injury to determine the restoring vascular endothelial injury by Ipt. In our previous study, the results indicated that H/R-EMVs induced by H/ R model to mimic I/R injury impaired the relaxation function of rat thoracic aorta rings [11]. However, there is no study on the prevention of impaired vascular relaxation function caused by H/R-EMVs. Ipt, as a novel anti-hypertensive drug, can antagonize hypoxia damage and protect the endothelial under hypoxia condition [12-13]. In this study, H/R-EMVs from HUVECs were isolated by ultracentrifugation, and H/R-EMVs were vesicle structures which in diameter between 0.1-1μm detected by TEM. Rat aortic rings were incubated with Ipt and H/R-EMVs. Th e results indicate that Ipt alleviates impairment of H/R-EMVs (10 μg/ml) on the relaxation of rat aortic rings in a superior level of concentration and reveal a concentration-dependent manner, which draw the conclusion that Ipt can alleviate the vascular endothelial dysfunction caused by H/R-EMVs.
NO, the main endothelium derived relaxing factor (EDRF), is crucial for maintaining the vascular endothelial functions. It was reported that I/R inju-ry reduced the expression of NO, which caused the dysfunction of vascular endothelial function [14]. Research suggests that both Ipt and MVs can impact the NO production [15-16]. We measured the NO production in rat aortic rings homogenate by Griess Reagent Assay. Our records showed that Ipt improved the expression of NO in rat aortic rings in a concentration-dependent manner mediated by ACh. Th e results proved that Ipt could alleviate the injury of vascular endothelial function caused by H/R-EMVs.
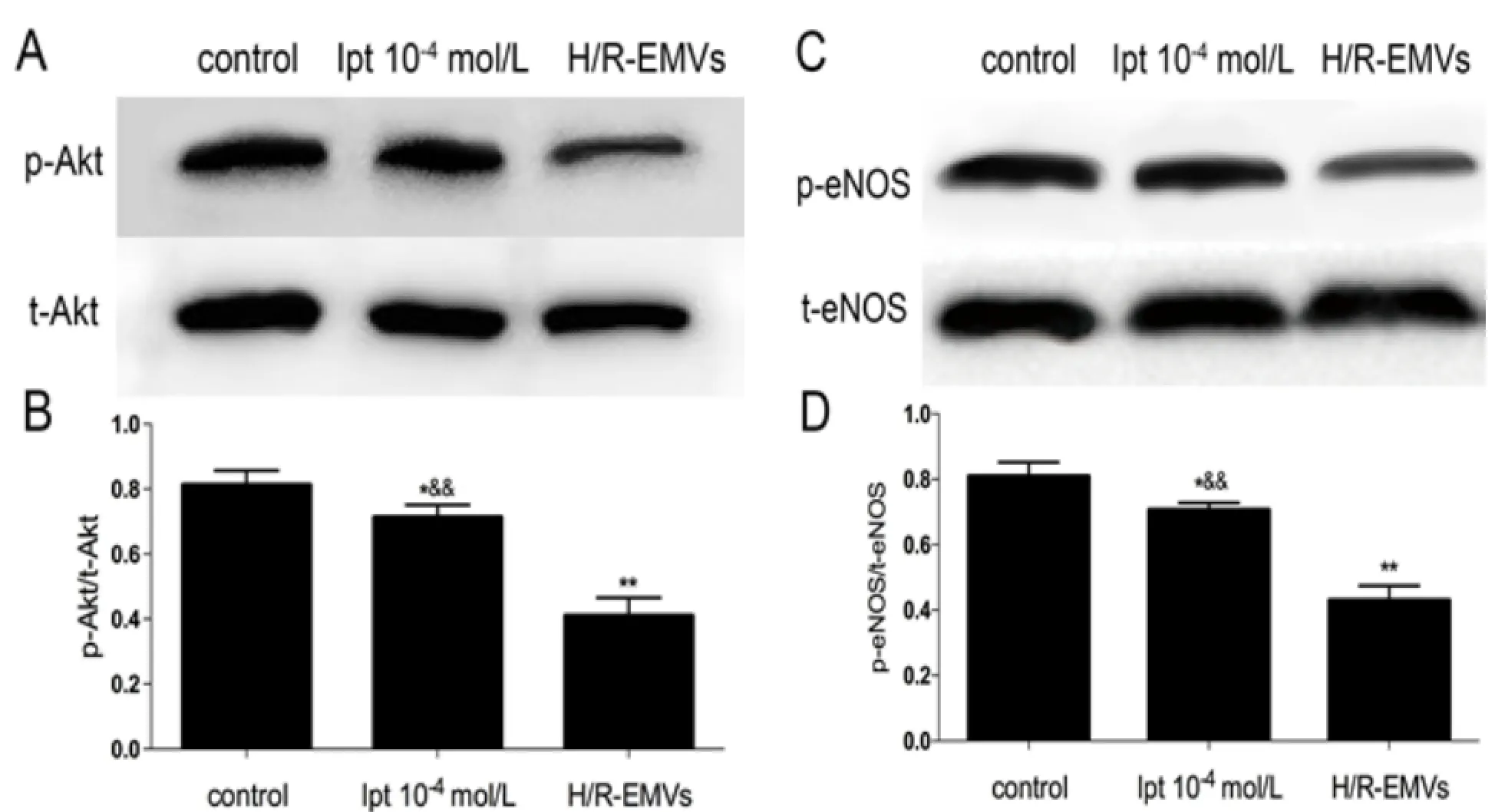
Fig. 4 t-Akt, p-Akt (Ser-473) and t-eNOS, p-eNOS (Ser-1177) expressions in thoracic aortic rings of rats (n = 5) after incubated with Ipt based on H/R-EMVs or H/R-EMVs individually for 4 hours. A: t-Akt and p-Akt (Ser-473) expression in thoracic aortic rings of rats (n = 10) was assayed by Western blotting. B: Relative densitometry of the expression of p-Akt (Ser-473) to t-Akt. C: t-eNOS and p-eNOS (Ser-1177) expression in thoracic aortic rings of rats (n = 10) was assayed by Western blotting. D: Relative densitometry of the expression of p-eNOS (Ser-1177) to t-eNOS. Data are representative of three independent experiments, values are means ± SD. *P<0.05, **P<0.01 vs control;&&P <0.01 vs H/R-EMVs.
Akt, as a vital signaling on apoptosis, proliferation, differentiation and metabolism, plays a crucial role in signal transduction of life processing. eNOS/Akt is a classical signal pathway mediating the level of NO. Akt, which phosphorylated by the phosphatidylinositol-3-kinase, can phosphorylate eNOS and release NO. It is confi rmed that H/R-EMVs can reduce the expression of NO through down-regulating the phosphorylation of eNOS [9]. Wu et al. have identified that Ipt could up-regulate the phosphorylation level of Akt/eNOS [17]. In this study, we found that Ipt up-regulated the phosphorylation of Akt (Ser-473) and eNOS (Ser-1177), while there was no signifi cant difference between total Akt and eNOS. It showed that the protection of Ipt was related to the Akt/ eNOS pathway.
In conclusion, our findings indicate that Ipt can ameliorate the relaxation of rat aortic rings through Akt/eNOS/NO pathways from damaging by H/ R-EMVs. The underlying mechanism involves the up-regulation of expression of NO, p-eNOS and p-Akt. The study indicates that H/R-EMVs should be taken into consideration in the pathogenesis of endothelial dysfunction, which provides a new target for the treatment of cardiovascular diseases accompanying with endothelial dysfunction. Ipt, which is an anti-hypertension effectively ameliorate the dysfunction of endothelial cells caused by H/R-EMVs also off er a method for the clinical treatments of hypertension and some other cardiovascular diseases. Further study should be conducted to clarify how the Ipt interact with H/R-EMVs since H/R-EMVs may also serve as a carrier in circulation which can deliver drugs, chemicals and biomolecules into target cells to activate a cascade biology process.
Acknowledgements
Th is work was supported by the Specialized Research Fund for the Doctoral Program of Higher Education of China (20101202110005), the Natural Science Foundation of Tianjin (11JCZDJC18300) and theResearch Foundation of Tianjin Municipal Education Commission (20110106).
1. Laresche C, Pelletier F, Garnache-Ottou F, et al. Increased levels of circulating microparticles are associated with increased procoagulant activity in patients with cutaneous malignant melanoma [J]. J Invest Dermatol, 2014, 134(1): 176-182.
2. Caporali A, Miscianinov V, Saif J, et al. MicroRNA transport in cardiovascular complication of diabetes [J]. Biochim Biophys Acta, 2016, 1861(12 pt B): 2111-2120.
3. Badimon L, Suades R, Fuentes E, et al. Role of platelet-derived microvesicles as crosstalk mediators in atherothrombosis and future pharmacology targets: a link between inflammation, atherosclerosis, and thrombosis [J]. Front Pharmacol, 2016, 7: 1-17.
4. Lawson C, Vicencio JM, Yellon DM, et al. Microvesicles and exosomes: New players in metabolic and cardiovascular disease [J]. J Endocrinol, 2016, 228(2): R57-R71.
5. Zhang Q, Shang M, Zhang M, et al. Microvesicles derived from hypoxia/reoxygenation-treated human umbilical vein endothelial cells promote apoptosis and oxidative stress in H9c2 cardiomyocytes [J]. BMC Cell Biol, 2016, 17(1): 25.
6. Miller VM, Lahr BD, Bailey KR, et al. Specifi c cell-derived microvesicles: Linking endothelial function to carotid artery intima-media thickness in low cardiovascular risk menopausal women [J]. Atherosclerosis, 2016, 246: 21-28.
7. Larson MC, Hillery CA, Hogg N. Circulating membrane-derived microvesicles in redox biology [J]. Free Radic Biol Med , 2014, 73(3): 214-228.
8. Chen JY, An R, Liu ZJ, et al. Therapeutic effects of mesenchymal stem cell-derived microvesicles on pulmonary arterial hypertension in rats [J]. Acta Pharmacol Sin, 2014, 35(9): 1121-1128.
9. Zhu R, Bi LQ, Wu SL, et al. Iptakalim attenuates hypoxia-induced pulmonary arterial hypertension in rats by endothelial function protection [J]. Mol Med Rep, 2015, 12(2): 2945-2952.
10. Yilmaz B, Usta C. Ellagic acid-induced endothelium-dependent and endothelium-independent vasorelaxation in rat thoracic aortic rings and the underlying mechanism [J]. Phyther Res, 2013, 27(2): 285-289.
11. Wang SX, Zhang Q, Shang M, et al. Microvesicles derived from hypoxia/reoxygenation-treated human umbilical vein endothelial cells impair relaxation of rat thoracic aortic rings [J]. Chin J Appl Physiol, 2014, 30(6): 560-566.
12. Huang JH, Han WZ, Jin X, et al. Th e selective dilatation eff ects of iptakalim on basilar and pulmonary arterioles in high-altitude hypoxic rats [J]. Chin J Appl Physiol, 2014, 30(1): 1-3.
13. Wang SY, Cui WY, Wang H. Th e new antihypertensive drug iptakalim activates ATP-sensitive potassium channels in the endothelium of resistance blood vessels [J]. Acta Pharmacol Sin, 2015, 36(12): 1444-1450. 14. Guo X, Cao W, Yao J, et al. Cardioprotective eff ects of tilianin in rat myocardial ischemia-reperfusion injury [J]. Mol Med Rep, 2015, 11(3): 2227-2233.
15. Zong F, Zuo XR, Wang Q, et al. Iptakalim rescues human pulmonary artery endothelial cells from hypoxia-induced nitric oxide system dysfunction [J]. Exp Th er Med, 2012, 3(3): 535-539.
16. Burger D, Turner M, Munkonda MN, et al. Endothelial microparticle-derived reactive oxygen species: role in endothelial signaling and vascular function [J]. Oxid Med Cell Longev, 2016, 2016: 12-15.
17. Wu Y, He MY, Ye JK, et al. Activation of ATP-sensitive potassium channels facilitates the function of human endothelial colony-forming cells via Ca2+/ Akt/eNOS pathway [J]. J Cell Mol Med, 2016. doi: 10.1111.15 /jcmm.13006. http://www.ncbi.nlm.nih. gov/pubmed/27709781.
doi 10.13459/j.cnki.cjap.2016.06.001
Yan-xia LIU, MD, PhD, Professor, Junqiu SONG, MD, PhD, Associate Professor, Department of Pharmacology, School of Basic Medical Sciences, Tianjin Medical University, 22 Qixiangtai Road, Heping District, Tianjin 300070, China. Tel/Fax: 86-22-83336660; E-mail: liu_yanxia126@126. com; song_junqiu@126.com.
2016-11-09; accepted 2016-11-21
杂志排行
中国应用生理学杂志的其它文章
- 5-HT1B受体亚型对小脑顶核介导的运动行为的影响*
- Association study between the angiotensin converting enzyme gene insertion/deletion polymorphism and Qinghai Han Chinese with congenital heart disease
- The infl uence of heterogeneity on the analysis of sleep-wake architecture in the single-prolonged stress rats
- Effect of creatine phosphate sodium on miRNA378, miRNA378* and calumenin mRNA in adriamycin-injured cardiomyocytes
- Changes of microcirculation in healthy volunteers and patients with septic shock in Xining
- 当归黄芪提取物对慢性腹膜功能衰竭大鼠腹膜功能、结构及TGF-β1表达的影响*
