Preparation and Lubricating Properties of A New Antibacterial Emulsion Containing Nano-TiO2for Cold Rolling Strips
2016-03-22
(School of Materials Science and Engineering, University of Science and Technology Beijing 100083)
Preparation and Lubricating Properties of A New Antibacterial Emulsion Containing Nano-TiO2for Cold Rolling Strips
Lu Yudi; Sun Jianlin; Zhang Bingtao
(School of Materials Science and Engineering, University of Science and Technology Beijing 100083)
A new kind of emulsion containing nano TiO2was developed through the dispersion experiment. A commercial emulsion and a prepared by our lab emulsion without nano particles were chosen as controls to test the tribological and antibacterial properties of this new emulsion. The load carrying capacity, friction coeffcient and average diameter of wear scars were tested by a four-ball machine and the comprehensive antifriction parameterωwas calculated. The wetting angle was also tested using a JC200C1 wetting angle tester. The micro surface and roughness of rolled strips were analyzed to investigate the tribological performance of the recommended new emulsion in strip production. It is shown that the new nano-emulsion possesses a higher load carrying capacity and wetting ability. Therefore the abrasive/plowing wear is reduced more effciently with the addition of nano particles, and the micro surface is improved. The density of bacteria in the emulsions was tested after the cold rolling experiment. The emulsion breaking ratio and bacteria density were also tested in different time intervals after the cold rolling experiment. The fnal pH values and bacteria density of different layers of emulsions were measured and the sediment was analyzed by TEM to evaluate the antibacterial behavior of this new emulsion. It is shown that the density of microbial colonies which led to a corruption of emulsions was decreased about 90% and the effective antibacterial period was prolonged.
nano TiO2; dispersion; emulsion, lubricant, antibacterial
1 Introduction
During the production of cold rolling strips, emulsions are common lubricants which not only can dissipate the heat produced by plastic deformation, but also reduce the rolling force and the energy consumption[1]. And the oil-phase of emulsions will be separated and reshaped as a lubricating flm on the cold rolling strips to improve the quality of rolling surface[2]. When it is used as a recycling lubricant, its working environment changes and microorganisms show an essential influence on the property of emulsion such as the concentration, pH value and ash content. Firstly, aerobiont decomposes the oilphase of emulsion during reproducing[3], and the lubricant property will worsen because of the decomposition of long carbon chain organic compounds into short carbon chain compounds. Then anaerobion such as the sulfate reducing bacteria can decompose the emulsion and release hydrogen sulfde which can cause the further deterioration of emulsion[4]. Together with the release of irritant gas, the pH value of emulsion decreases which increases the corrosion ability to transfer more iron powder into the emulsion as wear particles. The use of bactericide and adjustment of pH value are representative ways of anticorrosion. However, the composite bactericides are required to implement effective antibacterial measures[5], which may increase the cost of cold rolling strip production and waste liquid treatment. So it is required to fnd out a new kind of antibacterial emulsion which can inhibit the growth of bacteria in a long term and possess stable lubricant properties.
Nanoparticles, as a kind of new lubricant additive which possesses reliable properties such as the quantum-size effect, the small size effect, and the surface effect[6], have aroused a marked attention because of their special behavior in wear resistance, load carrying capacity and anti-friction ability[7-9]. Nano TiO2is one of those typical fne powder which has controllable particle size,nontoxic property, and extreme pressure lubrication performance[10-12]. Since 1985, Matsunaga[13]had found out that microbial cells including saccharomycetes and colibacillus could be sterilized by semiconductor powder through photocatalysis. The photocatalysts, in particular nano TiO2, have been paid more attention to function as bactericide thanks to their economical, effcient and stable properties[14]. Further researches have shown that the antibacterial property of nano TiO2could take effect under weak ultraviolet radiation[15], and the mixed powder of anatase and rutile presents a better photocatalysis property than any single crystal form of TiO2[16-17]. It has also been shown that the average particle size, concentration and element doping can influence the antibacterial ability signifcantly[18-20].
In the present work, a cold rolling emulsion was prepared and nano TiO2particles were dispersed in it while keeping the average particle size in a nanometer range through the adjustment of pH value. The tribological properties of emulsion were tested and compared with a commercial emulsion and an emulsion prepared by our lab without addition of nano particles. The antibacterial property was investigated and compared based on the cold rolling experimental results in more detail.
2 Experimental
2.1 Preparation of cold rolling emulsion
Mineral oil N15 was chosen as the base oil with its properties shown in Table 1.

Table 1 Properties of mineral oil N15
Span-60 was chosen as the emulsifier on account of it stability and antifoaming property. Sulfoaliphatic acid ester was chosen as the extreme-pressure additive, and sodium alkylsulfonate was chosen as the rust protection agent. The nano TiO2particles with a mean diameter of 20 nm, which were provided by the Degussa AG of Germany, were used in experiments. Glycol and SHMP were chosen as the dispersants. Figure 1 shows the process for preparation of the new developed emulsifed oil. Finally, the emulsifed oil with nano TiO2was added into the deionized water under stirring for 10 minutes at 25 ℃.

Figure 1 Preparation of emulsi fi ed oil containing nano TiO2
2.2 Dispersion experiment
The chemical reagent used in the pH adjustment was the diluted hydrochloric acid and calcium carbonate provided by the Sinopharm Chemical Reagent Co, Ltd. A pH meter was used to determine the pH value of solution. In order to find out the best pH value of dispersion, six samples were researched with the pH value varying from an acidic environment to an alkaline environment. Each sample was measured three times with the average value adopted. A WR-16IAQ zeta-meter made by the Malven Instruments Ltd. was used to determine the diameter and zeta potential of nano TiO2particles in emulsions. Each sample was measured twelve times with the average value adopted.
2.3 Tribological experiments
In order to evaluate the tribological properties of the new antibacterial emulsion, code-named as E-nano, which possessed the best degree of dispersion, two other emulsions were tested as controls. One was made from the emulsified oil and deionized water without using nano TiO2as the additive according to the procedure shown in Figure 1, and was code-named as E-none. The other was made from the commercial emulsifed oil and deionized water, code-named as E-commercial. All three emulsions had the same volume concentration and pH values determined through proper adjustment and were tested under the same condition.
The tribological parameters of the emulsions were tested on a four-ball tribological tester according to the test method GB/T 12583—1998. The load carrying capacity and wear scar diameter of steel balls were analyzed in order to evaluate the anti-friction properties of the selfprepared emulsion. The wear scars were investigated by a Zeiss AX10 optical microscope.
2.4 Cold rolling experiments
The wetting angle on a flat clean Q235 base slab was tested before cold rolling using the JC200C1 wetting angle tester at a temperature of about 25 ℃. Each wetting angle was measured 5 times to minimize errors.
The rolling experiment was tested using a Φ40/170 mm 4-Hi reversing mill. The roughness measurement and micro surface observation were studied using an Olympus OLS400 laser scanning confocal microscope. Strips used in the experiments had the dimension covering a thickness of 1.2 mm, a length of 300 mm, and a width of 100 mm.
2.5 Antibacterial experiments
The estimation of total bacterial density count was performed immediately after the cold rolling experiment using an Easicult dip slide tester featuring good effciency and accuracy[21]. The Easicult TTC dip tester was provided by the Orion Diagnositica Company of Finland. Each emulsion was tested three times with the results compared with the model chart shown in Figure 2, and the samples to be inspected by the dip slide tester were labeled and stored at room temperature in a dry place for 48 hours before testing.
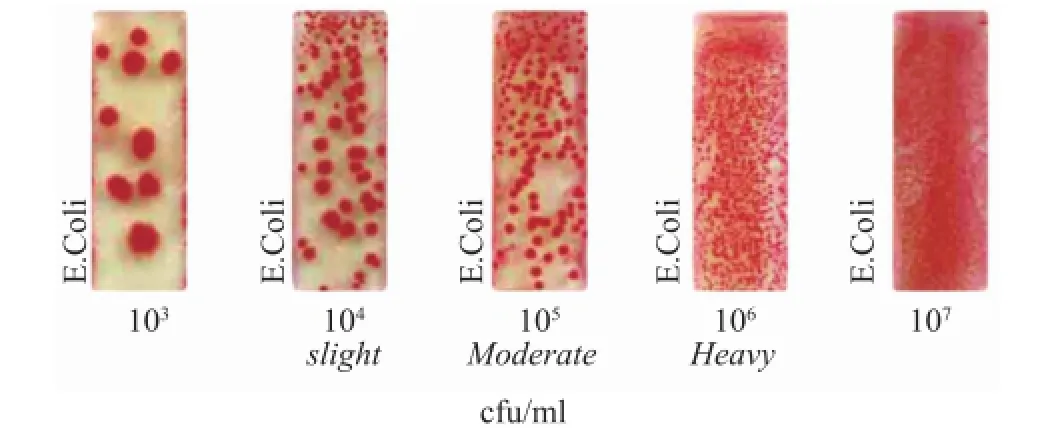
Figure 2 Model density chart of microbial count in powers of ten
The rolled emulsions were stored in test tubes which were sterilized at 121 ℃ for 30 minutes, and the total bacterial count was checked on the SW-CJ-1D clean bench after 1 day, 3 days, 6 days, 10 days, and 14 days, respectively. The pH values of emulsions were tested after 14 days of storage, and the sediment of nano emulsion was analyzed by a Tecnai G2 F20 TEM. The instruments used were sterilized to reduce errors.
3 Results and Discussion
3.1 Effect of pH value on dispersion of nano TiO2
The nano TiO2particles in this experiment had a mean diameter of 20 nm, which possessed high surface energy and tended to aggregate in the emulsion. During the adjustment, the pH value change of each emulsion is shown in Table 2, and each sample should have the same concentration of nanoparticles and dispersant.
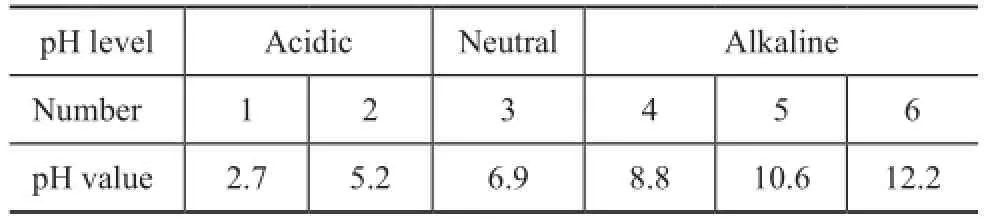
Table 2 pH value of emulsions containing nano TiO2
The DLVO theory has expressed the nature of dispersion[22], and the energy barrier arises because of the van der Waals force and the double layer electrical forces. Upon being wetted in the solution, the surface of nanoparticles becomes complex by the reaction between the surface groups and the base-fuid, which can be expressed by the Stern model[23]. The dispersion behavior of nano TiO2can be markedly different due to the electrostatic effect, the van der Waals force, and the structure. The pH value plays an important role on it. Figure 3 shows the average size and zeta potential of nanoparticles in emulsions with different pH values.

Figure 3 Average size and zeta potential of nanoparticles with different pH values
In comparison with the sample having a pH value of 6.9, those samples in acidic environment possess higher concentration of hydrogen ions, thus the nano TiO2particles, the surface of which is electronegative in nature, are surrounded with higher concentration of counterions. The electric potential of Stern plane (zeta potential) will decrease because the entry of more counterions into the Stern layer.
Based on the DLVO theory, the stability of nano TiO2suspension is maintained due to the magnitude of electrostatic repulsion and van der Waals attractive force. If the electrostatic repulsion of particles is lower than the van der Waals force, particles tend to aggregation through the Brownian motion. So the dispersion of nano TiO2becomes better with the increase of absolute value of zeta potential, which can take place due to the increase of pH value till 10.6, and the average size of particles may reach 234 nm.
3.2 Antifriction ability of new emulsion with nano TiO2
In order to make sure that the self-prepared emulsion containing nano TiO2particles possessed lubricant property that could meet the requirements of real production, the tribological parameters of E-nano, E-commercial and E-none were tested through a 30-minute friction experiment, with the results shown in Table 3. It can be found that the load carrying capacity increased with the adding of nano TiO2, which was by about 5% higher than E-commercial and by 11% higher than E-none. However, with E-commercial used as the lubricant, steel balls possessed the smallest wear scar diameter. Upon considering that the antifriction ability depends on both the friction dimension and the load carrying capacity, the parameterωis put forward[24],

wherePBis the maximum non-seizure load,Dis the average diameter of wear scar. It can be concluded that the antifriction ability increases with the value of parameterω. Thus the antifriction ability of E-nano is better than E-commercial, and both of them are better than E-none.
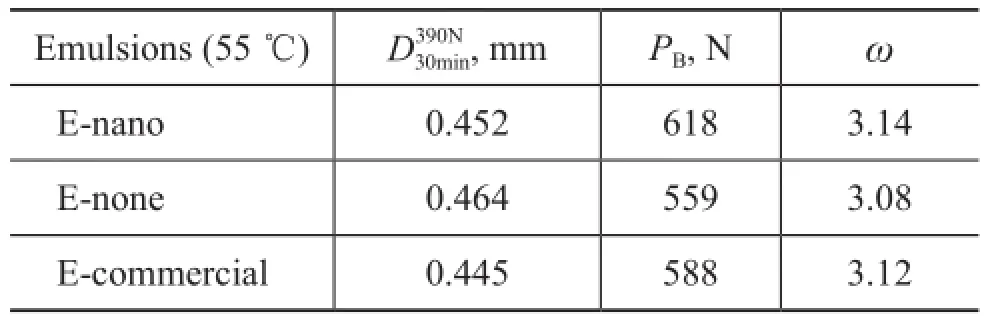
Table 3 Tribological parameters of emulsions
3.3 Cold rolling experiment for testing emulsions
Before the cold rolling experiments, the wetting angle and viscosity of three emulsions (E-nano, E-none, and E-commercial) were tested. Figure 4 shows the wetting performance and the value of wetting angle.

Figure 4 Wetting performance of emulsions on Q235 base slab
With the addition of nano particles, the electric interaction and steric effect between nano TiO2and polymer molecules have hindered the movement of base fuid. However, the free energy of emulsion decreased a lot, because of the strong unordered and random Brownian motion of nano particles. Thus the wetting angle decreased with the addition of nano particles. The wetting angle denotes the wetting ability of emulsion while it is used as a lubricating oil, and emulsions with better wetting ability are easier to reshape as lubricant flm which can reduce the friction and wear defects of surface. The micrographs of surface of rolled strips are shown in Figure 5.
Defects such as scar and plowing could be seen on the micro surface of samples, which were distributed randomly. More black spots could be seen on the surface of strip when the E-none was used as the lubricant, indicating to the failure of lubrication. The heat released by friction and deformation had caused extreme heating in the deformation zone, which could break the lubricant film and lead to an adhesive wear through the direct contact of roller and strip. The same kind of wear happened on the strip when E-commercial was used as the lubricant too, and the particles that were separated from the surface caused the abrasive wear, which could be identified as plowings. Those main types of wear occurred on the sur-face with E-nano used as the lubricant, but the wear was slighter. And the metal structure was elongated along the rolling direction more obviously, because of the minimum rolling gauge shown in Table 4, in which the roughness of micro surface is presented too.
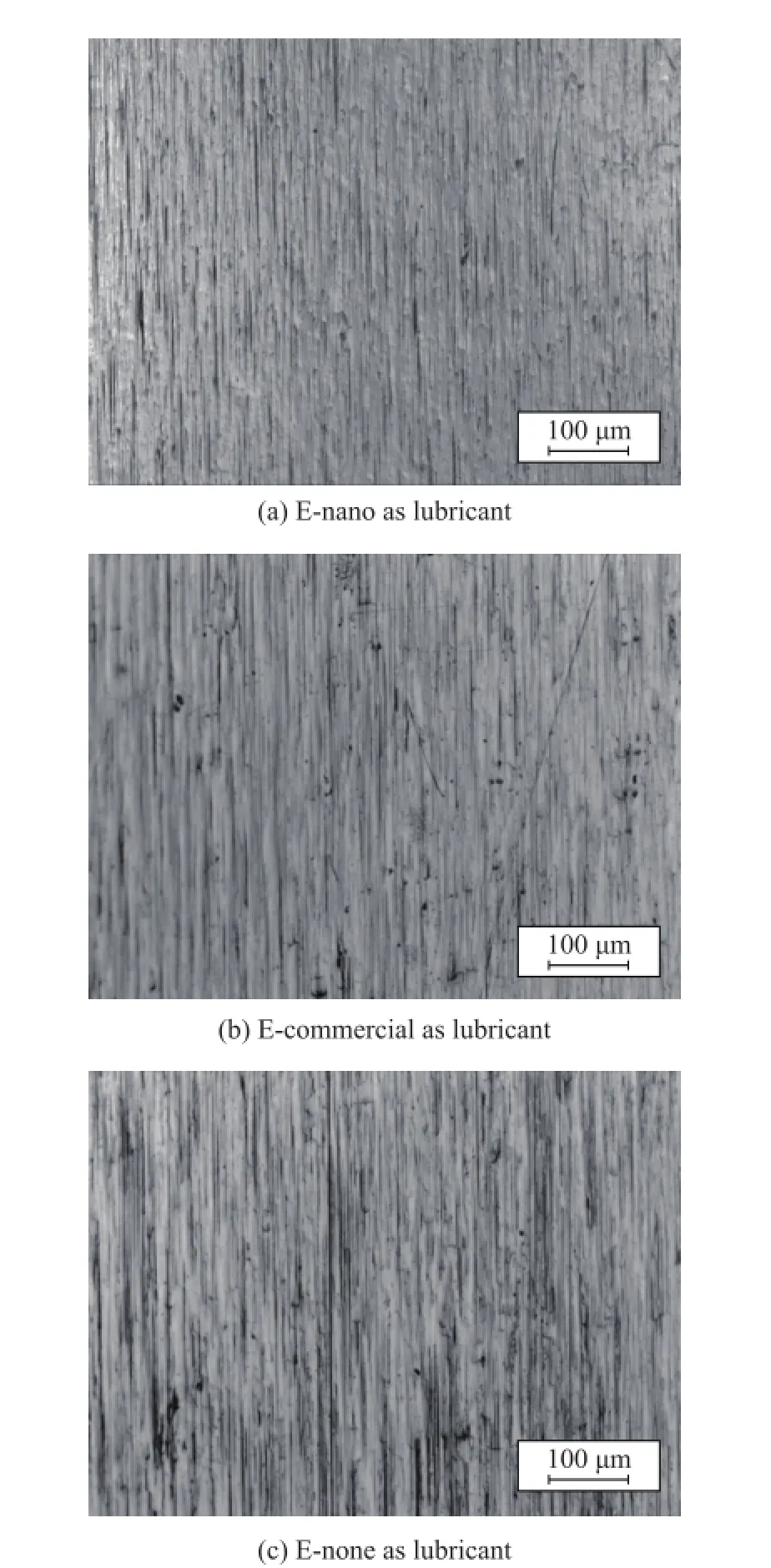
Figure 5 Optical photographs of cold rolled strips

Table 4 Minimum thickness and surface roughness of cold rolled strips
The addition of nano TiO2had increased the extreme pressure of emulsion, while the abrasive wear was alleviated and less wear particles were separated from the lubricant. Then, the abrasive wear was alleviated too, because the mean diameter of nano particles dispersed in the emulsion was smaller than the average roughness of surface, which could refll the defects. The strip rolled with E-nano serving as the lubricant possessed a thickness of minimum rolling gauge, which indicated that the largest grain deformation along the rolling direction was based on the constant-volume principle.
3.4 Antibacterial properties of emulsion with nano TiO2
The cold rolling experiments were conducted with the lubrication provided by three emulsions in the order of E-none, E-commercial and E-nano. Compared with the model chart in Figure 2, the density of bacteria in powers of ten was counted. The photograph of bacterial colonies and results of count are shown in Figure 6.

Figure 6 Photograph and bacteria density count of E-nano (a), E-commercial (b), and E-none (c)
Compared with the model density chart, the E-none emulsion had indicated the approximate microbial density while cold rolling was performed without any addition of bactericide. And the E-commercial emulsion containing the bactericide had decreased the density of bacteria by one order of magnitude. The E-nano emulsion demonstrated its antibacterial performance on reducing the density of bacteria by one order of magnitude too. However, the bacterial colonies on the tester were bigger than those in E-commercial groups, denoting that these bacterial colonies might be the fusion of smaller ones. Thus the number of bacterial colonies on the tester could be morethan the value counted although it remained at the same order of magnitude.
Since the duration of antibacterial ability can indicate the lifetime of emulsions directly, the density of bacteria and the emulsion breaking ratio were tested across 1 day, 3 days, 6 days 10, and 14 days, respectively, after cold rolling experiments. The emulsion breaking ratio is defned as
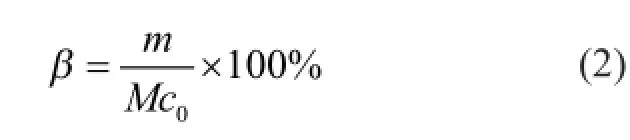
wheremis the mass of oil phase on the top of emulsion,Mis the mass of emulsion, andc0is the mass fraction of base oil in emulsion before cold rolling experiment. Figure 7 shows the behavior of microbial multiplication and emulsion breaking ratio in different time. The antifriction parameter ω based on Equation (1) after 14-day storage is shown in Figure 7 (b) too. Compared with the samples before storage, the antifriction ability of all three emulsions decreased. However, there was very minor difference in antifriction ability between E-nano and E-commercial although E-nano was better. Through fourteen days of storage after cold rolling experiment, the E-commercial emulsion and E-none emulsion remained stable basically, and a little sediment could be seen at the bottom of E-nano emulsion.

Figure 7 Bacterial density, emulsion breaking ratio and fi nal antifriction ability of tested emulsions in two weeks
As for E-none, the density of bacteria increased after four days, which presented an explosive growth to reach 107CFU/ml and then began to decrease after the tenth day. The emulsion breaking ratio showed the same trend, and the rate of emulsion breaking which was represented by the slope of curve began to increase extremely and reached a maximum near the sixth day. However, after the tenth day of cold rolling experiment, both the density of bacteria colonies and the rate of emulsion breaking declined. At the incipient stage of bacterial reproduction, the bacterial density increased explosively and the metabolism was vigorous so that the rate of oil decomposition and emulsion breaking were very fast. And then, with the consumption of nutriments and accumulation of toxic metabolites, the cell activity was restrained.
As regards the E-nano emulsion and E-commercial emulsion, the density of microbial colonies remained stable in the first six days. And in the E-commercial emulsion, the bacterial level even showed a decreasing trend, as the microbial colonies became smaller following the testing in the third day. However, the density of microbial colonies increased in the next few days and reached a 106order of magnitude, as compared with the 105order of magnitude in the E-nano emulsion. Upon considering Figure 7 (a) and (b), the sixth day for storing the emulsion was a dividing line, in which the density of microbial colonies exploded and the emulsion breaking started both in E-commercial and E-nano samples. The ratio of emulsion breaking reached 50.7% in the E-none emulsion, when the fluid was divided into two phases accompanied with the release of a foul stench of rotten eggs. With the lapse of time, the emulsion breaking ratio of E-commercial caught up with and surpassed that of E-nano emulsion and reached 32.7% ultimately.
To investigate the self-reacting antibacterial behavior of E-nano emulsion, the bacteria density of different layers were tested with the results shown in Table 5 using E-commercial as the control.

Table 5 Bacteria density in different layer of emulsions
In the E-commercial emulsion, the bacteria density was equal in every layer. And in the E-nano emulsion, the bacteria density in the bottom layer was much larger than the other layers. The distribution of bacteria is decreasing from the bottom to the top.
It is known that theBacillus subtilisis the main bacterium that can decompose the mineral oil[25], and the surface ofBacillus subtilisis provided with positive charges[26]. And since in the micro structure of TiO2crystal, the chemical bonds between titanium and oxygen atoms are in unequal length, thus the hydrone can easily absorb and dissociate out hydroxyls. In the alkaline environment, the reaction on the surface of TiO2can be explained as follows:

So the bacteria are attracted because the surface of TiO2is electronegative, and the reproduction is limited. At the same time, the hydrolysis reaction happens when the SHMP is contained in the emulsion, which can be explained as follows:

The pH value of E-commercial was identifed to be 5.2, because the metabolites of bacteria rendered the emulsion acidic. According to Equation 4, the reaction tends to move along the direction of hydrolysis, so the addition of SHMP not only can disperse nano particles but also remain in the alkaline environment of emulsions, which can restrict the growth of bacteria too.
The photocatalytic activity of nano TiO2has also contributed to the antibacterial ability, although the cold rolling was conducted in a sunlight environment, with weak ultraviolet irradiation. The micro structure and elemental analysis of the sediments in E-nano emulsion is shown in Figure 8, as evidenced by the TEM observation. It can be seen from Figure 8 that black balls (particles containing iron) were mixed and agglomerated with white balls (TiO2particles), as verifed by the elemental analysis. It is known that Fe3+ions can inhibit the hole-electron recombination, acting as the photogenerated hole (h+) and the electron (e-) trap[27], which will accelerate the electron transfer and enhance the photocatalytic activity of nano TiO2. The antibacterial behavior of photocatalytic TiO2can be explained as[28]follows:

Figure 8 Micro structure (a) and elemental analysis (b) of sediments in E-nano emulsion
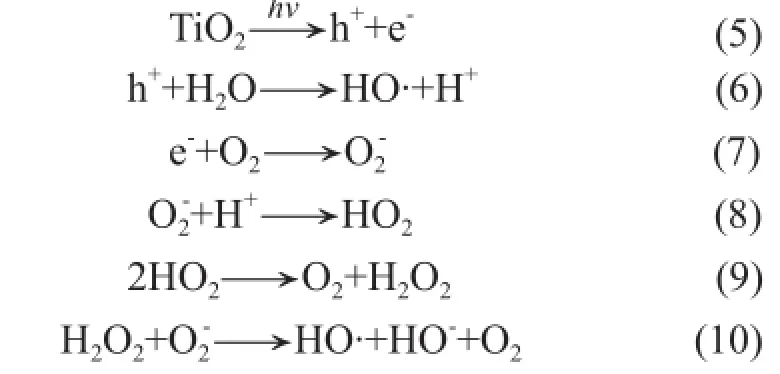
The water molecule and oxygen gas molecule reacted with photogenerated hole (h+) and an electron (e-) of TiO2on the surface of particles, and the end product hydroxyl radical (OH·) could degrade organics into carbon dioxide and water so that the reproduction of bacteria was restricted.
4 Conclusions
Nano TiO2particles upon being dispersed in the new developed emulsion stably retains an average size of 234 nm, and the pH value plays an important role on the dispersion of nano TiO2through changing the thickness of flm surrounding the solid spheres, which also changes the zeta potential of particles.
Compared with a commercial emulsion, the new developed emulsion possesses a better tribological property mainly in terms of the load carrying capacity, the wetting ability and the antifriction properties. The micro surface of rolled strips is improved.
As regards this new emulsion containing nano TiO2, the density of microbial colonies is decreased and the effective antibacterial duration is prolonged as compared with the traditional emulsion containing chemical bactericide. The surface of nano TiO2is electronegative in nature which can attract bacteria leading to emulsionbreaking and the worn particles containing iron species are transferred into the emulsion which can contribute to the photocatalytic activity of TiO2under weak ultraviolet radiation to enhance the antibacterial ability of photocatalytic TiO2.Also, this new antibacterial emulsion can maintain the pH value through its self-reacting adjustment, which ensures the stability and property of the lubricant system.
Acknowledgement: This work was supported by the National Natural Science Foundation of China (contract/grant number: 51274037) affliated to the project: “The research of lubrication model and interaction between nano-lubricating particles and rolling deformed surface.”
[1] Sun J, Wang Y, Rui L, et al. Investigation on relationship between lubricating performance and stability of emulsion for cold strip rolling[J]. China Petroleum Processing & Petrochemical Technology, 2010, 12(3): 54-58
[2] Wang Y, Dai E, Zhuang X, et al. Research on lubrication behavior and mechanism of nano-copper used in emulsions for strip cold rolling[J]. China Petroleum Processing & Petrochemical Technology, 2012, 14(1): 61-67
[3] Huang P, Zhou S. Microorganism control in hot rolling emulsion [J]. Aluminium Fabrication, 2006, (6): 19-21 (in Chinese)
[4] Shen B. Extending the service life of emulsion for energy conservation and environmental protection [J]. New Technology & New Process, 2010, (12): 64-67 (in Chinese)
[5] Tang X. Study on the biological stability of emulsion type water based metal working fluid [D]. Shenyang: Northeastern University, 2004 (in Chinese)
[6] Lee C G, Hwang Y J, Choi Y M, et al. A study on the tribological characteristics of graphite nano lubricants[J]. International Journal of Precision Engineering & Manufacturing, 2009, 10: 85-90
[7] Qiang C, Xue W, Wang Z, et al. Preparation of watersoluble nanographite and its application in water-based cutting fuid[J]. Nanoscale Research Letters, 2013, 8(1): 1-8
[8] Rapoport L, Leshchinsky V, Lapsker I, et al. Tribological properties of WS2nanoparticles under mixed lubrication [J]. Wear, 2003, 255(7-12): 785-793
[9] Kim S H, Su H C, Lee N E, et al. Adhesion properties of Cu/Cr films on polyimide substrate treated by dielectric barrier discharge plasma [J]. Surface & Coatings Technology, 2005, 193(1): 101-106
[10] Huo Y Q, Yan Y T, Liu X X, et al. Preparation and tribological properties of monodispersed nano-SiO2particles as additive in lubricating oil [J]. Tribology, 2005, 25(1): 34-38
[11] Zhang Y, Fu S, Tao D. The tribological properties of several ultra-fine particles in semifluid grease [J]. Lubrication Engineering, 2002(6): 43-44, 46 (in Chinese)
[12] Xia X, Tang J, Fan C. Lubrication technology of nano material for rolling bearing [J]. Bearing, 2004(9): 37-39 (in Chinese)
[13] Matsunaga T, Tomoda R, Nakajima T, et al. Photoelectrochemical sterilization of microbial cells by semiconductor powders [J]. FEMS Microbiology Letters, 1985, 29(1-2): 211-214
[14] Lin M, Xia D. Photocatalysts for water disinfection: New developments and opportunities [J]. Guangdong Chemical Industry, 2015, 42(15): 120-121 (in Chinese)
[15] Hur J S, Koh Y. Bactericidal activity and water purifcation of immobilized TiO2photocatalyst in bean sprout cultivation [J]. Biotechnology Letters, 2002, 24(1): 23-25
[16] Li H, Xu B, Fan Y. Dramatic activity of mixed-phase TiO2photocatalyst synthesized by hydrothermal method[J]. Chemical Physics Letters, 2013, 558(4): 66-71
[17] Xu H Y, Liu W C, Shi J, et al. Photocatalytic discoloration of methyl orange by anatase/schorl composite: optimization using response surface method [J]. Environmental Science & Pollution Research, 2014, 21(2): 1582-1591
[18] Zhang Q, Gao L, Guo J. Photocatalytic activity of nanosized TiO2[J]. Journal of Inorganic Materials, 2000, 15(3): 556-560
[19] Min C, Chung H, Choi W, et al. Linear correlation between inactivation of E. coli and OH radical concentration in TiO2photocatalytic disinfection[J]. Water Research, 2004, 38(4): 1069-1077
[20] Fei T, Zhu R, Feng O. Synergistic photocatalytic degradation of pyridine using precious metal supported TiO2with KBrO3[J]. Journal of Environmental Sciences, 2013, 25(11): 2299-2305
[21] Reinhardt D J, Nabors W, Kennedy C, et al. Limulus amoebocyte lysate and direct sampling methods for surveillance of operating nebulizers.[J]. Applied & Environmental Microbiology, 1981, 42(5): 850-855
[22] Biesheuvel P M. Electrostatic free energy of interacting ionizable double layers [J]. Journal of Colloid & Interface Science, 2004, 275(275): 514-522
[23] Singh M B, Kant R. Shape- and size-dependent electronic capacitance in nanostructured materials [J]. Proceedings of the Royal Society A: Mathematical Physical & Engineering Sciences, 2013, 469 (2158)
[24] Xiong X, Sun J, Wang B, et al. Tribological properties of new compound additives and their effects on the lubricity of cold-rolling emulsion [J]. Tribology, 2011, 31(2): 169-174 (in Chinese)
[25] Li Z, Gu G, Zhao C, et al. Degradation characteristics and community structure of a hydrocarbon degrading bacterial consortium [J]. China Petroleum Processing & Petrochemical Technology, 2015, 17(3): 15-24
[26] Qin X. Structural insight into unique properties of protoporphyrinogen oxidase from bacillus subtilis [D]. Tianjin: Nankai University, 2010 (in Chinese)
[27] Yu J C, Ho W, Lin J, et al. Photocatalytic activity, antibacterial effect, and photoinduced hydrophilicity of TiO2flms coated on a stainless steel substrate.[J]. Environmental Science & Technology, 2003, 37(10): 2296-301
[28] Nickheslat A, Amin M M, Izanloo H, et al. Phenol photocatalytic degradation by advanced oxidation process under ultraviolet radiation using titanium dioxide[J]. Journal of Environmental & Public Health, 2013, 2013(4): 815310
Received date: 2016-05-10; Accepted date: 2016-06-05.
Prof. Sun Jianlin, E-mail: sjl@ustb. edu.cn.
杂志排行
中国炼油与石油化工的其它文章
- Removal of Nitrogen Compounds from Shale Diesel Fraction Using Ionic Liquid [C4mim]HSO4
- Improved Ti-containing Mesoporous Silica Catalyst Synthesized by Using Anionic Surfactant as Co-template
- Synthesis of Hierarchically Porous FAU/γ-Al2O3Composites with Different Morphologies via Directing Agent Induced Method
- Study on CO2Absorption by Aqueous Benzylamine and Its Formulations with Monoethanolamine as a Component for Post-Combustion Capture Process
- Effect of Magnetic Field on Tribological Properties of Lubricating Oils with and without Tricresyl Phosphate
- The Effect of Surfactant on Synthesis of ZSM-5 in a Super-Concentrated System
