Study on CO2Absorption by Aqueous Benzylamine and Its Formulations with Monoethanolamine as a Component for Post-Combustion Capture Process
2016-03-22GaoJieYinJunZhuFeifeiChenXinTongMingKangWanzhongZhouYanboLuJun
Gao Jie; Yin Jun; Zhu Feifei; Chen Xin; Tong Ming; Kang Wanzhong; Zhou Yanbo; Lu Jun
(1. Key Laboratory of Coal Gasi fi cation and Energy Chemical Engineering of Ministry of Education, East China University of Science & Technology, Shanghai 200237; 2. SINOPEC Ningbo Engineering Co., Ltd., Ningbo 315103)
Study on CO2Absorption by Aqueous Benzylamine and Its Formulations with Monoethanolamine as a Component for Post-Combustion Capture Process
Gao Jie1; Yin Jun1; Zhu Feifei1; Chen Xin2; Tong Ming2; Kang Wanzhong2; Zhou Yanbo1; Lu Jun1
(1. Key Laboratory of Coal Gasi fi cation and Energy Chemical Engineering of Ministry of Education, East China University of Science & Technology, Shanghai 200237; 2. SINOPEC Ningbo Engineering Co., Ltd., Ningbo 315103)
Benzylamine (BZA) has been identifed as a promising candidate for CO2capture process; however the evaluation of BZA in the packed column was very few. Thus, in this work, the absorption and regeneration performance of unblended BZA solvent as well as a series of amine concentrations and ratios in the formulations were studied using a semibatch bubbling reactor. And due to the formation of ivory-white precipitates in solvents containing higher BZA ratios, a 4:1 molar ratio of MEA/BZA mixed solvent was used to study its performance in a pilot-scale test bed. The results showed that a higher BZA ratio in the MEA/BZA mixed solvent resulted in a faster absorption rate, a higher mass transfer and heat transfer rate and a better cyclic performance, but the mass transfer rate of BZA decreased more quickly than MEA with the increase of CO2loading of the solvents. In addition, at high CO2loading in the MEA/ BZA mixed solvent with a molar ratio of 4:1, the ivory-white precipitates were generated which could cause blockage of the packing in the absorber, the stripper and the liquid pipelines.
benzylamine (BZA), monoethanolamine (MEA), post combustion CO2capture, pilot plant, mass transfer
1 Introduction
At present, fossil fuels provide about 85% of the world’s total commercial energy needs[1]. Over the next 20 years, fossil fuels will likely continue to be the major energy source, which means that emissions of global greenhouse gas CO2will remain an issue well in the middle of this century[2]. Its influence on the greenhouse effect and its contribution to global warming are generally accepted. One possible option that has been identifed in an attempt to minimize CO2emissions from coal-fred power plants is the use of carbon capture and storage (CCS) technologies, specifically the post-combustion capture (PCC) technology, which has been identified as a crucial and key strategy in the development and implementation of short to medium term carbon reduction strategies from coal-fred power generation[3-4]. A literature review of CO2capture technologies indicates that a variety of methods are under investigation, including chemical absorption (such as use of amine compounds or ammonia derivative), physical adsorption and membrane separation[5-7]. Among these technologies, the absorption method using chemical solvents is considered to be the most favorable technology for application in coal fred power plants since it is suitable for capturing CO2concentrations as low as 15% in the fue gases, and it has high capture effciency, high selectivity and scale-up feasibility[8]. MEA (alkanolamine) has been functioning as the industrial standard solvent for CO2capture processes in several decades due to its simple chemical structure and rapid reaction rate with CO2. But several inherent issues related with MEA and similar solvents exist including large energy demand to strip CO2, signifcant corrosion, as well as considerableoxidative and thermal degradation rates. The above issues must be addressed in order to achieve and sustain a reasonable capture performance of chemical solvents[9].
The prospect for improved solvent performance can be achieved through the use of formulated solvents or amine blends containing two or more individual amine components which have been combined into a single solvent mixture. Typically, a rapid reacting amine such as MEA or piperazine (PZ) is combined into a blend with a slower reacting, but highly effcient amine in terms of its absorption capacity. Such solvents are rapidly gaining momentum as the next generation of CO2capture solvents by offering a compromise between the rapid kinetic performance and CO2capacity of the solvent[10-12].
Recently, BZA has been identifed as an interesting new CO2capture solvent in view of its similar fundamental reaction kinetics with CO2and absorption capacity to MEA, larger CO2cyclic capacity, miscibility with water[13], lower viscosity in aqueous solutions in the absence of CO2, and lower corrosivity. The corrosion rate and oxidative degradation of aqueous solution of BZA are lower, while its vapor pressure and boiling point are comparable to those of MEA[14]. Conway, et al.[9]reported that CO2absorption rate in the aqueous benzylamine solutions was signifcantly faster than in MEA solutions at similar concentrations and zero CO2loading but was severely limited by the formation of stable salts at high BZA concentrations. Formulations containing BZA/MEA and BZA/AMP demonstrated signifcantly faster absorption rates in CO2loaded solutions up to 0.3 mol/mol than in unblended MEA solutions at similar total amine concentration to the blends. However, these studies did not consider the issues of mass transfer and heat transfer for CO2absorption by BZA solvents in a packed column which was used in industrial pilot plant. Therefore, it is extremely necessary to get a set of accurate and reliable experimental data including a detailed CO2concentration and a system of temperature profles along the column. The CO2absorption implemented in a packed column, which has been widely used in commercial CO2capture, is a very practical approach to evaluate the performance of the BZA solvents, including the thermodynamics capacity, the reaction kinetics, and the mass and heat transfers data[15].
Therefore, in this study, the CO2absorption in BZA and BZA/MEA solvents under different concentrations were studied in a semi-batch bubbling reactor. Also a solvent mixture containing 4 moles of BZA and 1 mole of MEA was studied in a pilot scale CO2capture test bed and compared with the results of adsorption by 5 moles of MEA solvent. The experimental data obtained in this work are presented and discussed in this paper.
2 Theories
2.1 Chemical reactions
It has been reported that the reactions of CO2with primary or secondary amines can be explained by the wellestablished zwitterion mechanism. According to the zwitterion mechanism, the process of CO2absorption into the MEA or BZA solvent involves two-steps reactions, namely at first the formation of zwitterion and then the deprotonation of the zwitterion as follows[16-17]:
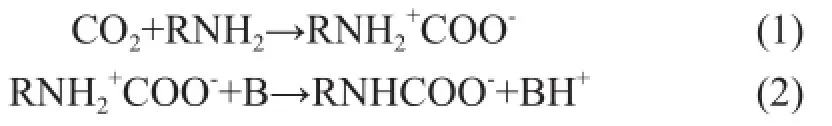
where RNH2is the MEA or BZA, B is any base existing in the solution, including MEA, OH-, H2O and BZA.
2.2 Data analysis
2.2.1 Mass transfer coef fi cient
Mass transfer occurs when a component A in a gas phase transfers across a gas—liquid interface into a liquid phase. The mass flux of component A (NA) at a steady state can be represented in terms of the gas-side mass transfer coeffcient (kG), the total pressure (P), and the gas phase driving force (yA-yA,i):

The mass fux can also be expressed in terms of the overall mass transfer coeffcient (KG) and the equilibrium mole fraction of component A in gas phase (yA*) as follows:

Then,KGcan be expressed as follows:

In a gas absorption apparatus such as a packed column, it is more useful to represent the rate of absorption in terms of the volumetric overall mass transfer coeffcients, represented by the termKGav, instead of the one based on the interfacial area unit because the gas—liquid interfacialarea cannot be measured accurately[17]. Therefore, the mass-transfer coeffcient based on the unit volume of the absorption column can be expressed as follows:
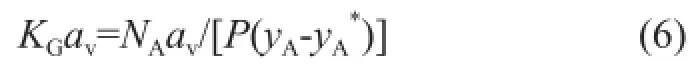
Upon considering that the absorption takes place in any height element (dz), the overall differential mass balance can be given as follows:
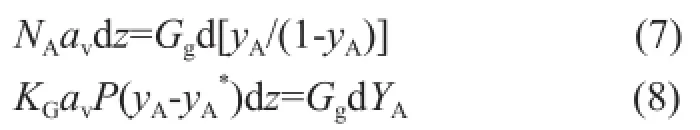
whereYArepresents the mole ratio of component A in the bulk gas andGgrepresents the molar flow rate of inert gas. The fnal equation that determines the overall mass transfer coeffcient,KGav, can then be defned as follows:
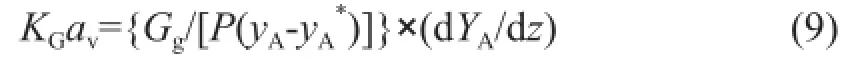
2.2.2 CO2recovery
The compositions of CO2in gases were measured at the inlet and outlet of the absorber by the gas analyzer, then the CO2recovery,η(%), was calculated as shown below:
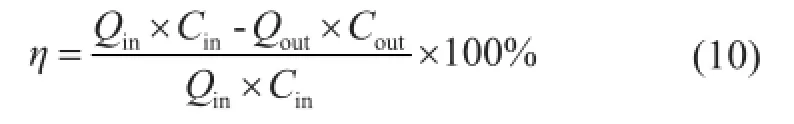
whereQinandQoutare the fow rate of the fue gas at the inlet and outlet (L/h) respectively;CinandCoutare the CO2volume fraction in the fue gas at the inlet and outlet, respectively;
3 Experimental
3.1 Materials
CO2and N2gases with a mole fraction of 0.999 and 0.999, respectively, were supplied by the Shanghai Shenkai Gas Company. Analytical grade MEA (with a purity of>99.9%), BZA (with a purity of >99.9%) were all used as purchased without further purification. The aqueous solution was prepared using distilled water.
3.2 CO2absorption experimental procedure
A semi-batch absorption apparatus used to measure the absorption performance of different solvents is shown in Figure 1. This experimental apparatus consisted mainly of three parts, viz.: the gas supply, the absorption reactor and the gas detection device. Firstly, the solution with a volume of 500 mL was located inside the reactor at the desired temperature. Then the gas flow rates of N2and CO2gases were controlled by mass fow controllers before being remixed and the total flow rate was controlled at 0.8 L/min. The flue gas was then routed through the by-pass tube and tested by the flue gas analyzer to maintain the CO2concentration at 15%. As the desired temperature of the reactor and the concentration of CO2were reached, the valve was opened to inject the fue gas into the reactor to start the absorption experiment. The fow rates of infuent gas and effuent gas from the reactor and the CO2concentration released from the reactor were measured and recorded. When the solvent was saturated, the absorption would be stopped. The CO2absorption rate was then determined from the difference between the fow rates of the infuent and effuent gas based on the following formula:

where,Rvis the CO2mole absorption rate the unit of which is mol/s,Pis the atmospheric pressure,VinandVoutrepresent the volume fow rate of inlet gas and outlet gas respectively andTis the reaction temperature.
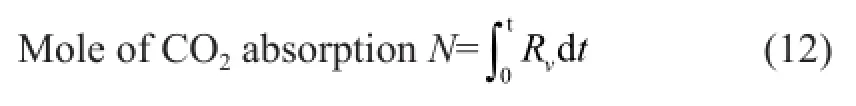
Figure 1 Experimental apparatus for CO2absorption
3.3 CO2regeneration experimental procedure
In the course of thermal regeneration, the solvent was heated from room temperature to 140 ℃ and this temperature was used for treating all the solvents. A three-necked flask placed in an oil bath was filled with 500 mL of saturated solvent. Two condensers were used over the fask to minimize the evaporation loss of the solvent. The temperature of the solvent during the regeneration process was measured through a thermometer. Each solvent was regenerated for the second time and the CO2absorption performance of the regenerated solvent was studied in the absorption apparatus.
3.4 A pilot scale test bed experimental procedure
The CO2capture test bed used in this study was designed on the basis of an aqueous solution containing 30 wt% of MEA. The test bed consisted of an absorber column to capture CO2and a stripper column to strip off CO2from the solvent. A DN 150 mm and 2.5-m high stainless steel column was used as both an absorber and a stripper. The absorber consisted of two packed beds filled with the Mellapale packing 500Y, 1.1 m and 0.9 m in height, respectively, and the stripper consisted of two packed beds flled with the Sulzer packing BX500, 1.1 m and 0.9 m in height, respectively. In the middle of the columns liquid re-distributors were placed to eliminate the wall effects. Along the absorber, five gas measurement points were installed to obtain the concentration profles of gas phase. Figure 2 gives the detailed fow diagram of the test bed. The gas fow rate of N2and CO2gases was controlled by mass fow controllers before being remixed and the fue gas was then routed through a by-pass tube and tested by the flue gas analyzer to maintain the CO2concentration at 15%. The lean solvent which was cooled by water to a desired temperature was fed to the top of the absorber by a constant liquid-fow pump to chemically react with the solvent. The rich solvent from the outlet of the absorber which was pre-heated to the designed temperature in the rich solvent tank was injected into the top of the stripper through a constant liquid-fow pump. In the stripper, the rich solvent that was injected into the upper part of the stripper moved down to the lower part and was stripped of by the steam generated from the lower part of the stripper. The CO2containing water moisture moved to the condenser to be separated from the condensate. The highpurity CO2was released and the condensate was injected to the stripper again. The hot lean solvent leaving the stripper was injected to the lean solvent tank to be cooled down. When the reaction reached a steady-state condition, the gas concentration profle, the liquid samples leaving the absorber were collected for investigating the absorption performance.
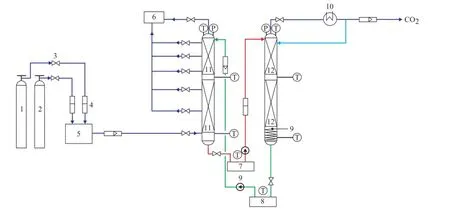
Figure 2 Process fl ow diagram of pilot plant for CO2capture
4 Results and Discussion
4.1 Initial mixed solvents selection criteria
4.1.1 Absorption performance in a semi-batch bubbling reactor
In order to compare the absorption properties of BZA solvent and its formulations with MEA, samples covering 5 mol of BZA, 5 mol of MEA, 4 mol of MEA/1 mol of BZA, 3 mol of MEA/2 mol of BZA, 2 mol of MEA/3 mol of BZA, and 1 mol of MEA/4 mol of BZA were studied in the absorption apparatus separately. The temperature of all experimental runs was 40 ℃.
Figure 3 shows the absorption rates (a) and absorption capacities (b) curves of different solvents. It can be observed that: (1) the initial absorption rates of all solvents were the highest and as the reaction time elapsed, the absorption rate of all solvents decreased because of being saturated; (2) at the beginning of the reaction, the initial absorption rate of the sample containing 5 mol of BZA solvent was the highest, the initial absorption rate decreased with a decreasing BZA ratio in the MEA/BZA solvent, and the initial absorption rate of the sample containing 5 mol of MEA solvent was the lowest which indicated that the CO2absorption rate of BZA was higher than that of MEA; (3) in the fnal stage of the reaction when CO2loading of all solvents were close to the saturated CO2loading, the absorption rate decreased quickly with an increasing BZA ratio in the MEA/BZA mixed solvent, the sample containing 5 mol of BZA solvent decreased most quickly while that of the sample containing 5 mol of MEA decreased least of all. The reason can be explained as follows: the BZA solvent could immediately form stable BZA-carbamate salts at high BZA concentration when it was mixed with CO2and at high CO2loadings which would hinder the contact and reaction between CO2and the solvent[13].
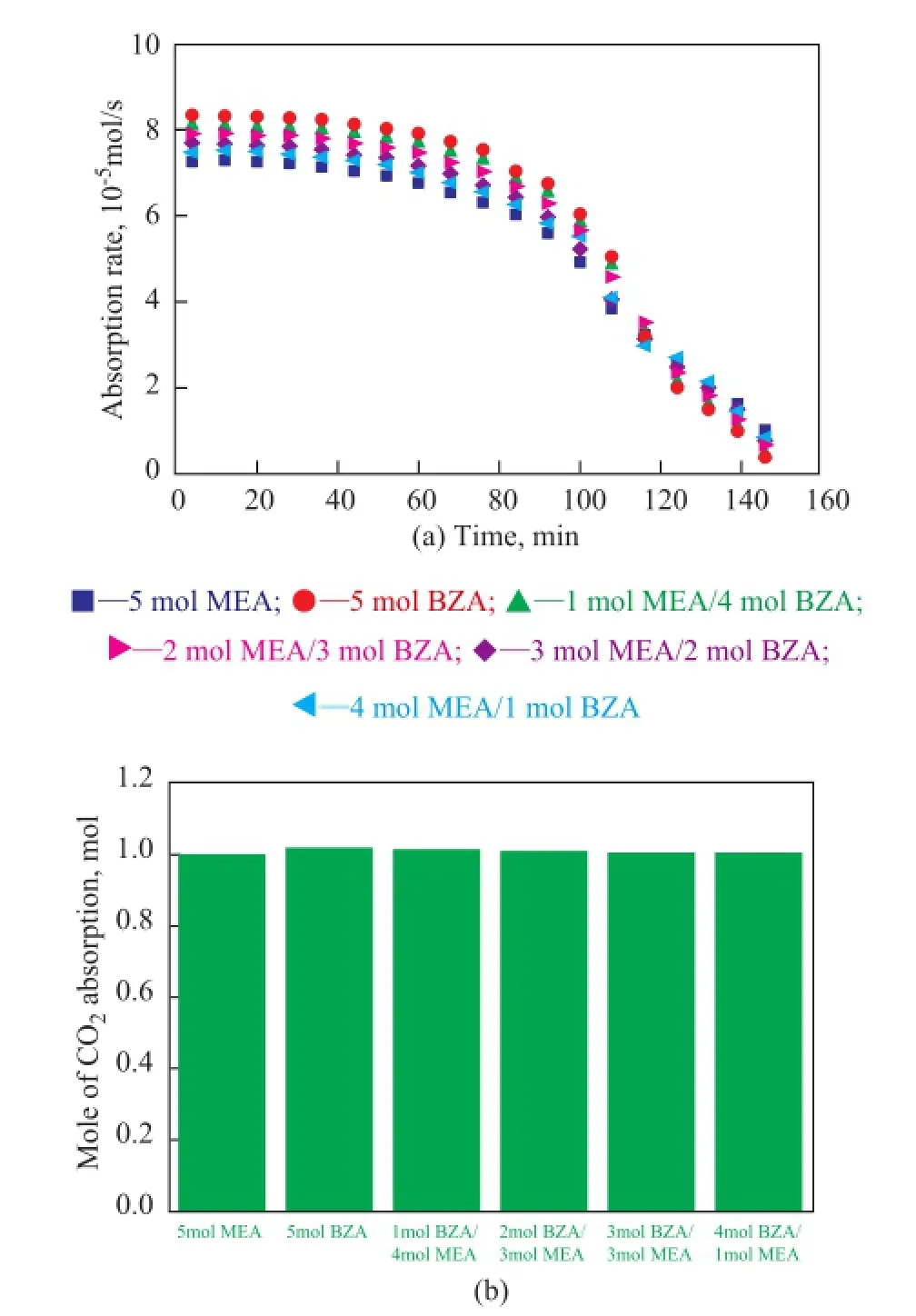
Figure 3 Comparison of CO2absorption rates (a) and CO2absorption capacity (b) between different solvents
It can be seen from Figure 3(b) that the absorption capacity of samples covering 5 mol of BZA, 5 mol of MEA, 4 mol of MEA/1 mol of BZA, 3 mol of MEA/2 mol of BZA, 2 mol of MEA/3 mol of BZA, and 1 mol of MEA/4 mol of BZA was almost the same which was in agreement with the study of Conway, et al.[13]which indicated that the saturated CO2loading of MEA and BZA was both equal to 0.5 mol of CO2per mol of alkanolamine.
4.1.2 Cyclic absorption performance in a semi-batch bubbling reactor
Due to the recycled use of solvents in the industry, the cyclic absorption capacity of absorbent becomes a very important factor for evaluating a solvent used in the industrial[18-20]. The CO2absorption capacity of fresh solvents (first time), solvents recovered after the first time of regeneration (second time), and solvents recovered after the second time of regeneration (third time) that had undergone the same reaction time were studied in this work. The results are shown in Figure 4. It can be seen that: (1) the absorption capacity of all fresh solvents was almost the same that had undergone the same reaction time; (2) after the frst time of regeneration, the absorption capacity of samples containing 5 mol of BZA, 5 mol of MEA, 4 mol of MEA/1 mol of BZA, 3 mol of MEA/2 mol of BZA, 2 mol of MEA/3 mol of BZA, and 1 mol of MEA/4 of mol BZA decreased by 0.348 mol, 0.179 mol, 0.192 mol, 0.230 mol, 0.278 mol and 0.307 mol on one mole of alkanolamine used, respectively, as compared to that of the fresh solvents; and (3) after the second time of regeneration, the absorption capacity decreased slightly as compared to the solvents recovered after the frst time regeneration. From the above-mentioned results, it can be known that a higher BZA ratio in MEA/BZA solvents resulted in a better cyclic performance.
But during the absorption reaction, the ivory-white precipitates were formed with an increasing BZA ratio in the sample. This occurred because the unblended BZA solvent could immediately form stable BZA-carbamate salts at high BZA concentrations when it met with CO2at high CO2loading[9]. Upon taking into account both the absorp-tion performance and precipitation behavior, the sample consisting of 4 mol of MEA/1 mol of BZA was used to study its performance in a pilot-scale test bed.
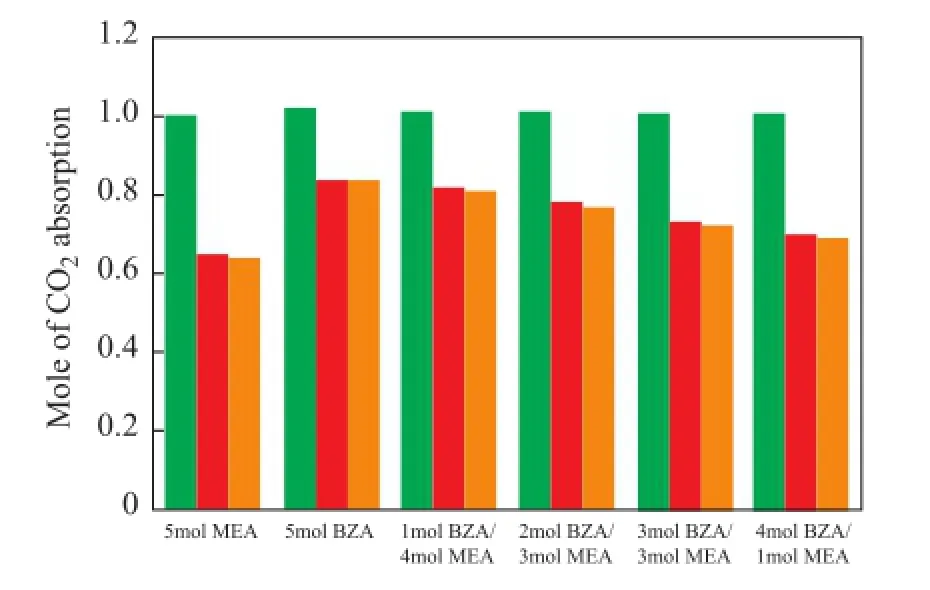
Figure 4 Comparison of cyclic CO2absorption capacity of different solvents
4.2 Performance of solvent composed of 4 mol of MEA/1 mol of BZA in a pilot-scale test bed
To evaluate the industrial potential of solvent composed of 4 mol of MEA/1 mol of BZA, its absorption performance was studied in a pilot-scale test bed, with the results compared with the solvent composed of 5 mol of MEA. The operating conditions are shown in Table 1.
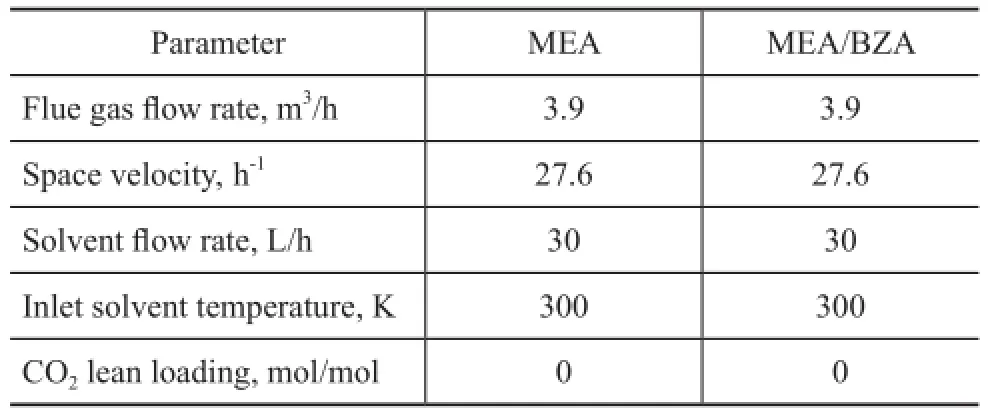
Table 1 Basic operating conditions for testing the solvent composed of 5 mol of MEA and the sample composed of 4 mol of MEA/1 mol of BZA
As shown in Figure 5, the CO2concentration at the column outlet decreased quickly along the height of the absorber for both of aqueous MEA solvent and MEA/BZA solvent, therefore, the CO2capture efficiency increased quickly. From the curve of the solvent composed of 5 mol of MEA, it can be seen that at a height of 1.36 m the CO2recovery from the aqueous MEA solvent maintained at a constant level equating to about 98.5% and it almost did not change with the increase of the height. By judging from the curve of the solvent composed of 4 mol of MEA/1 mol of BZA, it can be seen that at a height of 0.95 m the CO2recovery was about 98.5% and did not change with the increase of the height. So it can be concluded that at the same fue gas fow rate and solvent fow rate, the absorption of CO2into the solvent composed of 4 mol of MEA/1 mol of BZA was faster than the absorption of CO2into the solvent containing 5 mol of MEA.
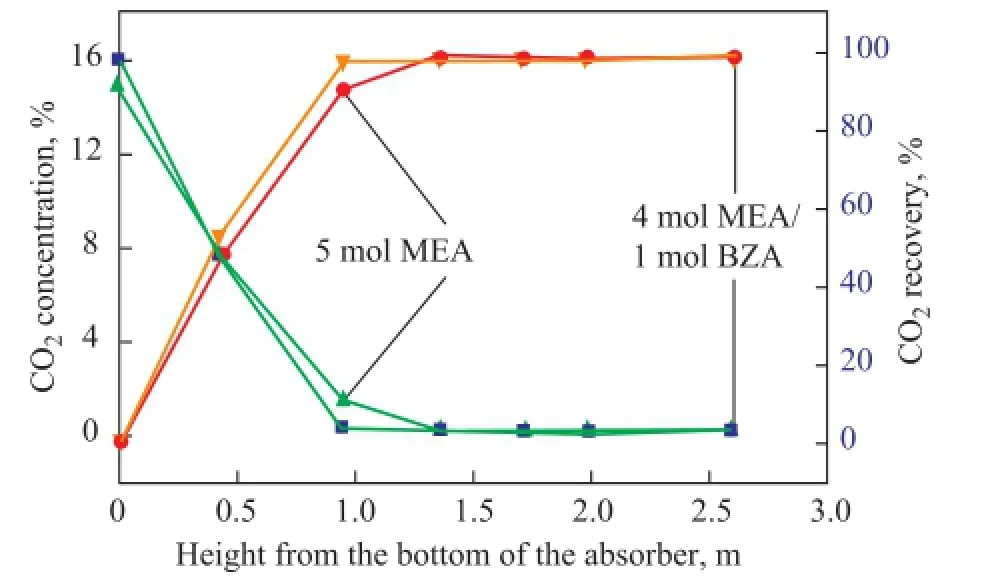
Figure 5 Comparison of the concentration of vented CO2and CO2capture ef fi ciency between MEA solvent and MEA/ BZA mixed solvent under the same operating conditions
As shown in Figure 6, theKGavvalue of the mixed solvent containing 4 mol of MEA/1 mol of BZA was higher than that of the solvent composed of 5 mol of MEA (3.12 kmol/(m3·h·kPa) and 2.62 kmol/(m3·h·kPa), respectively) which indicated that the mass transfer rate of BZA was faster than MEA.
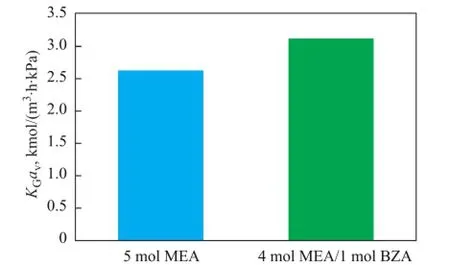
Figure 6 Comparison ofKGavbetween CO2absorption into MEA and MEA/BZA under the same condition
Figure 7 gives the temperature profles along the absorber of the mixed solvent containing 4 mol of MEA/1 mol of BZA and the solvent composed of 5 mol of MEA. It can be seen from Fig. 7 that: (1) the temperature of MEA solvent system frst increased and then decreased along the height of the absorber; (2) the temperature at the bottom of the absorber of MEA/BZA mixed solvent system was the highest and then the temperature decreased along theheight of the absorber; and (3) the highest temperature of MEA/BZA mixed solvent system was higher than that of MEA solvent system (44 ℃ and 36 ℃, respectively). The reasons can be explained as follows: (1) CO2in the fue gas fed into the bottom of the absorber could react with the solvent and a large amount of heat was released quickly to increase the system temperature and as the fue gas was discharged, only a small amount of CO2remained. So at the upper part of the absorber, the effect of the heat transfer between the absorber and the environment played a major role; and (2) the CO2absorption rate of the MEA/ BZA mixed solvent was faster than MEA solvent, so at the bottom of the absorber, MEA/BZA solvent could react with CO2instantly while releasing a large amount of heat than MEA solvent to increase the absorber temperature.
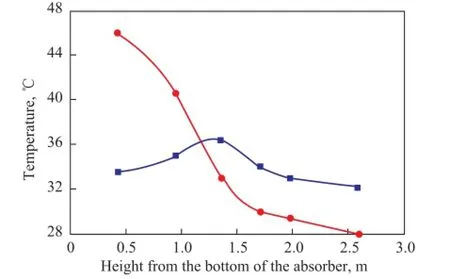
Figure 7 Comparison of absorber temperature pro fi le for CO2absorption between the solvents MEA and MEA/BZA operating under the same condition
Absorption experiments were conducted at different lean CO2loadings in the MEA solvent and the MEA/BZA mixed solvent by controlling the regeneration temperature of the stripper.KGavvalues of the MEA solvent and the MEA/BZA mixed solvent at different lean CO2loadings are shown in Figure 8. It can be seen that: (1) TheKGavvalue of mixed solvent composed of 4 mol of MEA/1 mol of BZA was by some 30% larger than the solvent composed of 5 mol of MEA; and (2) with the increase of the lean CO2loading, the KGavvalue of MEA/ BZA mixed solvent decreased more quickly than the MEA solvent. The reasons can be explained as follows: (1) BZA had a faster CO2absorption rate and mass transfer rate than MEA; and (2) a high CO2loading might infuence the physical arrangement of the solvent in solution, which, in turn, could directly influence the Henry constant, especially in BZA solvent[13].
In addition, at a high CO2loading to be absorbed by the mixed solvent composed of 4 mol of MEA/1 mol of BZA, the ivory-white precipitates were generated which could cause blockage of the packing in the absorber, the stripper and the liquid pipelines.
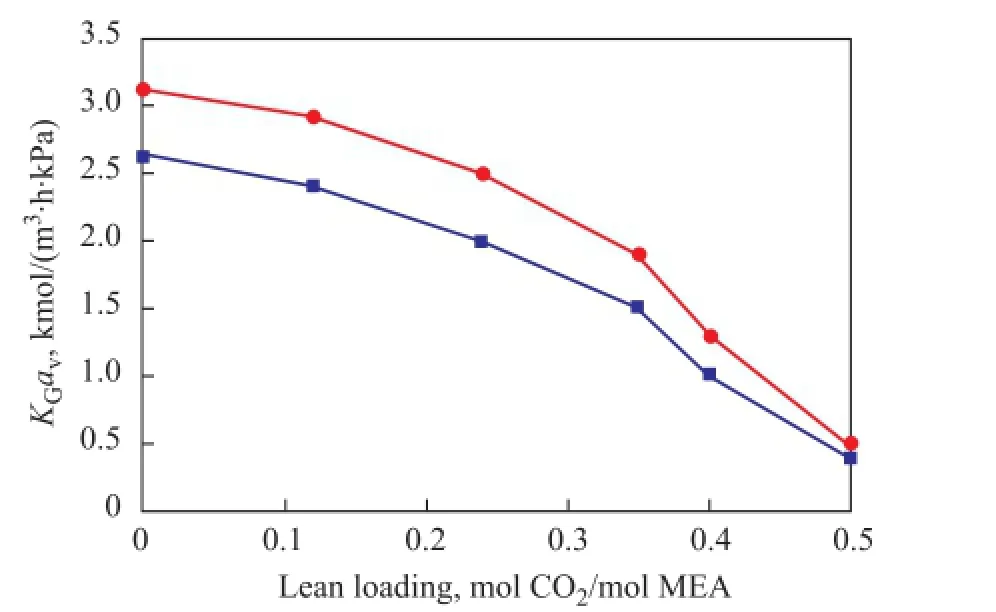
Figure 8 Comparison ofKGavvalue for CO2absorption between MEA and MEA/BZA as a function of CO2loading
5 Conclusions
The BZA solvent and its formulations with the MEA solvent were experimentally studied in a semi-batch bubbling reactor. The results showed that a higher BZA ratio in the MEA/BZA mixed solvents resulted in a faster absorption rate and a better cyclic performance. But due to the generation of ivory-white precipitates in solvents with higher BZA ratio, the mixed solvent containing 4 mol of MEA/1 mol of BZA was used to study its performance in a pilot-scale test bed. The results showed that BZA had a faster mass and heat transfer rate than MEA, but the mass transfer rate of BZA decreased more quickly than MEA with the increase of CO2loading in the solvents. In addition, at a high CO2loading to be treated by the mixed solvent containing 4 mol of MEA/1 mol of BZA, the ivory-white precipitates were generated which could cause blockage of the packing in the absorber, the stripper and the liquid pipelines. So judging from the results of the pilot-scale test bed experiments, BZA was not suitable for CO2capture in industrial pilot plant unless the problem of precipitation could be resolved.
Acknowledgement: This work was supported by the Sinopec Ningbo Engineering Co. Ltd. (No.l4850000-14-ZC0609-0003,H8XY-0032).
[1] Yang H, Xu Z, Fan M. Progress in carbon dioxide separation and capture: A review [J]. Journal of Environmental Sciences, 2008, 20(1): 14-27
[2] Chen P C, Luo Y X, Cai P W. CO2capture using monoethanolamine in a bubble-column scrubber [J]. Chemical Engineering Technology, 2015, 38(2): 274-282
[3] Kremer J, Galloy A, Strohle J. Continuous CO2capture in a 1-MWth carbonate looping pilot plant [J]. Chemical Engineering Technology, 2013, 36(9): 1518-1524
[4] Leung D Y C, Caramanna G, Maroto-Valer M M. An overview of current status of carbon dioxide capture and storage technologies [J]. Renewable and Sustainable Energy Reviews, 2014, 39(6): 426-443
[5] Liu Z, Teng Y, Zhang K. CO2adsorption performance of different amine-based siliceous MCM-41 materials [J]. Journal of Energy Chemistry, 2015, 24(3): 322-330
[6] Rahim N A, Ghasem N, Al-Marzouqi M. Absorption of CO2from natural gas using different amino acid salt solutions and regeneration using hollow fiber membrane contactors [J]. Journal of Natural Gas Science and Engineering, 2015, 26: 108-117
[7] Koronaki I P, Prentza L, Papaefthimiou V. Modeling of CO3capture via chemical absorption processes-An extensive literature review [J]. Renewable and Sustainable Energy Reviews, 2015, 50: 547-566
[8] Liang Y, Liu H, Rongwong W. Solubility, absorption heat and mass transfer studies of CO2absorption into aqueous solution of 1-dimethylamino-2-propanol [J]. Fuel, 2015, 144: 121-129
[9] Conway W, Beyad Y, Feron P. CO2absorption into aqueous amine blends containing benzylamine (BZA), monoethanolamine (MEA), and sterically hindered tertiary amines [J]. Energy Procedia, 2014, 63: 1835-1841
[10] Samanta A, Bandyopadhyay S S. Absorption of carbon dioxide into piperazine activated aqueousN-methyldiethanolamine [J]. Chemical Engineering Journal, 2011, 171(3): 734-741
[11] Mudhasakul S, Ku H M, Douglas P L. A simulation model of a CO2absorption process with methyldiethanolamine solvent and piperazine as an activator [J]. International Journal of Greenhouse Gas Control, 2013, 15: 134-141
[12] Ma’mun S, Svendsen H F, Hoff K A. Juliussen O. Selection of new absorbents for carbon dioxide capture [J]. Energy Conversion and Management, 2007, 48(1): 251-258
[13] Conway W, Beyad Y, Richner G. Rapid CO2absorption into aqueous benzylamine (BZA) solutions and its formulations with monoethanolamine (MEA), and 2-amino-2-methyl-1-propanol (AMP) as components for post combustion capture processes [J]. Chemical Engineering Journal, 2015, 264: 954-961
[14] Richner G, Puxty G, Carnal A. Thermokinetic properties and performance evaluation of benzylamine-based solvents for CO2capture [J]. Chemical Engineering Journal, 2015, 264: 230-240
[15] Fu K, Rongwong W, Liang Z. Experimental analyses of mass transfer and heat transfer of post-combustion CO2absorption using hybrid solvent MEA-MeOH in an absorber [J]. Chemical Engineering Journal, 2015, 260: 11-19
[16] Laddha S S, Danckwerts P V. Reaction of CO2with ethanolamines: kinetics from gas-absorption [J]. Chemical Engineering Science, 1981, 36(3): 479-482
[17] Naami A, Sema T, Edali M. Analysis and predictive correlation of mass transfer coeffcient KGavof blended MDEAMEA for use in post-combustion CO2capture [J]. International Journal of Greenhouse Gas Control, 2013, 19: 3-12
[18] Sema T, Naami A, Liang Z. A novel reactive 4-diethylamino-2-butanol solvent for capturing CO2in the aspect of absorption capacity, cyclic capacity, mass transfer, and reaction kinetics [J]. Energy Procedia, 2013, 37: 477-484
[19] Gao Jie, Yin Jun, Zhu Feifei, et al. Study on absorption and regeneration performance of novel hybrid solutions for CO2capture[J]. China Petroleum Processing and Petrochemical Technology, 2016, 18(1): 66-72
[20] Zhang Feng, Shen Benxian, Sun Hui, et al. Formulated solvent for organosulfurs removal from LPG in fber flm contactor[J]. Petroleum Processing and Petrochemicals, 2015, 46(6): 68-73 (in Chinese)
Received date: 2016-04-21; Accepted date: 2016-06-24.
Prof. Lu Jun, Telephone: +86-21-64252443, Fax: +86-21-64252737; E-mail: lujun@ecust.edu. cn.
杂志排行
中国炼油与石油化工的其它文章
- Synthesis of Hierarchically Porous FAU/γ-Al2O3Composites with Different Morphologies via Directing Agent Induced Method
- Puri fi cation of Aromatics over a PromisingCatalyst
- The Effect of Surfactant on Synthesis of ZSM-5 in a Super-Concentrated System
- Removal of Nitrogen Compounds from Shale Diesel Fraction Using Ionic Liquid [C4mim]HSO4
- Effect of Magnetic Field on Tribological Properties of Lubricating Oils with and without Tricresyl Phosphate
- Improved Ti-containing Mesoporous Silica Catalyst Synthesized by Using Anionic Surfactant as Co-template
