Improved Ti-containing Mesoporous Silica Catalyst Synthesized by Using Anionic Surfactant as Co-template
2016-03-22WangJingjingTengHongniXiaoWeiWangJinquLuJinmingYangJianhua
Wang Jingjing; Teng Hongni; Xiao Wei; Wang Jinqu, Lu Jinming, Yang Jianhua
(1. College of Chemical and Environmental Engineering, Shandong University of Science and Technology, Qingdao 266590; 2. Laboratory for Corrosion and Protection, Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016; 3. State Key Laboratory of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, Dalian 116024)
Improved Ti-containing Mesoporous Silica Catalyst Synthesized by Using Anionic Surfactant as Co-template
Wang Jingjing1; Teng Hongni1; Xiao Wei2; Wang Jinqu3, Lu Jinming3, Yang Jianhua3
(1. College of Chemical and Environmental Engineering, Shandong University of Science and Technology, Qingdao 266590; 2. Laboratory for Corrosion and Protection, Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016; 3. State Key Laboratory of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, Dalian 116024)
Ti-containing mesoporous silica materials (Ti-MSs) with isolated tetrahedrally coordinated Ti species have been widely applied in bulk molecular catalysis. Herein, Ti-MSs were synthesized using anionic surfactant SDS as the co-template. The SDS molecular assembled structures can interact with silica species through the interface hydrogen bonds leading to the formation of mesoporous silica structure with compact Ti-O bonds, lower hydrophilicity and low template cost. The infuence of adding SDS as the co-template on the oxidation of styrene with aqueous H2O2as the oxidant was investigated. Ti-MSs using SDS as the co-template showed better catalytic performance as compared with mesoporous titanium silicate synthesized with CTAB serving as the sole template. Moreover, the Ti-MSs synthesized at a Ti/Si ratio of 0.005 demonstrated an optimized performance for styrene oxidation with styrene conversion improved by 14.8%, benzaldehyde selectivity improved by 13.7% and styrene oxide selectivity improved by 9.2% when the reaction time was 6 h.
sol-gel preparation; mesoporous titanium silicate structure; anionic surfactant co-template; olefn oxidation; green catalyst
1 Introduction
Mesoporous materials have already been developed for dozens of years, as represented by MCM-41S at the Mobil Corporation in 1992[1-2]. Therein, the mesoporous titanium silicate molecular sieves have been proved to be effective catalysts in a wide range of oxidation reactions, especially for oxidation of bulky organic molecules thanks to their advantages of overcoming the mass transfer limitations, as compared to microporous titanium-containing silicate molecular sieves[3-4]. Much work has been focusing on the incorporation method and Ti amount on the catalyst activity for olefn oxidation[5-6]. However, the activity and selectivity improvements of mesoporous silica materials reported to date are lower than the theoretical speculation[7-9]. The higher hydrophilicity of mesoporous silica materials favors water adsorption and can poison the active sites of catalyst. Furthermore the surface hydrophilic properties hindered the diffusion between active sites and organic catalyst substrate[10]. Two different successful approaches, including the post-synthetic silylation and the one-step organic functionalization method, have been reported to be capable of enhancing the surface hydrophobicity of Ti-containing mesoporous silica[11-12]. Addition of salts, such as NaF, was also proved to be an effective approach[13]. However, less work has been focusing on a facile template route to control the surface hydrophilicity through the one step method[8].
Anionic surfactant SDS (sodium dodecyl sulfate) was widely employed because of its excellent interfacial stability and low cost[14]. Our work has proved that introducing SDS into the synthesis of mesoporous silica as the cotemplate can decrease the surface hydrophilic properties because of partial charge neutralization or decreasing charge density of the micelle in the mixed surfactant system[15]. As mentioned in the previous research, the surfactant-to-silica ratio was remarkably decreased as compared with the single template route. Furthermore, investigationon the synthesis approach may be theoretically important because the complex self-assembled phase behavior in CTAB/SDS solution may lead to new mesoporous structures which can favor the shape selective catalysis.
Therefore, in the present work, the Ti-containing mesoporous silicas (Ti-MSs) have been synthesized using a mixture of SDS and CTAB as supramolecular template under hydrothermal crystallization. The Ti-MSs were characterized and optimized by means of XRD, UV-vis, N2-adsorption/desorption, SEM, EDX, FT-IR and TEM analysis techniques to explore the most optimized material. Oxidation of styrene has been adopted as the probe reaction in order to determine the effect of rules related with the SDS co-template on the catalytic activity. The activity results have also been compared with Ti-MSs synthesized by the CTAB sole-template method and the involved reaction mechanism has been discussed.
2 Experimental
2.1 Chemicals
Cetyltrimethylammonium bromide (CTAB, C16H33N(CH3)3Br) was purchased from the Beijing Aoboxing Biotech Company, Ltd.; tetraethyl orthosilicate (TEOS) was purchased from the Tianjin Kermel Reagent Co. Ltd.; titanium (IV) isopropoxide (TIP) was purchased from Acros; sodium dodecyl sulfate (SDS, C12H25OSO3Na) was purchased from the Tianjin Bodi Chemical Holding Co., Ltd.; and sodium hydroxide (NaOH) was purchased from the Tianjin Kermel Reagent Co., Ltd. All chemicals were used as received without any further purifcation. The deionized water used in this study was self-made.
2.2 Synthesis of Ti-MSs with SDS serving as co-template
A typical procedure for preparation of Ti-MSs is as follows: Firstly, CTAB and NaOH were dissolved in deionized water under vigorous stirring at 308 K. SDS were then added, after the reagents were dissolved. The TEOS solution was dropwise added after the above reagents were well mixed at 308 K under magnetic stirring. Then, the temperature was reduced to 298 K and a certain amount of TIP was added dropwise as the titanium source. After having been stirred for 2 h, the resulting synthetic gel was heated under static hydrothermal conditions at 373 K in a stainless steel autoclave for 72 h. The precipitated products were fltered, washed with deionized water to be neutral and dried in air at 333 K overnight. Finally, the as-synthesized samples were calcined at 823 K for 6 h to remove the template. The molar composition of the initial gel mixture had a ratio of SiO2:CTAB:SDS:NaOH:H2O:TIP= 1:0.152:0.025:0.5:62:x.
2.3 Characterization
The as-synthesized mesoporous Ti-MSs molecular sieve was characterized by X-ray powder diffraction (XRD) on a Rigaku-Dmax 2400 diffractometer equipped with graphite monochromatized Cu Kα radiation in the 2θangle range from 0.6° to 10°. The nitrogen adsorption and desorption isotherms were carried out with an Autosorb-1 adsorption analyzer (Quantachrome Instruments) at 77 K. The surface area and pore size distribution was calculated by applying the multipoint BET analysis and the Barrett-Joyner-Halenda (BJH) method, respectively. The transmission electron microscopy (TEM) analysis was carried out on a Philips Tecnai G2 20 instrument, operating at 200 kV. For TEM measurements, all mesoporous materials were crushed in an agate mortar, dispersed in ethanol, and deposited on a microgrid. The scanning electron microscopy (SEM) images were taken with a Nova Nano-SEM 450 microscope (FEI Company) at an acceleration voltage of 15 kV and a working distance of 10 mm after gold plating. The EDAX Genesis system, with an SUTW detector equipped with SEM equipment, was used to carry out the EDX analysis in order to confrm the presence and quantify Si and Ti species. The Ti contents in titanium silicate samples were determined using an inductively coupled plasma-optical emission spectrometer (Optima 2000 DV).
Ultraviolet-visible (UV-vis) spectra were acquired using a Hitachi 340 spectrometer with a di ff use refectance mode. The nature of acid sites (Brönsted and Lewis acids) of the catalyst samples was characterized by in situ FTIR spectroscopy with chemisorbed pyridine in drift mode on a Bruker EQUINOX55 spectrometer with a scanning range of 400 cm-1—4 000 cm-1. The in situ pyridine (Py) adsorbed IR spectra were derived from a spectra taken from samples pressed into self-supporting discs, whichwere placed in an IR cell, and treated at 298 K for 5 min for pyridine adsorption. The IR spectra were recorded at different temperatures including 423 K, 573 K and 723 K for pyridine adsorption, respectively.
2.4 Catalytic reactions
The oxidation of styrene with an aqueous solution of H2O2was carried out in a 100 mL glass roundbottomed fask equipped with a condenser under vigorous stirring. In a typical run, 0.5 g of styrene, 10 mL of acetonitrile, and 0.3 g of catalyst were mixed in the fask and heated to 333 K. Aqueous H2O2(2.25 mmol, 30%) was added to the mixture to start the reaction. The catalysts were separated by centrifugation and the products were analyzed by a gas chromatograph (Agilent 6850) equipped with a HP-1 capillary column, 30 m in length, and a FID detector. The products were quantifed using cyclooctane (1 g) as the internal standard. All the catalysts were dried at 393 K to remove the adsorbed water before each reaction.
3 Results and Discussion
3.1 XRD characterization of Ti-MSs
Highly ordered mesoporous MCM-48 molecular sieve has been successfully synthesized using mixed surfactant consisting of CTAB and anionic SDS as the template at a low surfactant-to-silica ratio of 0.177[15]. Then, a certain amount of Ti was added in the synthesis process and all the titanium-containing mesoporous molecular sieve samples synthesized with the mixed template were assessed by X-ray powder diffraction (Figure 1). The phase of the calcined sample without Ti showed four peaks for the (211), (220), (420), and (332) planes corresponding to the Ia3d space group of cubic mesoporous phase MCM-48 as depicted in Figure 1a. When a small amount of TIP was added, the Ti-MSs synthesized thereby were mesoporous silica with wormlike porous structure as shown in Figure 1(b, c, and d)[11]. The mesoporous structure turned to be a disordered structure with increase in Ti/Si molar ratio as depicted in Figure 1(e and f), which was ascribed to the difference in hydrolysis rate between TIP and TEOS[16]. The mesoporous structure vanished when the Ti/Si ratio increased to 0.015[7]. The above X-ray diffraction results indicate that the mesoporous could be partly destroyed by the Ti ions introduced in the framework. The above mentioned phenomena should be considered in choosing the optimized synthesis condition.
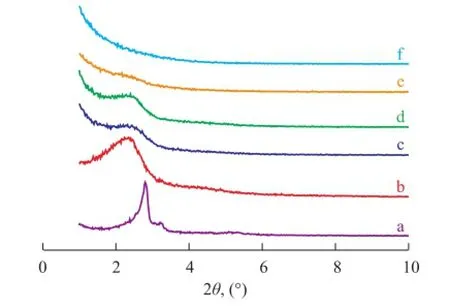
Figure 1 XRD patterns of mesoporous titanium silicates synthesized with different Ti/Si molar ratios
3.2 IR and UV-vis spectra
In order to confrm the incorporation and coordination of Ti species in the framework of mesoporous silica sphere, the UV-vis spectra are listed in Figure 2. The UV-vis spectrum of pure silica sample indicated no adsorption band in Figure 2a. The UV-Vis spectrum of anatase TiO2showed a broad peack between 200 nm and 350 nm in Figure 2g. However, all samples containing Ti species exhibited a band centered at 210 nm—220 nm attributed to mono-atomically dispersed tetrahedral Ti species, which was presented in Figure 2b—2f[17-18]. The above results indicated that the tetrahedral Ti species were successfully incorporated into the mesoporous silica framework via the mixed template route. The spectra of Ti-MSs samples with various Ti/Si ratios presented a wide shoulder at 250 nm—300 nm, revealing the formation of penta-coordinated Ti species due to the interaction of Ti species with moisture on the polymerized hexa-coordinated Ti species[19]. The band intensity increased with the increase of Ti/Si ratio from 0.002 to 0.005 and then became stable with a further increase of Ti amount. Furthermore, the existence of a band at 330 nm indicates that anatase TiO2was formed in the sample. Upon combining the UV-vis spectra with the X-ray measurement results, Ti-MSs with a Ti/Si ratioof 0.005 would be used in catalytic reaction for further research because it showed better UV-vis band in all the samples mentioned above.
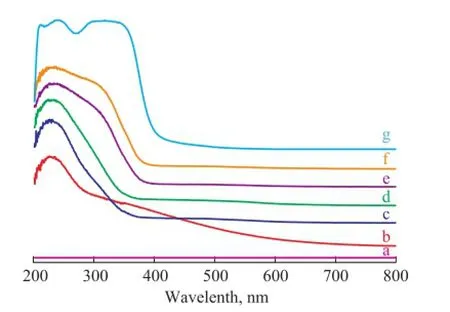
Figure 2 Effect of Ti/Si molar ratio on UV-vis spectra of the synthesized Ti-MSs
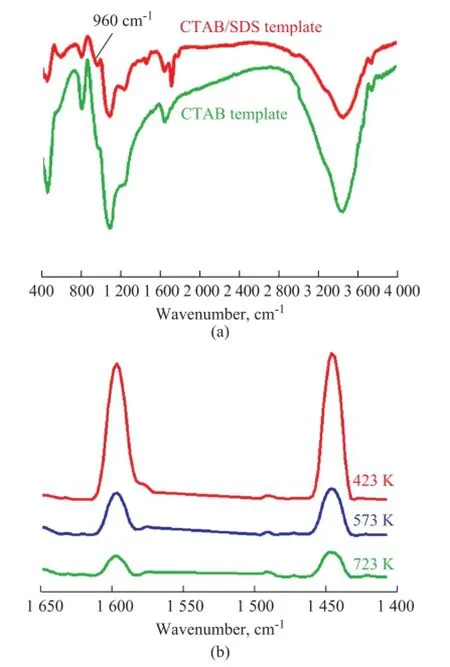
Figure 3 FT-IR spectra. (a) and pyridine adsorbed FT-IR (b) of mesoporous titanium-silicate synthesized at a Ti/Si molar ratio of 0.005
Ti-MSs with a Ti/Si ratio of 0.005 was further characterized by FT-IR spectrometry with the results presented in Figure 3a. A strong band at 950—975 cm−1was clearly observed in the FT-IR spectra of Ti-MSs samples which were assigned to the stretching vibration of the SiO4tetrahedral bond with Ti atoms. However, this absorption band was frst assigned to then(SiOH) vibration of silanol groups. Thus, this band only can be taken as a reasonable proof of the Ti species incorporated into Ti-MSs[20].
The Brönsted and Lewis acidity of the catalysts was distinguished by the technique of pyridine adsorption in situ FT-IR spectroscopy as shown in Figure 3. The IR absorbance intensity of bands at 1 445 cm-1which was assigned to the weak hydrogen bond interaction between Si-OH group and pyridine (Py) indicated to the existence of Lewis acidity[21]. However, no obvious signal was found at around 1 540 cm-1, denoting that almost no Brönsted acidity existed in the material. A weak peak was found at 1 490 cm-1which should be ascribed to a lower Ti incorporation of the samples.
3.3 TEM and SEM images of Ti-MSs
The molar composition of the Ti-MSs with better mesoporous structure and tetra-coordinated Ti species was equal to SiO2:CTAB:SDS:NaOH:H2O:TIP= 1:0.152:0.025:0.5:62:0.005. The SEM image of the above synthesized Ti-MSs shown in Figure 4a indicates that the as-synthesized block is constructed from small particles. To verify the porous structure of the particles, the calcined Ti-MSs sample was characterized by TEM measurements. The TEM images are shown in Figure 4 (b, c), which presents an ordered arrangement of wormlike porosity. The TEM images show a good agreement with the powder XRD results.
The information concerning titanium dispersion inside the synthesized silica particles was obtained by EDX spectroscopy on the same instrument. As shown in Figure 5, the peaks of titanium, silicon and oxygen elements all appeared in both spectra, while the peak of titanium could only be observed after the grafting process. The results indicate that titanium was successfully incorporated in the framework of mesoporous silica. The observation shows that the distribution of Ti ions in Ti-MSs framework was stabilized along the scan line in Figure 5a. The atomic percentage of titanium was also characterized by ICP, with the molar ratio of Ti/Si in the materials presented in Table 2.
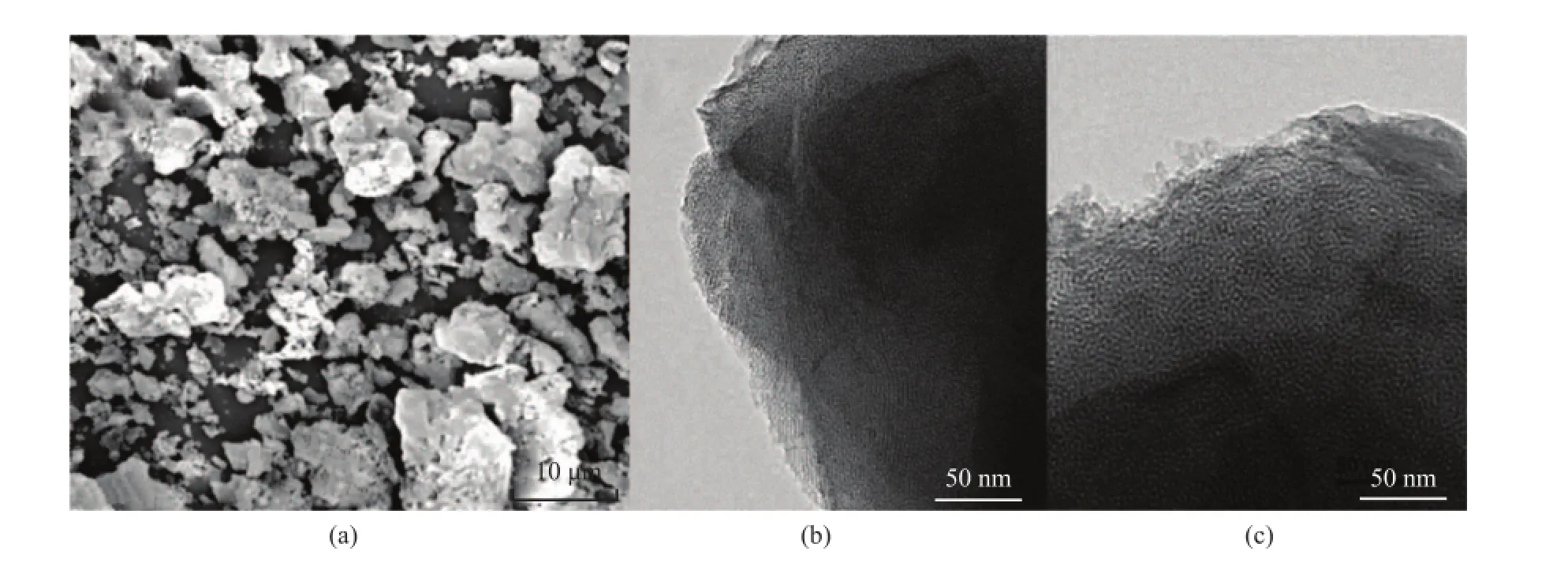
Figure 4 SEM images (a) and TEM images (b, c) of Ti-MSs synthesized using cationic and anionic surfactant template at a Ti/Si molar ratio of 0.005
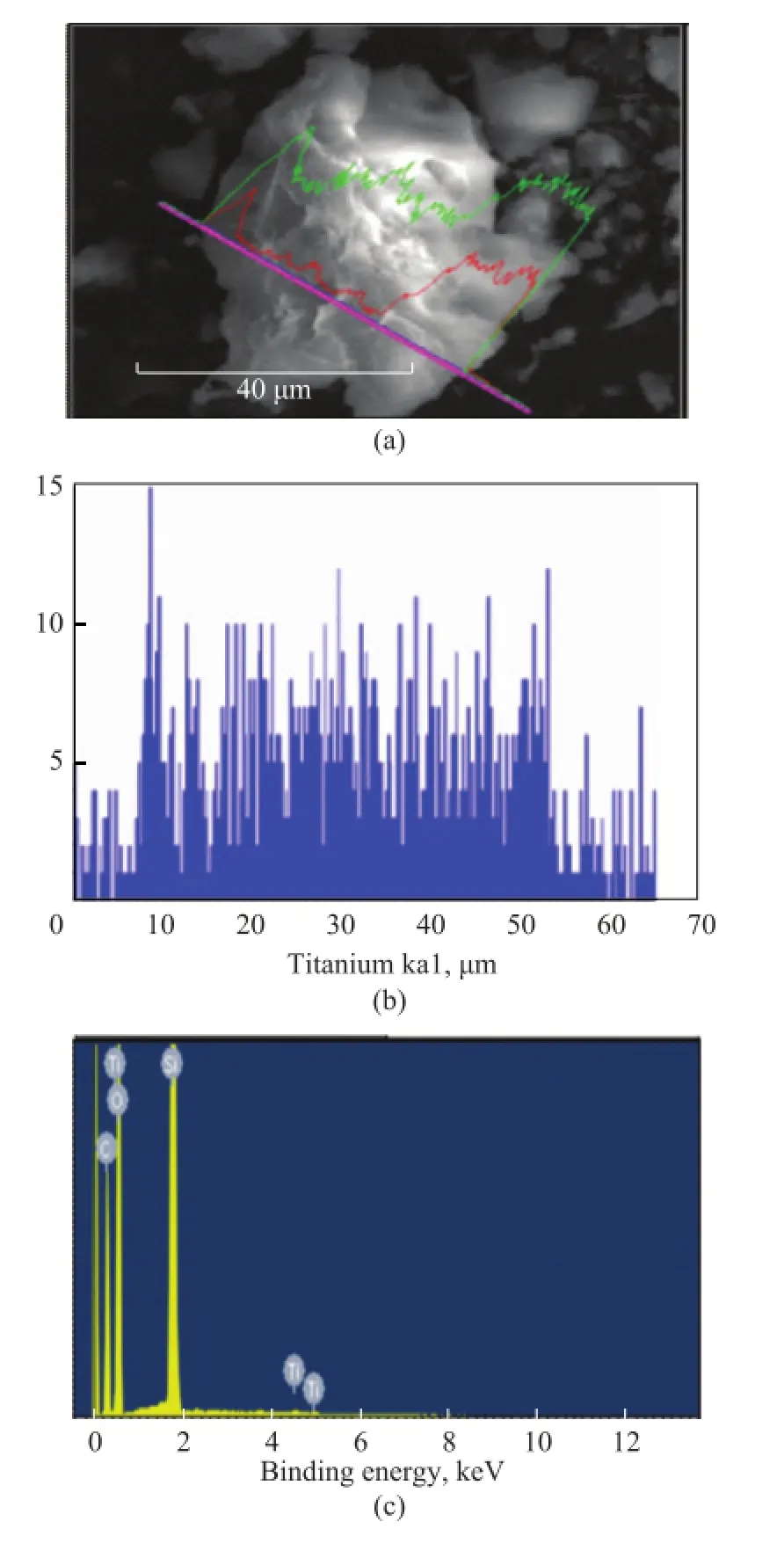
Figure 5 SEM (a) and EDX (b, c) images of synthesized Ticontaining mesoporous silicate at a Ti/Si molar ratio of 0.005
3.4 N2adsorption-desorption isotherms of Ti-MSs
The calcined Ti-MSs with better structure and the mesoporous silica synthesized without adding SDS were further characterized by N2adsorption-desorption isotherms in Figure 6. The samples present a hysteresis loop at high value of relative pressure (p/p0) ranging from 0.45 to 1, indicating to the existence of the ink-bottle type mesopores according to literature reports[22-24]. The hysteresisloop which covered a broad range of relative pressures ranging from around 0.45 to 1.0 is consistent with the wide mesoporous size distribution within the as-prepared Ti-MSs as shown in Figure 6 (insert). As shown in Table 1, Ti-MSs synthesized with CTAB/SDS used as the template showed higher BET surface areas and total pore volume as compared with the CTAB sole template method. The calculation results of correspondingSBETandVmesoporeof Ti-MSs with and without adding SDS are shown in Table 1. Adding SDS used as the co-template increased theSBETand Vmesopore of synthesized Ti-MSs which could further promote the catalyst activity. The BET surface area of Ti-MSs with a better structure was 740.86 m2/g and the pore volume was 0.68 cm3/g. The average pore size, calculated from the desorption curve using the BJH model, was about 3.18 nm.
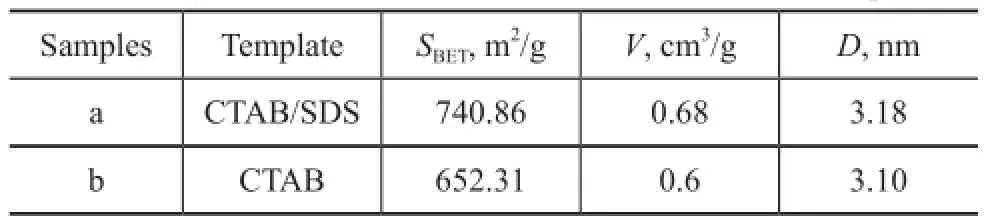
Table 1 BET surface area and pore volume data of Ti-MSs synthesized with or without SDS as co-template
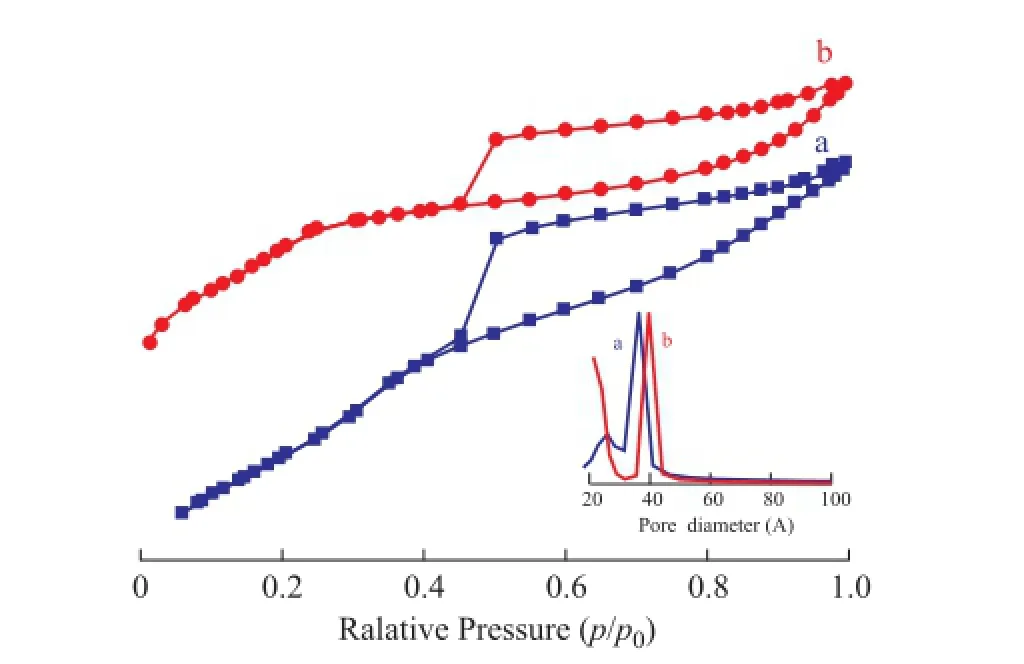
Figure 6 N2adsorption and desorption isotherms and poresize distribution (inset) of samples synthesized with (a) and without (b) SDS as a co-template
3.5 Catalytic activity of Ti-MSs
Finally, the catalytic performance of the as-synthesized Ti-MSs was evaluated during liquid-phase oxidation of styrene using 30% H2O2as an oxidant in order to find out how could the SDS co-template influence the catalyst activity of Ti-MSs. Benzaldehyde and styrene oxide were identifed as main products, together with a certain amount of over-oxidized products such as benzoic acid. The styrene conversion, epoxide selectivity, and molar ratio of Ti/Si synthesized in the presence of catalyst samples with or without employing SDS as co-template are summarized in Tables 2. In contrast, Ti-MSs synthesized without using SDS as co-template and Ti-MCM-41 prepared with CTAB used as a sole template according to the literature[7], are presented in Table 2. The conversion of styrene and the selectivity for styrene oxide were 25.6% and 10.2%, respectively, when the Ti-MCM-41 sample that was synthesized with CTAB serving as the sole template was used as the catalyst. The catalyst activity of Ti-MSs synthesized with SDS serving as the co-template could achieve a 40.4% conversion of styrene, a 54.2% selectivity for benzaldehyde and a 19.4% selectivity for styrene oxide, and was proved to be superior to Ti-MCM-41. It is worth noting that the catalyst selectivity for benzaldehyde is better than the nano-sized Ti-MSs as reported in the former research although the Ti/Si ratio is much lower[25]. Furthermore, results of recycling experiments indicate that the conversion of styrene and the selectivity for styrene oxide decreased obviously after one cycle and the Ti-MS slost its catalyst activity after 3 cycles.
As shown in Figure 3a, adding SDS co-template could decrease the surface hydrophilicity of materials which would facilitate the accessibility of active site and increase the catalytic performance according to literature research outcome[8]. The high surface area and pore volume of Ti-MSs could make more Ti sites of Ti-MSs activated, resulting in higher catalytic performance. Unfortunately, the selectivity of epoxide products was low due to the relative large pore size in Ti-MSs (Table 1) which would be more effective for oxidation of large molecular olefns. Further work is expected to introduce bulk molecular olefns to obtain an improved catalyst performance.
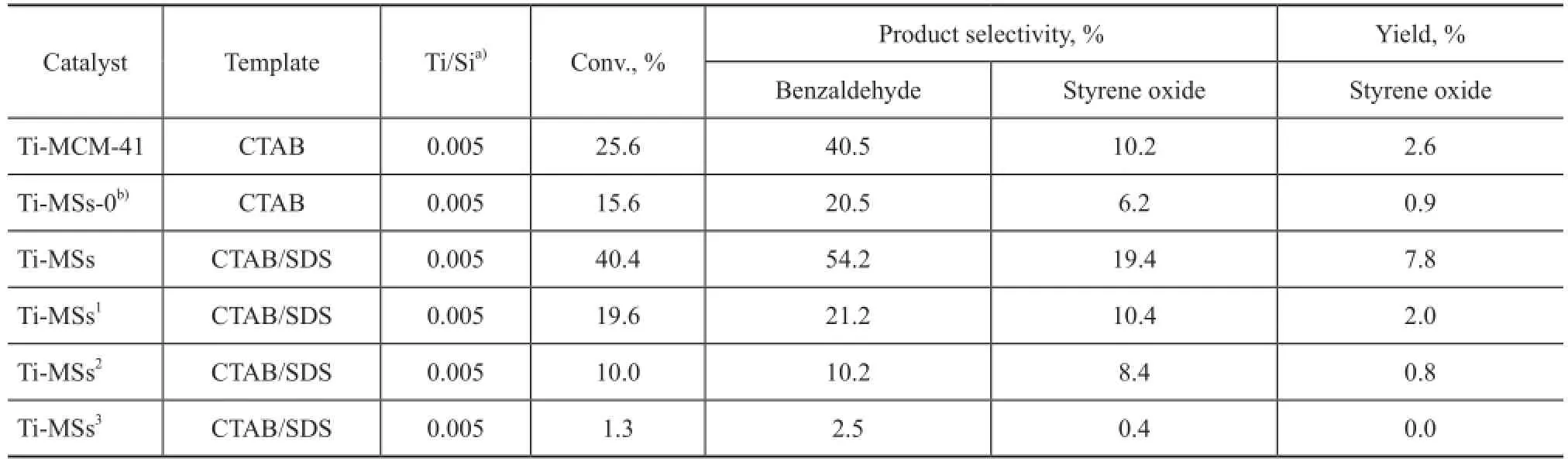
Table 2 Effect of catalytic activity of mesoporous titanium-silicate with H2O2used as oxidant on styrene conversion
4 Conclusions
The Ti-containing mesoporous silicate (Ti-MSs) with an ordered mesoporous structure was successfully synthesized using SDS as a co-template at a Ti/Si ratio of 0.005. The results show that the BET surface area was as high as 740.86 m2/g and the average pore diameter reached 3.18 nm. Ti-MSs showed high catalytic performance to achieve a styrene conversion of 40.4%, a 54.2% selectivity for benzaldehyde and a 19.4% selectivity for styrene oxide as compared with those achieved by Ti-MCM-41 particles. The better performance of the catalysts using SDS as the co-template was ascribed to the high BET surface area and the decrease in surface hydrophilic properties which could facilitate the accessibility of active sites. Further application of the obtained Ti-MSs with special structure will provide insights in bulk molecular catalysis due to effcient mass transport of guest molecules.
Acknowledgments: This study was fnancially supported by the Scientifc Research Foundation of Shandong University of Science and Technology for Recruited Talents (No. 2014RCJJ017).
[1] Kresge C T, Leonowicz M E, Roth W J, et al. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism[J]. Nature, 1992, 359(6397): 710-712
[2] Beck J S, Vartuli J C, Roth W J, et al. A new family of mesoporous molecular sieves prepared with liquid-crystal templates[J]. J Am Chem Soc, 1992, 114(27): 10834-10843
[3] Tanev P T, Chibwe M, Pinnavaia T J. Titanium-containing mesoporous molecular sieves for catalytic oxidation of aromatic-compounds[J]. Nature, 1994, 368(6469): 321-323
[4] Laha S C, Kumar R. Promoter-induced synthesis of MCM-41 type mesoporous materials including Ti- and V-MCM-41 and their catalytic properties in oxidation reactions[J]. Micropor Mesopor Mat, 2002, 53(1/3): 163-177
[5] Berube F, Nohair B, Kleitz F, et al. Controlled postgrafting of titanium chelates for improved synthesis of Ti-SBA-15 epoxidation catalysts[J]. Chem Mater, 2010, 22(6): 1988-2000
[6] Wang Y Y, Wang G, Yang M, et al. Highly effcient olefn oxidation catalysts based on regular nano-particles of titanium-containing mesoporous molecular sieves[J]. J Colloid Interf Sci, 2011, 353: 519-523
[7] Lin K F, Pescarmona P P, Vandepitte H, et al. Synthesis and catalytic activity of Ti-MCM-41 nanoparticles with highly active titanium sites[J]. J Catal, 2008, 254(1): 64-70
[8] Yu H, Haruhisa U, Takashi K, et al. Controlled synthesis and surface hydrophilic properties of Ti-containing mesoporous silica thin films using various structure-directing agents[J]. J Phys Chem C, 2011, 115(31): 15410-15415
[9] Xiao F, Han Y, Yu Y, et al. Hydrothermally stable ordered mesoporous titanosilicates with highly active catalytic sites[J]. J Am Chem Soc, 2002, 124 (6): 888-889
[10] Li X F, Xu Z H, Gao H X, et al. Preparation, characterization, and catalytic performance of a novel methylrich Ti-HMS mesoporous molecular sieve with high hydrophobicity[J]. Science China-Chemistry, 2010, 53(6): 1337-1345
[11] Fang X Q, Wang Q, Zheng A M, et al. Fluorine-planted titanosilicate with enhanced catalytic activity in alkene epoxidation with hydrogen peroxide[J]. Catalysis Science & Technology, 2012, 2(12): 2433-2435
[12] Kamegawa T, Suzuki N, Tsuji K, et al. Preparation of hydrophobically modified single-site Ti-containing mesoporous silica (TiSBA-15) and their enhanced catalytic performance[J]. Catalysis Today, 2011, 175(1): 393-397
[13] Fujiki J, Yamada H, Yogo K. Enhanced adsorption of carbon dioxide on surface-modified mesoporous silicasupported tetraethylenepentamine: Role of surface chemical structure[J]. Microporous and Mesoporous Materials , 2015, 215: 76-83
[14] Yoshii N, Fujimoto K, Okazaki S. Molecular dynamics study of the structure of anionic SDS, cationic DTAC, zwitterionic DDAO, and nonionic C12E8spherical micelles in solution[J]. Journal of Molecular Liquids, 2016, 217: 99-102.
[15] Wang J J, Lu J M, Yang J H, et al. Synthesis of ordered MCM-48 by introducing economical anionic surfactant as co-template[J]. Mater Lett, 2012, 78: 199-201
[16] Lin K, Pescarmona P P, Houthoofd K, et al. Direct roomtemperature synthesis of methyl-functionalized Ti-MCM-41 nanoparticles and their catalytic performance in epoxidation[J]. Journal of Catalysis, 2009, 263: 75-82
[17] Tabacchi G, Gianotti E, Fois E, et al. Understanding the vibrational and electronic features of Ti (IV) sites in mesoporous silicas by integratedab initioand spectroscopic investigations[J]. J Phys Chem C, 2007, 111(13):4946-4955
[18] Gianotti E, Bisio C, Marchese L, et al. Ti (IV) catalytic centers grafted on different siliceous materials: spectroscopic and catalytic study[J]. J Phys Chem C, 2007, 111(13): 5083-5089
[19] Peng R, Zhao D, Dimitrijevic N M, et al. Room temperature synthesis of Ti-MCM-48 and Ti-MCM-41 mesoporous materials and their performance on photocatalytic splitting of water[J]. J Phys Chem C, 2012, 116(1): 1605-1613
[20] Guidotti M, Ravasio N, Psaro R, et al. Epoxidation on titanium containing silicates: Do structural features really affect the catalytic performance?[J] J Catal, 2003, 214(2): 242-250
[21] Chen S Y, Mochizuki T, Abe Y, et al. Ti-incorporated SBA-15 mesoporous silica as an effcient and robust Lewis solid acid catalyst for the production of high-quality biodiesel fuels[J]. Applied Catalysis B: Environmental, 2014, 148-149: 344-356
[22] Qin Z, Shen B, Gao X, et al. Mesoporous Y zeolite with homogeneous aluminum distribution obtained by sequential desilication–dealumination and its performance in the catalytic cracking of cumene and 1,3,5-triisopropylbenzene[J]. J Catal, 2011, 278(2): 266-275
[23] Zelenka T. Adsorption and desorption of nitrogen at 77 K on micro- and mesoporous materials: Study of transport kinetics[J]. Microporous and Mesoporous Materials, 2016, 227: 202-209
[24] Springuel-Huet M A, Bonardet J L, Gedeon A, et al. Mechanical properties of mesoporous silicas and aluminasilicas MCM-41 and SBA-15 studied by N2adsorption and129Xe NMR[J]. Micropor Mesopor Mat, 2001, 44: 775-784
[25] Wang J J, Lu J M, Yang J H, et al. Ti containing mesoporous silica submicrometer-sphere with tunable particle size for styrene oxidation[J]. Applied Surface Science, 2013, 283(11): 794-801
Received date: 2016-03-19 Accepted date: 2016-06-24.
Dr. Wang Jingjing, Telephone: +86-532-86057104; E-mail: wjj20082001@163.com.
杂志排行
中国炼油与石油化工的其它文章
- Removal of Nitrogen Compounds from Shale Diesel Fraction Using Ionic Liquid [C4mim]HSO4
- Puri fi cation of Aromatics over a PromisingCatalyst
- Synthesis of Hierarchically Porous FAU/γ-Al2O3Composites with Different Morphologies via Directing Agent Induced Method
- Study on CO2Absorption by Aqueous Benzylamine and Its Formulations with Monoethanolamine as a Component for Post-Combustion Capture Process
- Effect of Magnetic Field on Tribological Properties of Lubricating Oils with and without Tricresyl Phosphate
- The Effect of Surfactant on Synthesis of ZSM-5 in a Super-Concentrated System
