Removal of Nitrogen Compounds from Shale Diesel Fraction Using Ionic Liquid [C4mim]HSO4
2016-03-22LiuJieMaBo
Liu Jie; Ma Bo
(1. College of Chemical Engineering, China University of Petroleum, Qingdao 266580; 2. College of Chemistry, Chemical Engineering and Environmental Engineering, Liaoning Shihua University, Fushun 113001)
Removal of Nitrogen Compounds from Shale Diesel Fraction Using Ionic Liquid [C4mim]HSO4
Liu Jie1,2; Ma Bo1,2
(1. College of Chemical Engineering, China University of Petroleum, Qingdao 266580; 2. College of Chemistry, Chemical Engineering and Environmental Engineering, Liaoning Shihua University, Fushun 113001)
Ionic liquid (IL) 1-butyl-3-methylimidazolium hydrosulphate ([C4mim]HSO4) was synthesized and its denitrogenation performance was investigated for diesel fraction with high content of nitride from oil shale. The effects of the temperature, the mass ratio of oil to IL, the mass ratio of water to IL, the extraction time, the settling time and the regeneration of IL on the N-removal effciency were studied. Experimental results showed that the ionic liquid [C4mim]HSO4exhibited excellent denitrogenation performance, and about a 90% basic N-extraction effciency and a 71% total N-extraction effciency were achieved under the conditions covering a temperature of 30 ℃, an oil/IL mass ratio of 7:1, a H2O/ IL mass ratio of 2:1, an extraction time of 20 min and a settling time of 120 min. In addition, the basic N-removal effciency can still reach 74% during fve recycles of the ionic liquid.
ionic liquid; denitrogenation; shale diesel fraction
1 Introduction
With the shortage of global petroleum resources, the unconventional resources, such as oil sands bitumen, extraheavy oil, and oil shale, have been attracting more and more interest across the world[1]. Oil shale, generally defned as the sedimentary rock rich in kerogen[2], is already an important energy source in a few countries and is expected to be one of promising alternatives to petroleum resource because of its high abundance. It is conservatively estimated that there are at least 10 trillion tons of oil shale resources around the world, and the liquid oil extracted from oil shale through the process of retorting is called shale oil and its resource is about 50% more than the total known conventional oil resources which is about 0.27 trillion tons[3-5]. Compared with the conventional petroleum resources, shale oil is a rather complex liquid organic mixture, containing thousands of hydrocarbons and oxygen-, sulfur- and nitrogen-containing organic compounds[6-7], and it is well known that the high heteroatomic concentration in shale oils has an adverse influence on their potential exploitation as substitute transport fuels[8]. For example, shale oil from the Fushun oil shale contains a large amount of basic nitrides such as pyridine, quinoline and their derivatives, and neutral nitrides, e.g. carbazole, indole and their derivatives[9], as shown in Figure 1. The compounds containing nitrogen (N-compounds) not only can reduce the storage stability of oil product, resulting in the increase of resins and black color, but also can lead to NOx emission during combustion of fuel oil, which can pollute the environment[10-11]. Therefore the development of upgrading processes to remove heteroatoms from shale oil has become the important areas of studies. The catalytic hydrogenation method has also been applied to shale oils to remove N-compounds[12-14], however, the technology cannot remove certain heterocyclic species effectively because of the steric hindrance encountered by these compounds on the surface of the catalyst, and moreover, the N-compounds can affect the activity of hydrogenation catalyst and make the desulfurization effect worsen[15-16]. To lower S-/N-content in fuel oils to an ultralow level using hydrogenation technology, harsher operating conditions (e. g. high pressure and high hydrogen/oilratio, etc.) are needed. So selective removal of nitrogen compounds from feeds prior to hydrogenation strongly enhances further deep desulfurization and increases the catalyst lifetime[17]. Non-hydrogenation denitrogenation technology has attracted much attention from researchers because of its small equipment investment, simple process and low operating cost[18-20].
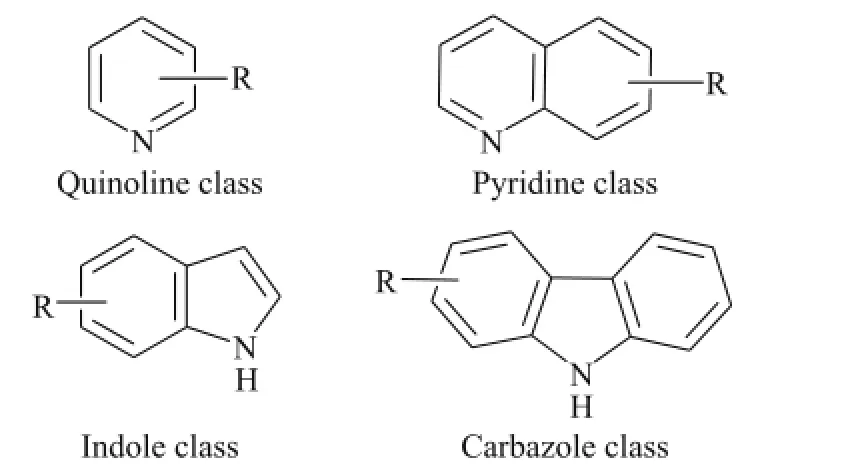
Figure 1 N-compounds present in shale oil
In recent years, the research on removal of N-compounds with ionic liquids (ILs) has made a defnite progress[21-24]. As a green solvent, ILs consist mostly of large organic cations and organic/inorganic anions. Compared with the conventional extraction solvents, ILs have some advantages[25-26], such as high thermal/chemical stability over a wide temperature range, non-volatility (a desirable property for avoiding fugitive emissions in engineering applications), immiscible with fuel oils, higher affinity to N-compounds and good recyclability. However, the studies on denitrogenation with ILs were mainly confned to model oil, and their denitrogenation performance applied on actual oil was rarely studied. In addition, the denitrogenation of shale oil containing a large amount of nitrides with ionic liquid has not been reported. Therefore, in this study, 1-butyl-3-methylimidazolium hydrosulphate ([C4mim]HSO4) was synthesized and used as the extractant to remove N-compounds from diesel distillate derived from the Fushun shale oil, and the work may provide a new approach for shale oil denitrogenation.
2 Experimental
2.1 Reagent and apparatus used in experiments
2.1.1 Experimental reagent
N-Methylimidazole (99%) was purchased from the Zhejiang Kaiyue Chemical Plant. 1-Bromobutane (99%) was purchased from the Shanghai Laboratory Reagent Co., Ltd. Ethyl acetate (99%) and acetone (99%) were supplied by the Tianjin Damao Chemical Plant. Sodium hydrosulfate (99%) was purchased from the Sinopharm Chemical Reagent Co., Ltd.N-Methylimidazole was further purifed by distillation, and other chemicals were used as received without purifcation.
2.1.2 Experimental apparatus
The experimental devices included: a constant temperature magnetic heating stirrer DF-101S (Gongyi City Instrument Co., Ltd, China); a TSN-5000 series fluorescence nitrogen/sulfur analyzer (Jiangfen Electroanalytical Co., Ltd., China); an electronic balance FA2104N (with a precision of 0.000 1 g, Shanghai Jingke Scientific Instruments Co., Ltd., China); an automatic potentiometric titrator ZD-2(A) (Shanghai Dapu Instruments Co., Ltd., China); a vacuum oven ZK-82J (Shanghai Experimental Instrument Factory, China); a Cary 600 series FTIR spectrometer (American Agilent Technologies Corp.); a nuclear magnetic resonance spectrometer type Varian Mercury-Plus 300BB (American Varian ).
The FT-IR and1H NMR characterization of ionic liquid was carried out in the Fushun Research Institute of Petroleum and Petrochemicals (FRIPP).
2.2 Preparation of ionic liquid
1-Butyl-3-methylimidazolium hydrosulphate ([C4mim] HSO4) was synthesized using the method described in the literature[27], with the synthesis route shown in Scheme 1.1H NMR (500MHz, DMSO): δ0.895 (3H, t), 1.257 (2H, m), 1.802 (2H, m), 3.907 (3H, s), 4.234 (2H, t), 7.790 (1H, s), 7.865 (1H, s), 9.325 (1H, s).
FT-IR (KBr, disc):ν3 437, 3 135, 3 061, 2 958, 2 871, 1 642, 1 560, 1 460, 1 425, 13 33, 1 118, 1 048, 831 cm-1.
2.3 Experimental feedstock
The shale oil used in the present study was obtained from Fushun, and it contained about 40% of diesel distillate. The diesel distillate with a boiling range of 200—350 ℃ was obtained by fractionating the shale oil on a true-boiling-point distillation apparatus and was used as the experimental feedstock, with its main properties shown in Table 1.
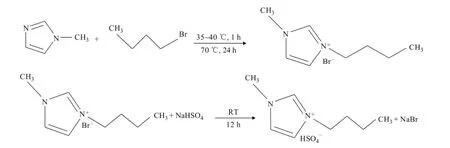
Scheme 1 Synthesis route of [C4mim]HSO4
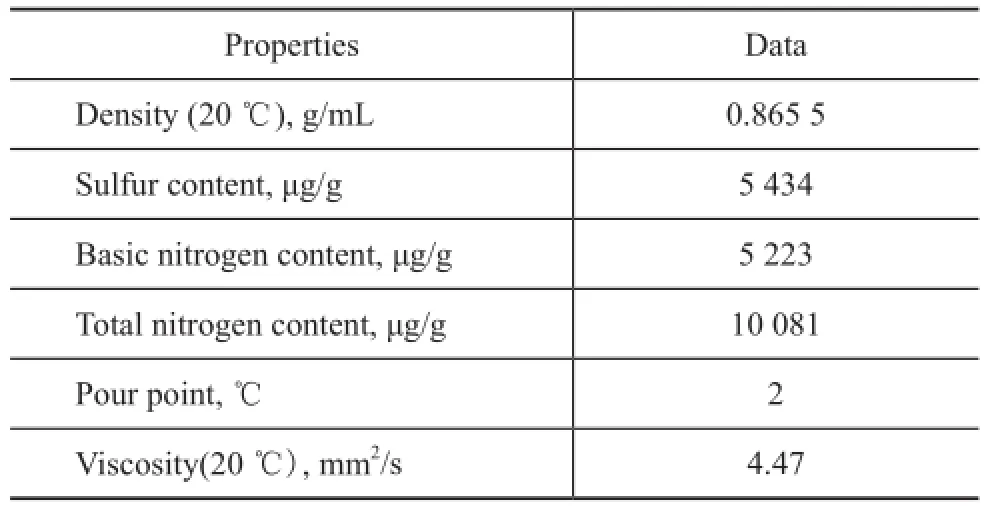
Table 1 Properties of diesel fraction from Fushun shale oil
2.4 Denitrogenation experiment procedure and N-content analysis
In a typical experiment, the diesel fraction, IL and water were placed in a 50 mL beaker and were magnetically stirred at a specified temperature. After the reaction continued to take place within a specified time, the reaction mixture was subject to settling and stratifcation and the nitrogen content in the upper oil phase was analyzed.
The determination of basic nitrogen content adopted the perchloric acid-glacial acetic acid titration method (SH/T 0162—1992, China), and the total nitrogen content was analyzed on a TSN-5000 series fluorescence nitrogen/ sulfur analyzer equipped with a liquid auto-sampler. The extraction effciency (E) of N-compounds is determined according to the following formula:
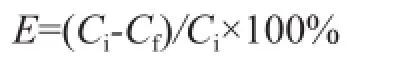
whereCiandCfare the initial and final total (basic) nitrogen contents in diesel fraction.
2.5 Regeneration of IL
Recycle experiments were performed using carbon tetrachloride as a back extractant. After the denitrogenation experiment was fnished, the oil phase was separated from IL by a separating funnel. The IL layer was washed with a same quantity of carbon tetrachloride for 3—5 times, and was then evaporated under vacuum to remove carbon tetrachloride. The regenerated IL was used for further extraction of fresh shale diesel fraction under the same operating conditions to investigate its denitrogenation performance.
3 Results and Discussion
3.1 Effect of temperature on N-removal ef fi ciency
Five sets of experiments at various temperatures were carried out to investigate the effect of extraction temperature on N-removal efficiency, with the experimental results shown in Figure 2.
It can be seen from Figure 2 that the total N-removal efficiency tended to fluctuate with different extraction temperatures. However, the fuctuation was not very obvious, and there was only a difference of 6.2% between the highest N-removal effciency at 40 ℃ and the lowest N-removal effciency at 80 ℃. Basic N-removal effciency decreased slightly with an increasing temperature. In the process of denitrogenation with [C4mim]HSO4, the anion [HSO4]-played a very important role, because the hydrogen bond interaction between it and N-compounds existed[28]. Especially for basic N-compounds which contained lone pair electrons on N atoms, the H+provided by the [HSO4]-anion attacked the lone pair electrons of the N atom, which was followed by a complex reaction between the H+and N-compound. So [C4mim]HSO4showed good denitrogenation performance for removing basic N-compounds.
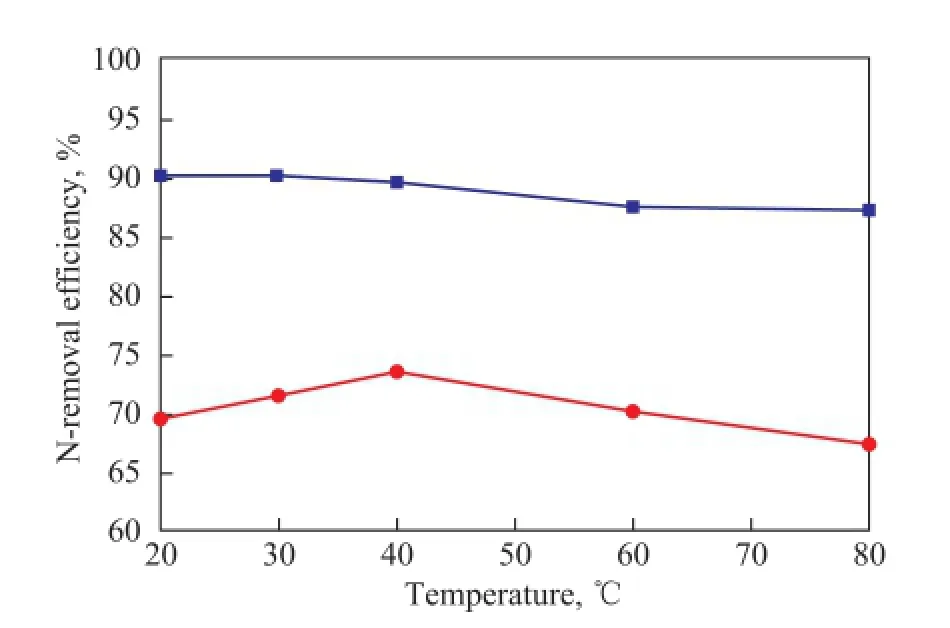
Figure 2 Effect of temperature on N-removal ef fi ciency
Although the viscosity of IL reduced with the increase of temperature, which was beneficial to the sufficient contact between IL and N-compounds in diesel fraction, leading to an increased total N-removal efficiency, while the increase of temperature was not conductive to hydrogen bond interaction and complex reaction (exothermic reaction) towards the positive direction. So denitrogenation with the ionic liquid can be conducted at or above the room temperature, and the temperature was determined as 30 ℃ in the study in order to consume less energy and make it more viable for industrial applications. The study on denitrogenation of shale diesel fraction with ionic liquid was performed for the first time, and the extraction effciency of total N-compounds and basic N-compounds could reach above 70% and 90%, respectively. Compared with other non-hydrodenitrogenation methods for treating shale oil[29], higher total N-extraction efficiency was achieved at lower temperature with less denitrogenation agent used.
3.2 Effect of oil/IL mass ratio on N-removal ef fi ciency
The oil/IL mass ratios considered covered 3:1, 7:1, 10:1, 20:1 and 30:1, with the results depicted in Figure 3. It can be seen that oil/IL mass ratio had a very signifcant effect on the N-removal effciency. The effciency for removal of basic nitrogen and total nitrogen reduced from 93.26%, 72.94% to 46.74%, 40.01%, respectively when the oil/IL mass ratio increased from 3:1 to 30:1. Obviously, the less the ionic liquid was, the smaller the probability of contact between the nitrogen compounds and IL would be, and it was not favorable to the denitrogenation reaction. Upon considering the cost of ionic liquid as well as the denitrogenation effect, the suitable oil/IL mass ratio was determined as 7:1 in the study.
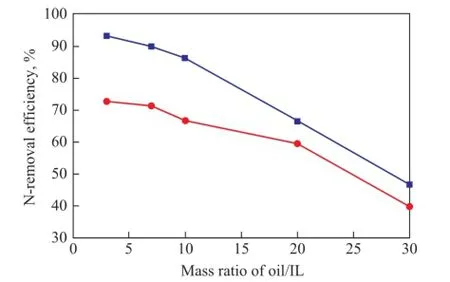
Figure 3 Effect of mass ratio of oil /IL on N-removal ef fi ciency
3.3 Effect of water/IL mass ratio on N-removal ef fi ciency
The effect of H2O/IL mass ratio on denitrogenation was shown in Figure 4. As it can be seen from Figure 4, when the H2O/IL mass ratio was 0, i.e., without water, the N-removal efficiency was the lowest, which can be attributed to the sticking of ionic liquid with higher viscosity to the bottle in the process of denitrogenation experiment, leading to insufficient contact with the diesel fraction. The addition of deionized water can reduce the viscosity of ionic liquid, which can make it mix with diesel fraction more suffciently, resulting in an increased N-removal efficiency. However, when the H2O/IL mass ratio washigher than 2, the effciency for removal of basic and total nitrogen compounds all decreased with the increase of the amount of water usage, which was resulted from poorer layering effect because the addition of too much water made surface tension between oil and water increased. So the suitable H2O/IL mass ratio was selected as 2:1 in this study.
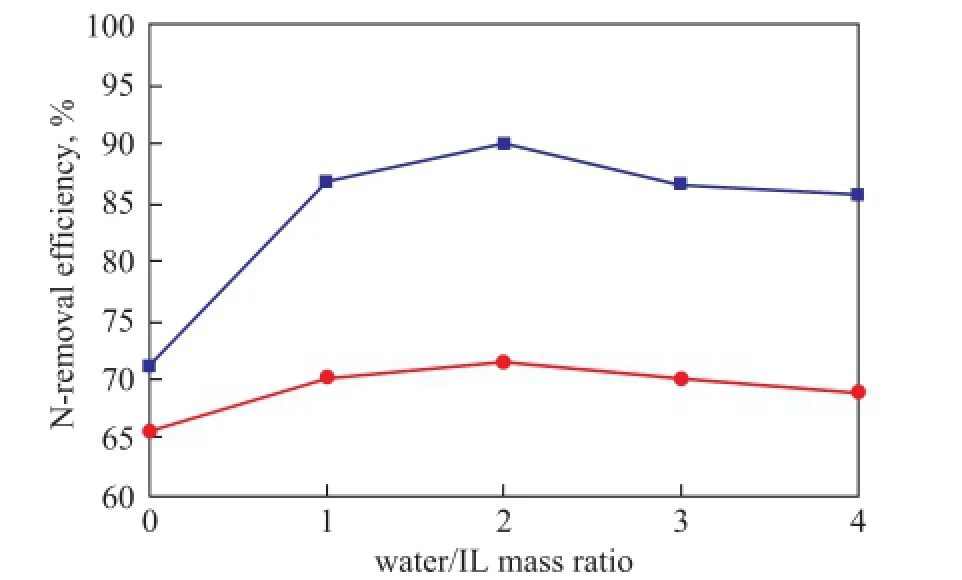
Figure 4 Effect of water/IL mass ratio on N-removal ef fi ciency
3.4 Effect of extraction time on N-removal ef fi ciency
Figure 5 presents the denitrogenation performance of [C4mim]HSO4for shale diesel distillate at different extraction time.
It can be seen from Figure 5 that the basic and total N-removal effciency increased slightly with the extension of extraction time. For example, the efficiency for extraction of basic and total nitrogen compounds increased from 88.47% and 68.31% at 5 min to 89.98% and 71.33% at 20 min, respectively. Moreover, the N-removal rate did not change basically after 20 min, which indicated that the extraction process reached an equilibrium in a shorter time because of faster mass transfer and reaction between [C4mim]HSO4and N-compounds. The extraction time was set at 20 min in the study to save the operating time and increase the effciency.
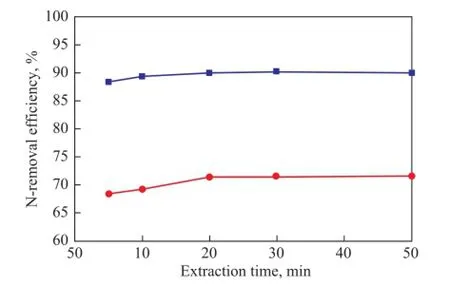
Figure 5 Effect of extraction time on N-removal ef fi ciency
3.5 Effect of settling time on N-removal ef fi ciency
It was very necessary to make IL containing undesirable components (e.g. N-compounds) separated from diesel fraction by the settling and stratification process to improve the denitrogenation effect. The effect of settling time on N-removal effciency is shown in Figure 6. Obviously, when the settling time was extended from 30 min to 120 min, the effciency for extraction of basic and total nitrogen compounds increased from 85.12% and 62.56% to 89.98% and 71.33%, respectively, and the N-extraction effciency remained nearly constant at a settling time of longer than 120 min. So 120 min can be regarded as the suitable time for the separation of IL and the diesel fraction.
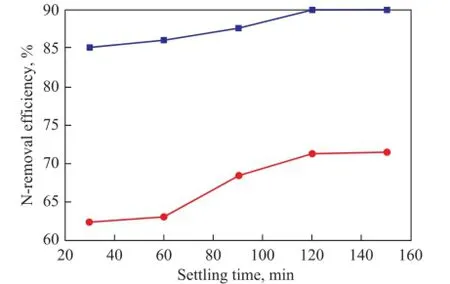
Figure 6 Effect of settling time on N-removal ef fi ciency
3.6 Regeneration of IL
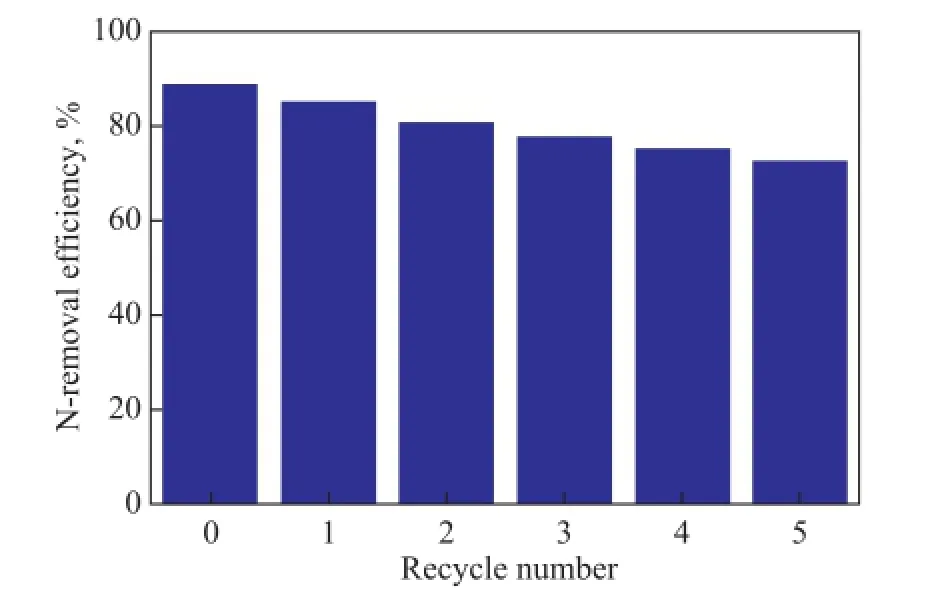
Figure 7 The relationship between basic N-extraction ef fi ciency and recycle number
The recycling of IL was very important for industrial application. The denitrogenation performance of [C4mim] HSO4after its regeneration is shown in Figure 7. The basic nitrogen content in carbon tetrachloride after the last washing cycle of used ionic liquid was determined to be 50 μg/g, which showed that some basic N-compounds contained in ionic liquid had been transferred to carbon tetrachloride. As it can be seen from Figure 7, the re-generated ionic liquid [C4mim]HSO4still had a definite ability to remove basic N-compounds and about a 74% N-removal effciency was retained after 5 recycles, which showed that most of N-compounds contained in IL could be removed by washing with carbon tetrachloride and its denitrogenation performance was relatively stable. Upon considering the slight decrease in denitrogenation performance of [C4mim]HSO4after regeneration, more effcient regeneration method needs to be further studied in the future.
3.7 Properties of re fi ned diesel fraction
The properties of the diesel fraction obtained by refning with the ionic liquid [C4mim]HSO4are shown in Table 2. As it can be seen from Table 2, the sulfur content, density and pour point of refned oil were decreased and the oil quality was improved after denitrogenation with ionic liquid.
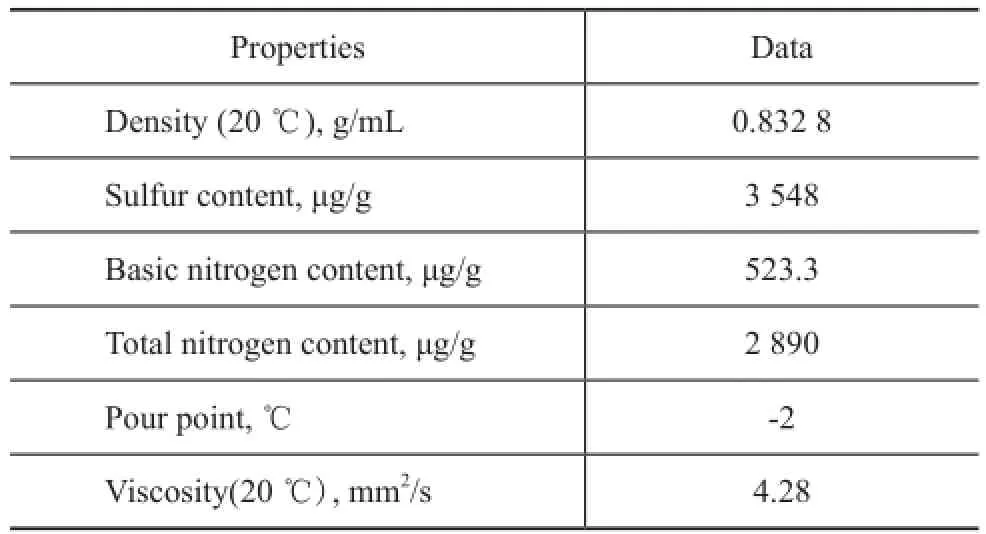
Table 2 Properties of refined diesel fraction
4 Conclusions
For diesel fraction originated from Fushun shale oil containing a large amount of nitrogen compounds, e. g., about 0.52% of basic nitrogen content and 1.01% of total nitrogen content, its denitrogenation pretreatment is very necessary prior to hydrogenation to reduce the operating cost and energy consumption. The ionic liquid [C4mim]HSO4is synthesized and applied to denitrogenation of shale diesel fraction. Under the suitable operating conditions, i. e., a temperature of 30 ℃, an extraction time of 20 min, a H2O/IL mass ratio of 2:1, an oil/IL mass ratio of 7:1 and a settling time of 120 min, an effciency for removal of about 90% of basic nitrogen compounds and 71% of total nitrogen compounds is achieved and the basic N-removal effciency can still reach 74% after the ionic liquid is recycled for 5 times. Moreover, the sulfur content, density and pour point of refned oil are also decreased after denitrogenation with the ionic liquid. Compared with the conventional solvent, the denitrogenation performance of [C4mim]HSO4is competitive. All these results indicate that ILs have the potential to become an environmentally benign pre-treatment alternative for denitrogenation of the diesel fraction from shale oil.
Acknowledgements. The authors thank the Fushun Research Institute of Petroleum and Petrochemicals, SINOPEC, for supporting the ionic liquids characterization in this work.
[1] Fu J M, Klein G C, Smith D F, et al. Comprehensive compositional analysis of hydrotreated and untreated nitrogenconcentrated fractions from syncrude oil by electron ionization, feld desorption ionization and electrospray ionization ultrahigh-resolution FT-ICR mass spectrometry [J]. Energy & Fuel, 2006, 20(3): 1235-1241
[2] Jin J M, Kim S, Birdwell J E. Molecular characterization and comparison of shale oils generated by different pyrolysis methods [J]. Energy & Fuels, 2012, 26(26): 1054-1062
[3] Hou J. L, Ma Y, Li S Y, et al. Development and utilization of oil shale worldwide[J]. Chemical Industry and Engineering Progress, 2015, 34(5): 1183-1190 (in Chinese)
[4] Dyni J R. Geology and resources of some world oil shale deposits [J]. Oil Shale, 2003, 20(3): 193-252
[5] Li D M, Tang D Z, Yang Y F. Advances in oil-shale resources: development and utilization [J]. Petroleum Exploration and Development, 2006, 33(6): 657-661 (in Chinese)
[6] Tong J H, Liu J G, Han X X, et al. Characterization of nitrogen-containing species in Huadian shale oil by electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry [J]. Fuel, 2013, 104(2): 365-371
[7] Bae E J, Na J G, Chung S H, et al. Identifcation of about 30000 chemical components in shale oils by electrospray ionization (ESI) and atmospheric pressure photoionization (APPI) coupled with 15 T Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS) and a comparison to conventional oil [J]. Energy & Fuels, 2010, 24(4): 2563-2569
[8] Williams P T, Chishti H M. Reaction of nitrogen and sul-phur compounds during catalytic hydrotreatment of shale oil [J]. Fuel, 2001, 80(7): 957-963
[9] Chen Xiaobo, Li Teng, Liu Yibin, et al. Characterization of nitrogen compounds in vacuum residue and their structure comparison with coker gas oil[J]. China Petroleum Processing and Petrochemical Technology, 2014, 16(3): 33-41
[10] Zhang J, Xu J, Qian J, et al. Denitrogenation of straightrun diesel with complexing extraction [J]. Petroleum Science and Technology, 2013, 31(8): 777-782
[11] Almarri M, Ma X L, Song C S. Selective adsorption for removal of nitrogen compounds from liquid hydrocarbon streams over carbon- and alumina-based adsorbents [J]. Industrial & Engineering Chemistry Research, 2009, 48(2): 951-960
[12] Yu H, Li S Y, Jin G Z. Hydrotreating of the diesel distillate from Huadian shale oil for production of clean fuel [J]. Journal of Fuel Chemistry and Technology, 2010, 38(3): 297-301 (in Chinese)
[13] Yu H, Li S Y, Jin G Z. Kinetics of hydrodesulfurization of diesel distillate from Fushun shale oil [J]. Acta Petrolei Sinica (Petroleum Processing Section), 2011, 27(6): 924-928 (in Chinese)
[14] Yu H, Li S Y, Jin G Z. Hydrodesulfurization and hydrodenitrogenation of diesel distillate from Fushun shale oil[J]. Oil Shale, 2010, 27(2): 126-134
[15] Beltramone A R, Crossley S, Resasco D E, et al. Inhibition of the hydrogenation and hydrodesulfurization reactions by nitrogen compounds over NiMo/Al2O3[J]. Catalysis Letters, 2008, 123(3/4): 181-185
[16] Asumana C, Yu G R, Guan Y W, et al. Extractive denitrogenation of fuel oils with dicyanamide-based ionic liquids [J]. Green Chemistry, 2011, 13(11): 3300-3305
[17] Palou R M, Luque R. Applications of ionic liquids in the removal of contaminants from refnery feedstocks: an industrial perspective [J]. Energy & Environment Science, 2014, 7(8): 2414-2447
[18] Li G X, Han D Y, Cao Z B, et al. Study on new processing technology of Fushun shale oil [J]. Modern Chemical Industry, 2011, 31(2): 74-78 (in Chinese)
[19] Zhang Z M, Zhao D Z, Zhang H M, et al. Removal of basic nitrogen compounds from shale oil [J]. Journal of Liaoning Shihua University, 2011, 31(3): 24-27 (in Chinese)
[20] Xu M, Chen D F, Xiao S Q, et al. Experimental study on denitrogenation process of Daqing shale oil [J]. Acta Petrolei Sinica (Petroleum Processing Section), 2012, 28(1): 55-59 (in Chinese)
[21] Wang H, Xie C X, Yu S T, et al. Removal of non-basic nitrogen in model oil with functionalized acidic ionic liquid [J]. Journal of Fuel Chemistry and Technology, 2014, 42(1): 55-59 (in Chinese)
[22] Xie L L, Favre-Reguillon A, Pellet-Rostaing S, et al. Selective extraction and identification of neutral nitrogen compounds contained in straight-run diesel feed using chloride based ionic liquid [J]. Industrial & Engineering Chemistry Research, 2008, 47(22): 8801-8807
[23] Wang H, Xie C X, Yu S T, et al. Denitrifcation of simulated oil by extraction with H2PO4-based ionic liquids[J]. Chemical Engineering Journal, 2014, 237(1): 286-290
[24] Chen X C, Yuan S, Abdeltawab A A, et al. Extractive desulfurization and denitrogenation of fuels using functional acidic ionic liquids[J]. Separation and Purification Technology, 2014, 133: 187-193
[25] Wang Baofeng, Han Shaohua, Zhang Jinjun. Hydrothermal liquefaction of wheat straw in sub-critical water/ethanol with ionic liquid for bio-oil production[J]. China Petroleum Processing and Petrochemical Technology, 2015, 17(4): 81-88
[26] Wang Haojie, He Jianxun, Yang Cairong, et al. Deep extractive desulfurization of gasoline with ionic liquids based on metal halide[J]. China Petroleum Processing and Petrochemical Technology, 2014, 16(2): 65-70
[27] Xiao J, Wang Q, Zhou M D, et al. Extractive and oxidative desulfurization of fuel oils using hydrosulfate based ionic liquids [J]. Journal of Petrochemical Universities, 2013, 26(1):21-24 (in Chinese)
[28] Eun S H, Alexey Z, Jelliarko P, et al. Zn-containing ionic liquids for the extractive denitrogenation of a model oil: a mechanistic consideration[J]. Energy & Fuels, 2009, 23(6): 3032-3038
[29] Han D Y, Li G X, Cao Z B, et al. A study on the denitrogenation of Fushun shale oil [J]. Energy Sources, Part A, 2013, 35(7): 622-628
Received date: 2016-03-09; Accepted date: 2016-04-24.
Dr. Liu Jie, E-mail: lj13898309829 @163.com.
杂志排行
中国炼油与石油化工的其它文章
- Synthesis of Hierarchically Porous FAU/γ-Al2O3Composites with Different Morphologies via Directing Agent Induced Method
- Puri fi cation of Aromatics over a PromisingCatalyst
- The Effect of Surfactant on Synthesis of ZSM-5 in a Super-Concentrated System
- Study on CO2Absorption by Aqueous Benzylamine and Its Formulations with Monoethanolamine as a Component for Post-Combustion Capture Process
- Effect of Magnetic Field on Tribological Properties of Lubricating Oils with and without Tricresyl Phosphate
- Improved Ti-containing Mesoporous Silica Catalyst Synthesized by Using Anionic Surfactant as Co-template
