The Effect of Surfactant on Synthesis of ZSM-5 in a Super-Concentrated System
2016-03-22LiHaiyanQinLihongGaoGuangboSunFamin
Li Haiyan; Qin Lihong; Gao Guangbo; Sun Famin
(1. Daqing Chemical Research Center, Petrochemical Research Institute, PetroChina Company Limited, Daqing 163714; 2. China University of Petroleum, Beijing 102249; 3. Liaohe Oil fi eld Company Production Operation Division, PetroChina Company Limited, Panjin 124010)
The Effect of Surfactant on Synthesis of ZSM-5 in a Super-Concentrated System
Li Haiyan1; Qin Lihong2; Gao Guangbo3; Sun Famin1
(1. Daqing Chemical Research Center, Petrochemical Research Institute, PetroChina Company Limited, Daqing 163714; 2. China University of Petroleum, Beijing 102249; 3. Liaohe Oil fi eld Company Production Operation Division, PetroChina Company Limited, Panjin 124010)
ZSM-5 zeolite was synthesized in a super-concentrated system using different kinds of surfactants. The ZSM-5 samples were characterized by XRD, SEM, FT-IR and BET techniques. The surfactant could change the properties of ZSM-5 zeolite, including the crystallinity, the crystal grain size, the surface area, the pore volume and the Si/Al mole ratio.
ZSM-5; super concentrated; surfactant
1 Introduction
ZSM-5 zeolite used as the catalyst exhibits excellent features such as thermal stability, resistance to deactivation, high acidic activity and molecular shape selectivity. In many applications particularly FCC[1], gasoline aromatization[2], MTO[3-4], MTP[5-7], alkylation[8-10]etc., ZSM-5 zeolite dominates many established and most of new processes. In general, ZSM-5 is synthesized in a hydrothermal system[11-12]. The pathway of the crystallization process of MFI-type zeolite is infuenced by different variables[13-15], viz.: the silicon and aluminum source, the aluminum content, the template/silicon ratio, the nature of cations existing in the synthesis medium, the alkalinity, the temperature of crystallization, the presence of seeds, the water content, etc. However, the production cost of ZSM-5 zeolite is a problem that infuences the extensive use of ZSM-5. The H2O/SiO2molar ratio adopted in the conventional hydrothermal method is about 20—500 generally. The high H2O/SiO2molar ratio for hydrothermal method has the defects such as low solid content, low synthesis efficiency, and more wastewater discharge. In this paper, a method for synthesis of super-concentrated system is designed[16]. This method has the advantages such as short crystallization time, less wastewater, high synthesis effciency, etc. The effect of surfactant on synthesis of ZSM-5 zeolite in the super-concentrated system is mainly studied.
2 Experimental
The synthesis of ZSM-5 zeolite was carried out in a superconcentrated system. The molar ratio of components in the prepared mixture covered: 25—200 SiO2/Al2O3, 0.05—0.5 Na2O/SiO2, 1.0—3.0 H2O/SiO2, 0.0003—0.0023 SDS/SiO2, and 0.018—0.045 OP-10/SiO2. The abbreviation SDS denotes the surfactant sodium dodecylsulfate. The trade name OP-10 denotes the surfactant nonylphenol polyoxyethylene ether.
Sodium aluminate and sodium hydroxide were dissolved in distilled water to form the aqueous solution A. The surfactants and ZSM-5 seeds were added to the aqueous solution A under stirring at room temperature for 10—30 min to obtain the aqueous solution B. The solid silica was added to the aqueous solution B to obtain the final mixture. The fnal mixture was stirred at room temperature for 30—60 min and was transferred into a teflonlined stainless-steel autoclave prior to being subject to crystallization by thermal treatment under autogenous pressure and static conditions at 170 ℃ for 24 h. Thereafter, the autoclave was quenched immediately with cold water. The solid product was separated by centrifugation, washed several times with distilled water, and then driedovernight at 110 ℃ and calcined in air at 550 ℃ for 4—6 h.
The powder XRD patterns of zeolite samples were recorded using a Philips X’pert Model MPD diffractometer operating at 40 kV and 30 mA using CuKα radiation over a 2θrange from 1° to 46°. The crystallinity was determined from the peak area between 2θ=22°—25° using a highly crystalline ZSM-5 sample as the reference. Nitrogen isotherms at 77 K were determined using a volumetric adsorption apparatus (Micromeritics, ASAP 2010). The surface area was estimated according to the BET method. The pore size distribution was obtained via applying the BJH model with cylindrical geometry of the pores and by using the Harkins and Jura equation for determining the adsorbed layer thickness. The morphology and size of the crystallites were determined from the scanning electron microscopy images taken with a scanning microscope (Cambridge S-360). The FT-IR spectra were recorded on an infrared spectrometer (Nicolet FT-IR-560 plus).
3 Results and Discussion
3.1 Effect of surfactant on crystallinity and pore structure
To study the effect of surfactant on the crystallinity of ZSM-5 zeolite synthesized in the super-concentrated system, Figure 1 shows the X-ray diffraction patterns of the as-synthesized samples using different types of surfactants. Only the ZSM-5 phase was formed judging from the patterns of the samples. Table 1 illustrates the effect of different types of surfactants on the crystallinity and crystal grain size. The results show that the crystallinity of ZSM-5 increased greatly, when surfactants such as SDS and OP-10 were added during the synthesis of ZSM-5. The crystallinity of ZSM-5 increased from 85% to 90%, when only SDS was added. The crystallinity of ZSM-5 further increased from 90% to 112%, when OP-10 was also added on the basis of SDS.
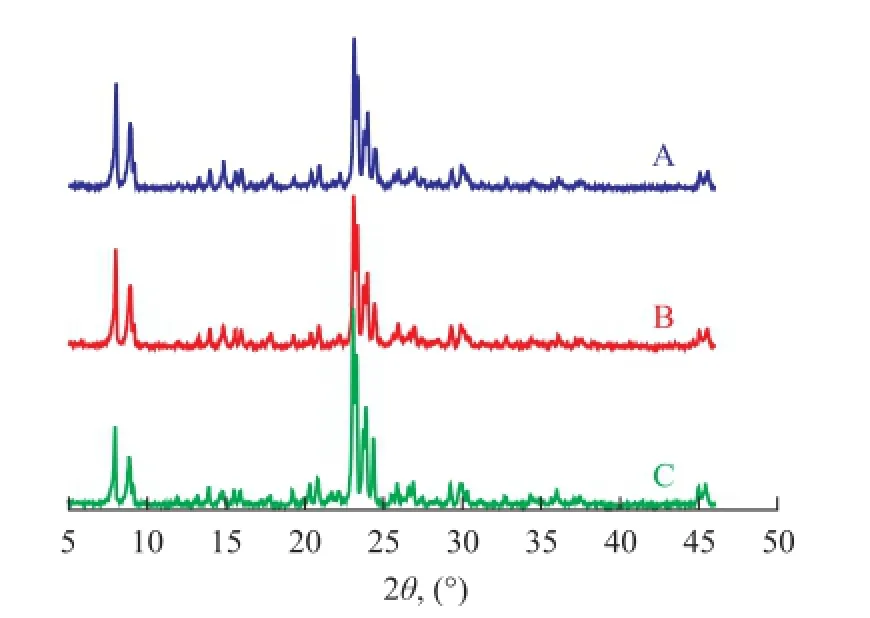
Figure 1 Effect of the surfactant on crystallinity
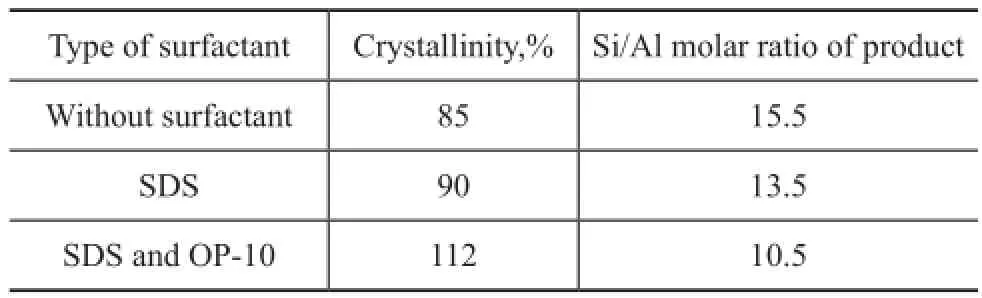
Table 1 Effect of the surfactant on crystallinity
Table 2 illustrates the effect of surfactant on the surface area and pore volume of ZSM-5. The BET surface area of ZSM-5 increased in the following order: 269.0 m²/g (without addition of surfactant) <283.2 m²/g (with addition of SDS)<409.2 m²/g (with addition of SDS and OP-10). When both SDS and OP-10 were added during the synthesis of ZSM-5, the external surface area of ZSM-5 reached 58.96 m²/g and the micropore surface area increased from 231.8 m²/g to 350.2 m²/g. The micropore volume and external micropore volume all increased greatly.
3.2 Effect of surfactant type on Si/Al molar ratio
Figure 2 displays the IR spectra of the ZSM-5 samples synthesized with different types of surfactants such as SDS and OP-10. The results show that the absorption bands of the samples were seen at about 550, 1 080, 1 230, 790, and 450 cm-1. There was only ZSM-5 phase formed. The adsorption band at about 1230 cm-1shifted to thelower wavenumber when the surfactants were added into the prepared mixture in sequence. It is well known that the Al-O bond was longer in the silica tetrahedron and the electronegativity of Al atom was weaker than the electronegativity of Si atom. The Si atom and the Al atom had the similar behavior. As a result, the decreasing Si/Al molar ratio led to lower force constant activity between Si atom and Al atom. Therefore, the characteristic peaks of ZSM-5 shifted to lower wavenumber. According to the changes in vibration frequency of ZSM-5 framework, we can calculate the Si/Al molar ratio of the ZSM-5 zeolite. The framework vibration led to bending vibration at the outside of tetrahedron, which could infuence the crystalline structure of ZSM-5 sensitively. Furthermore, the Si/Al molar ratio can influence the bonds at around 1 230 cm-1more sensitively. On the other hand, the XRD patterns (Figure 3) followed the same rules. The X-ray characteristic diffraction peaks at 22°—25° shifted to the lower degree when different surfactants were added during the synthesis of ZSM-5. The results illustrate that the Si/Al molar ratio of the ZSM-5 sample varied with the IR spectra too. The use of surfactants such as SDS and OP-10 decreased the Si/Al molar ratio of the ZSM-5 sample partly. Table 1 illustrates that the Si/Al molar ratio of the ZSM-5 product also decreased with the use of different surfactants as evidenced by chemical analyses.
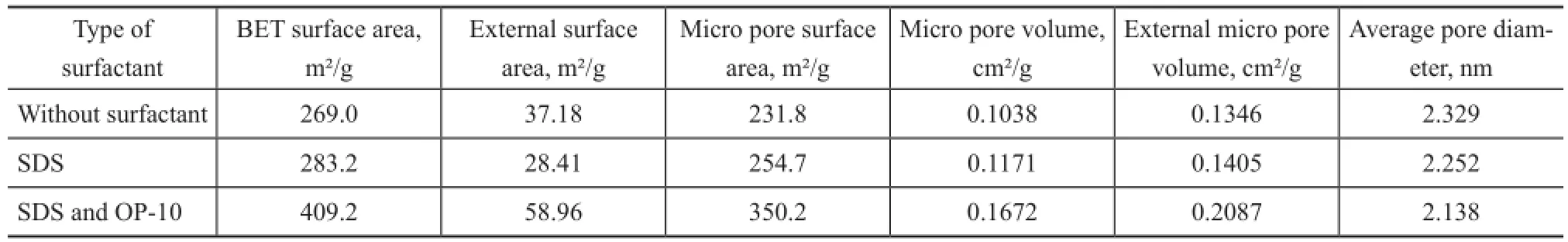
Table 2 Effect of the surfactant on surface area and pore structure
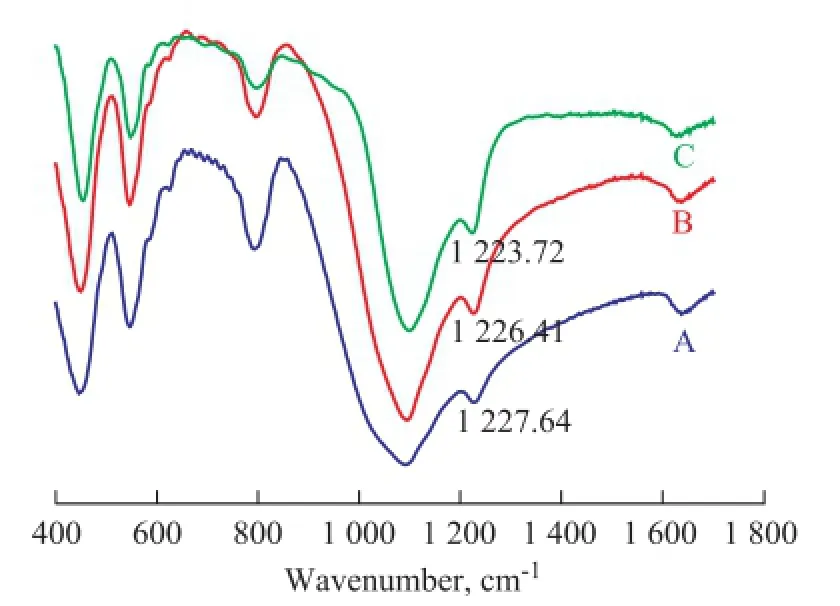
Figure 2 IR spectra of as-synthesized samples
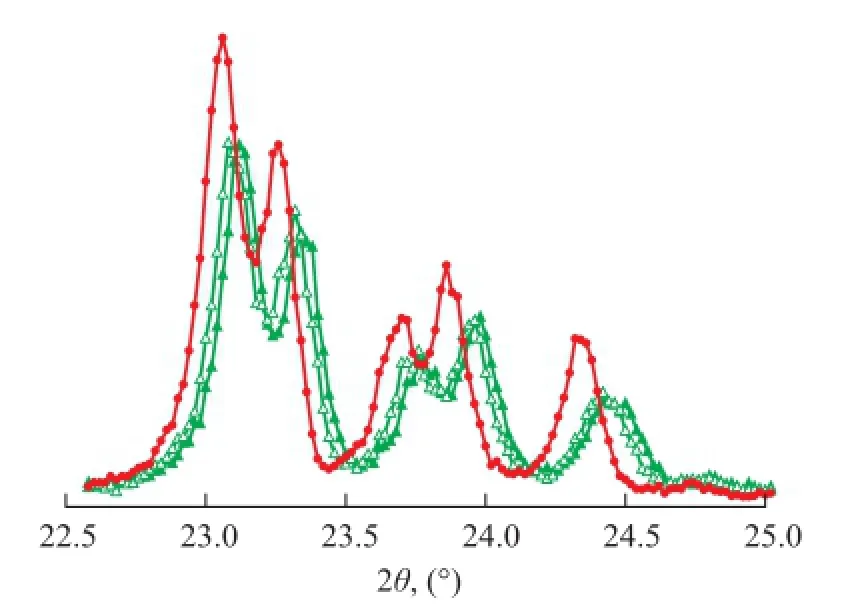
Figure 3 XRD pattern of as-synthesized samples
3.3 Effect of surfactant type on morphology
Figure 4 shows the SEM images of the as-synthesized ZSM-5 samples using different types of surfactants in the super-concentrated system. The results show that the crystal grain of ZSM-5 samples had the morphology of hexagonal prism–shaped crystals. The crystal grain size of ZSM-5 synthesized without surfactant was 8 μm. However, the crystal grain size of ZSM-5 sample synthesized with SDS became smaller and was equal to 6 μm. Furthermore, the crystal grain size of ZSM-5 sample synthesized with a mixture of SDS and OP-10 became the smallest and was only 3 μm. Although these ZSM-5 samples all had the morphology of hexagonal prism–shaped crystals, the crystal grain of ZSM-5 sample (Figure 4C) synthesized with SDS and OP-10 was more uniform as compared to other samples (Figure 4A and 4B). Figure 4C shows that each edge of the hexagonal prism-shaped crystal grain had the same length and the crystal grain had a better symmetric structure.
[11]黄廓,姜飞.国际主流媒体发展战略研究及其对中国国际传播的启示[J].现代传播(中国传媒大学学报),2013(2)
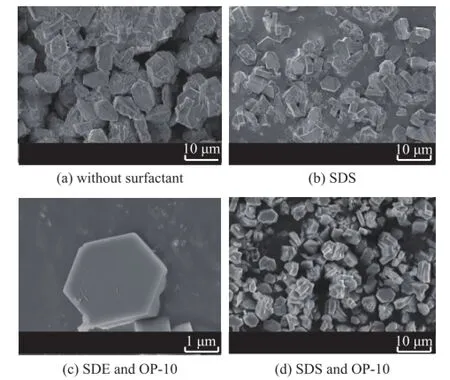
Figure 4 SEM images of samples synthesized using different types of surfactants
3.4 Effect of surfactant/silica mole ratio on the property of ZSM-5
The effect of surfactant/silica mole ratio on the crystal-linity of ZSM-5 samples was studied. Figure 5 shows the XRD patterns of samples synthesized with different SDS/ silica mole ratios ranging from zero to 0.002 3. Figure 6 shows the XRD patterns of ZSM-5 samples synthesized with different OP-10/silica mole ratios ranging from zero to 0.045. The results show that there were only the characteristic diffraction peaks of ZSM-5 in the XRD patterns of samples and the crystallinity of samples was infuenced by the surfactant/silica mole ratio greatly. When the two types of surfactants (SDS and OP-10) were used, the crystallinity of ZSM-5 samples varied with different SDS/silica mole ratio (Figure 5). However, there was an optimum SDS/silica mole ratio. When the ZSM-5 sample was synthesized at a SDS/ silica mole ratio equating to 0.0007, the crystallinity of ZSM-5 was the highest. When the SDS/silica mole ratio remained at a constant value of 0.0007, we only changed the OP-10/silica mole ratio (Figure 6). The results show that the crystallinity of ZSM-5 increased with the OP-10/silica mole ratio. When the OP-10/silica mole ratio reached 0.036, the crystallinity of ZSM-5 sample did not increase any more.
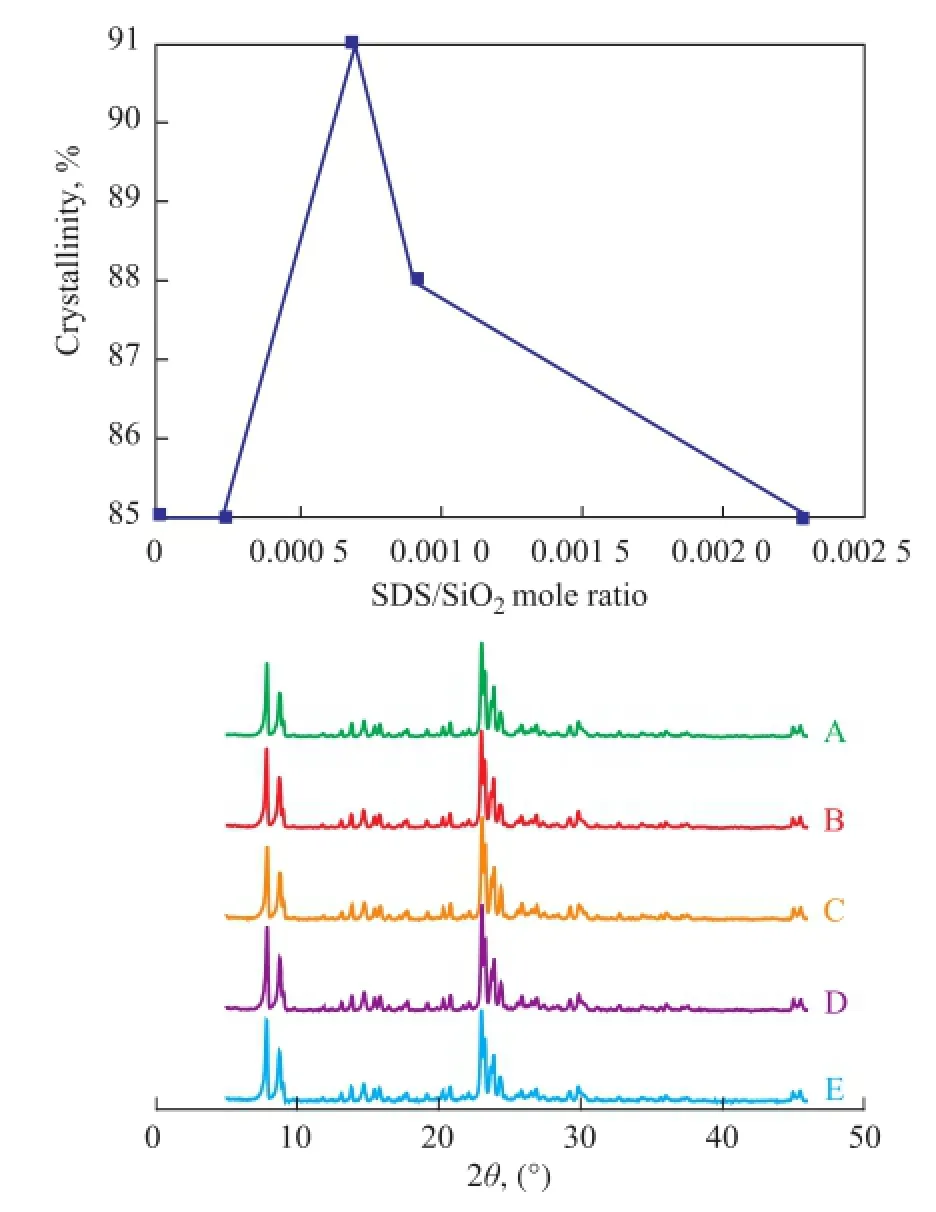
Figure 5 Effect of SDS/silica mole ratio on crystallinity
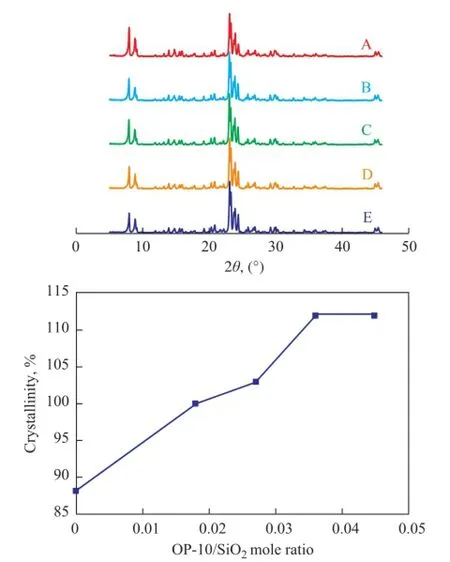
Figure 6 Effect of OP-10/silica mole ratio on crystallinity of ZSM-5
Figure 7 shows the IR spectra of the as-synthesized ZSM-5 samples synthesized with OP-10 and SDS. The results show that the characteristic adsorption band at around 1 225 cm-1shifted to the lower wavenumber when the OP-10/silica mole ratio increased from 0 to 0.045. This could indirectly prove that the framework Si/Al mole ratio of ZSM-5 also changed with the shifting of adsorption band. When the OP-10/silica mole ratio increased to 0.045, the characteristic adsorption band at 1 224.43cm-1shifted to 1 220.83 cm-1.
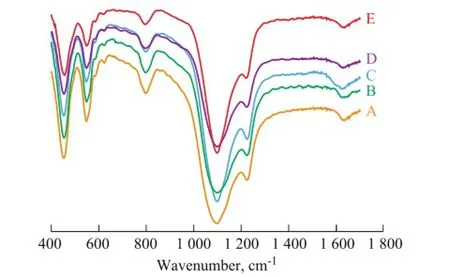
Figure 7 IR spectra of ZSM-5 samples with different OP-10/silica mole ratio
Figure 8 shows the SEM images of the as-synthesized ZSM-5 samples using different OP-10/silica mole ratios. The results show that the crystal grain size became smaller with an increasing OP-10/silica mole ratio used.
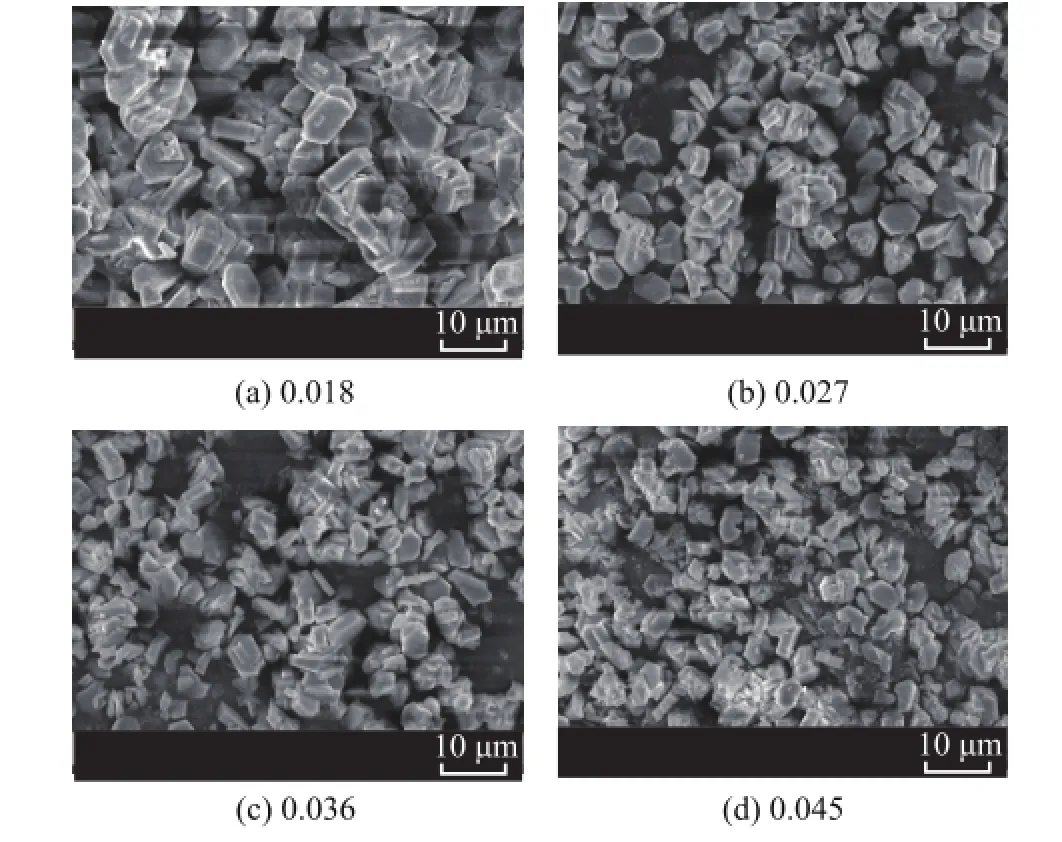
Figure 8 Effect of OP-10/silica mole ratio on morphology of ZSM-5
3.5 Exploration of surfactant mechanism on the synthesis of ZSM-5
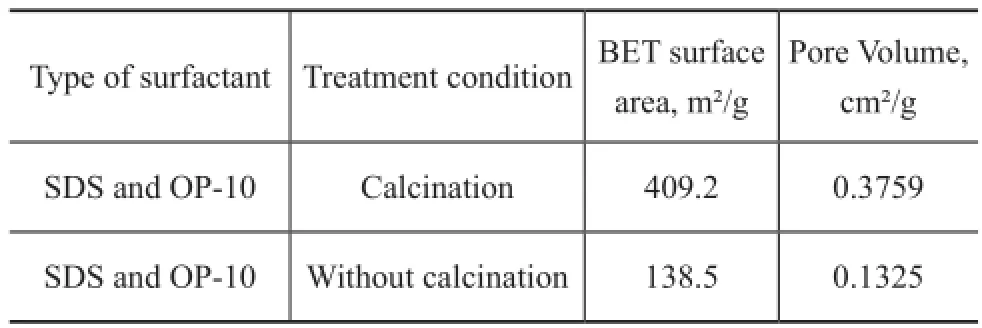
Table 3 The BET results of ZSM-5 product
4 Conclusions
The effect of surfactants on synthesis of ZSM-5 was investigated in a super-concentrated system. The XRD and FT-IR analyses confrmed that there was only the ZSM-5 phase in the as-synthesized samples using different surfactants and different surfactant/silica mole ratios. The crystallinity of ZSM-5 decreased with different types of surfactants in the following order: (OP-10 and SDS)>SDS>no surfactant. The SEM images show that the crystal grain size of ZSM-5 could be infuenced by different types of surfactants and increased in the following order depending on the type of surfactants: (OP-10 and SDS)<SDS<no surfactant. The exploration of surfactant mechanism on the synthesis of ZSM-5 was implemented. When the concentration of surfactant such as SDS and OP-10 increased in the aqueous solution, the micelle density of aqueous solution increased too. The ZSM-5 crystal nuclei were easier to grow along the micelles of surfactant.
Acknowledgements: Thanks are due to the National Natural Science Foundation (20473039) for the support of this work. We would like to thank Professor Dou Tao for this work.
[1] Komvokis V G, Iliopoulou E F, Vasalos I A, et al. Development of optimized Cu-ZSM-5 deNOx catalytic materials both for HC-SCR applications and as FCC catalytic additives[J]. Applied Catalysis A: General, 2007, 325(2): 345-352
[2] Zhang Peiqing, Guo Xinwen, Guo Hongchen, et al. Study of the performance of modifed nano-scale ZSM-5 zeolite on olefns reduction in FCC gasoline[J]. Journal of Molecular Catalysis A: Chemical, 2007, 261(2): 139-146
[3] Noronha L A, Aguiar E F S, Mota C J A. Conversion of chloromethane to light olefins catalyzed by ZSM-5 zeolites[J]. Catalysis Today, 2005, 101(1): 9-13
[4] Mads Kaarsholm, Finn Joensen, Jesper Nerlov, et al. Phosphorous modifed ZSM-5: Deactivation and product distribution for MTO[J]. Chemical Engineering Science, 2007, 62(18): 5527-5532
[5] Zhao Tiansheng, Tomokazu Takemoto, Noritatsu Tsubaki. Direct synthesis of propylene and light olefins from dimethyl ether catalyzed by modifed H-ZSM-5[J]. Catalysis Communications, 2006, 7(9): 647-650
[6] Morten Bjorgen, Stian Svelle, Finn Joensen, et al. Conversion of methanol to hydrocarbons over zeolite H-ZSM-5: On the origin of the olefnic species[J]. Journal of Catalysis, 2007, 249(2): 193-205
[7] Zhan Jianghong, Nie Hongyuan, Pei Bei, et al. Synthesis and evaluation of ZSM-5 zeolite catalyst for MTP[J]. Petroleum Processing and Petrochemicals, 2015, 46(4): 46-50 (in Chinese)
[8] Liu Dongmei, Kong Feifei, Zhai Yuchun, et al. Secondary crystallization of Na2CO3-modified HZSM-5 zeolites with tetrapropylammonium hydroxide and their catalytic performance in thiophene alkylation reaction[J]. China Petroleum Processing and Petrochemical Technology, 2015, 17(3): 53-60
[9] Zhao Xuebin, Zeng Feng, Zhao Bin, et al. Alkylation activity of benzene with syngas over Cu-based catalysts[J]. China Petroleum Processing and Petrochemical Technology, 2015, 17(1): 31-38
[10] Zhang Baozhong, Liu Xiaopeng. Catalytic performance of MFI/MFI core-shell zeolites in benzene methylation[J]. China Petroleum Processing and Petrochemical Technology, 2014, 16(4): 94-99
[11] Kim S D, Noh S H, Seong K H, et al. Compositional and kinetic study on the rapid crystallization of ZSM-5 in the absence of organic template under stirring[J]. Microporous and Mesoporous Materials, 2004, 72(1/3): 185-192
[12] Ghiaci M, Seyedeyn-Azad F, Kia R. Fast and effcient synthesis of ZSM-5 in a broad range of SiO2/Al2O3without using seeding gel[J]. Materials Research Bulletin, 2004, 39(9): 1257-1264
[13] Zhao Tianbo, Wang Jia, Xu Xin, et al. Preparation of hierarchically trimodal-porous ZSM-5 composites through steam-assisted conversion of macroporous aluminosilica gel with two different quaternary ammonium hydroxides[J]. China Petroleum Processing and Petrochemical Technology, 2015, 17(1): 48-58
[14] Sun Li, Zhang Qiang, Li Chunyi, et al. Effect of crystllization conditions on properties of ZSM-34[J]. Petroleum Processing and Petrochemicals, 2015, 46(12): 5-9 (in Chinese)
[15] Wei Min, Kong Feifei, Ding Yueye, et al. Study on preparation of micro-mesoporous ZSM-5 zeolite by alkali treatment and proformance in hydrodesulfurization of FCC gasoline[J]. Petroleum Processing and Petrochemicals, 2015, 46(10): 61-66 (in Chinese)
[16] Van Grieken R, Sotelo J L, Menendez J M, et al. Anomalous crystallization mechanism in the synthesis of nanocrystalline ZSM-5[J]. Microporous and Mesoporous Materials, 2000, 39(1/2): 135-147
[17] Behrens P. Voids in variable chemical surroundings: mesoporous metal oxides[J]. Angewandte Chemie International Edition, 1996, 35(35): 515-518
[18] Huo Q, Margolese D I, Ciesla U, et al. Generalized synthesis of periodic surfactant/inorganic composite materials[J]. Nature, 1994, 368(6469): 317-321
[19] Stucky G D, Huo Qisheng, Firouzi A, et al. Directed synthesis of organic/inorganic composite structure[J]. Studies in Surface Science and Catalysis, 1997, 105: 3-28
Received date: 2016-03-22; Accepted date: 2016-06-24.
Dr. Li Haiyan, Telephone: +86-459-6743152; E-mail: lhy459@petrochina.com.cn.
猜你喜欢
杂志排行
中国炼油与石油化工的其它文章
- Effect of Magnetic Field on Tribological Properties of Lubricating Oils with and without Tricresyl Phosphate
- Preparation and Lubricating Properties of A New Antibacterial Emulsion Containing Nano-TiO2for Cold Rolling Strips
- Prediction of Coke Yield of FCC Unit Using Different Arti fi cial Neural Network Models
- An Extraction Process for Optimal Utilization of Naphtha Based on Molecule Management
- Hydrodynamic Characteristics in an External Loop Airlift Slurry Reactor
- Microwave-Assisted Synthesis of Poly(Aspartic Acid-Itaconic Acid) Copolymer and Its Characterization
