Tracking Catalytic Esteri fi cation of Naphthenic Acids in Crude Oil by ESI FT-ICR MS
2016-03-22LiXiaohuiWuBenchengZhuJianhuaTaoXiujuan
Li Xiaohui; Wu Bencheng; Zhu Jianhua; Tao Xiujuan
(1. College of Chemistry and Chemical Engineering, Xi’an Shiyou University, Xi’an 710065; 2. College of Chemical Engineering, China University of Petroleum, Beijing 102249; 3. Key Laboratory of Auxiliary Chemistry and Technology for Chemical Industry, Shaanxi University of Science and Technology, Xi’an 710021)
Tracking Catalytic Esteri fi cation of Naphthenic Acids in Crude Oil by ESI FT-ICR MS
Li Xiaohui1; Wu Bencheng2; Zhu Jianhua2; Tao Xiujuan3
(1. College of Chemistry and Chemical Engineering, Xi’an Shiyou University, Xi’an 710065; 2. College of Chemical Engineering, China University of Petroleum, Beijing 102249; 3. Key Laboratory of Auxiliary Chemistry and Technology for Chemical Industry, Shaanxi University of Science and Technology, Xi’an 710021)
The catalytic esterifcation reaction was used to decrease total acid number (TAN) of crude oil by converting naphthenic acids to naphthenic acid esters in the presence of Zn-Al hydrotalcite used as the catalyst and glycol used as the reactant. The crude oil and its corresponding esterifed oil were characterized by the negative-ion electrospray ionization (ESI) Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS). Six acidic class species, O2, O1, N1, N2, N1O1and N1O2were assigned in the negative-ion spectrum both in the crude oil and its esterifed oil. Among the identifed acidic compounds, the O2class was dominant. The relative abundance of O2class species was much higher than other acidic class species in crude oil, while it was signifcantly decreased after esterifcation. The most abundant O2class species had a carbon number of 30-34 and a double-bond equivalence (DBE) value of 5 before and after esterifcation. It could be concluded that the naphthenic acids in crude oil can be esterifed to lower its TAN value, and each of them seems to exhibit identical esterifcation effciency approximately due to the similar DBE versus the -carbon number distribution before and after esterifcation.
naphthenic acids; crude oil; esterifcation ; ESI FT-ICR MS
1 Introduction
Naphthenic acids in crude oil have been defined as carboxylic acids with one or more saturated ring structures, where five- and six-membered rings are most common. This defnition has become more loosely used to describe the range of organic acids found in crude oils[1]. When the total acid number (TAN) of crude oil exceeds 0.5 mg-KOH/g, it is considered to be acidic[2]. High TAN value means that there exist more acidic components which are responsible for liquid phase corrosion during crude oil transportation through pipelines and in refinery processing[3], which can result in higher TAN value of oil products[4]. As the supply of light crude oils is decreasing, the reliance will shift to highly acidic heavy oils or the so-called “opportunity crude oils” from the United States, China, Eastern Europe, and Venezuela[5]. It is very necessary to adopt proper measures to reduce the negative infuence when highly acidic heavy oils are used as the feedstocks of refineries, such as by improving the corrosion-resistant grade of the material for fabrication of refning equipment, mixing the highly acidic crude oil with less acidic one, using corrosion inhibitors, etc[6-8].
In recent years, catalytic esterification, as an interesting and novel method, which is promising in reducing the acidity of crude oil and improving the distillation efficiently, has been developed by some individuals and groups. Carboxylic acids are known to be reactive with alcohols in an equilibrium reaction. This method can convert naphthenic acids to naphthenic acid esters, almost without the loss of oil. Sartori, et al.[9]adopted catalytic esterification to reduce the content of naphthenic acids from highly acidic crude oil by using methanol as the reactant and lithium stearate or lithium palmitate as the catalyst. A deacidifcation effciency of 78%—89% could be obtained at a temperature of 350 ℃ and a reaction timeof 30 min. The catalytic esterification was also utilized by us to decrease the acidity of dewaxed vacuum gas oil (VGO) in order to upgrade the quality of lube base oil[10]. The catalytic esterifcation reaction was also employed by Wang, et al.[11]to decrease the TAN value of diesel fuel by using methanol as the alcohol reactant and SnO/Al2O3as the catalyst. Their experiments were completed in a fxed bed reactor, and the TAN value of the diesel fuel was reduced from 1.7 mgKOH/g to less than 0.1 mgKOH/g at a methanol/oil mass ratio of 0.010. Generally speaking, the study of using esterifcation to remove acidity of crude oil and its subfractions is usually conducted in laboratoryscale units, including the batch and fxed bed operation. Esterification method is a promising and efficient technique to decrease the TAN value of oil in a large-scale industrial process in the presence of an excellent catalyst with high catalytic activity, high stability and sufficient long catalyst life.
Recently, there is a growing interest in the chemical characterization of acidic components of crude oils and the distillates due to the problems caused by these components at the oil refneries. Normal analytical techniques, such as the Fourier transform infrared (FT-IR) and ultraviolet (UV) spectroscopy, can only provide information on the functional groups of species in oil which are inadequate in giving molecular structural information. Conventional mass spectrometry is commonly used to identify the molecular composition of petroleum, and a further reliable assignment of elemental compositions to mass spectrometric signals, however, requires a high mass resolution and mass accuracy[4]. The negative-ion electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry (ESI FT-ICR MS) has been successfully employed to analyze acidic polar compounds in petroleum and its fractions thanks to its high ionization selectivity, good resolving power and mass accuracy, which can be realized without derivatization or preconcentration of the sample along with minimal sample consumption[12-13]. Vaz, et al.[14]successfully predicted TAN value of crude oil based on a vast quantity of information provided by the negative-ion ESI FT-ICR MS using the related prediction models. Colati, et al.[15]monitored the liquid/liquid extraction of naphthenic acids in Brazilian crude oil using the negative-ion ESI FT-ICR MS, and found that the acidic extraction method at pH=14 was more effcient, and meanwhile the ESI FT-ICR MS analysis had revealed that the most abundant naphthenic acids were those with short alkyl chain lengths (<C44) and a DBE value of 3—4.
Relatively few studies on esterification using ESI FTICR MS have been reported. The self-esterification of two fulvic acid model compounds in methanolic solvents was studied via the negative-ion ESI-MS by Mclntyre, et al.[16]And they obtained some useful information which verified that the strongly acidic tetrahydrofurantetracarboxylic acid could self-catalyze its esterification with methanolic solvents. In recent years, esterification of naphthenic acids in crude oil and its subfractions was studied by some individuals and groups who mainly focused on the TAN value of oil superfcially. Therefore, in this work, a detailed characterization of crude oil before and after esterifcation was performed by the negative-ion ESI FT-ICR MS. This approach was helpful to obtaining a better understanding on naphthenic acids esterifcation in crude oil by revealing some detailed information of the compositional changes, which might be useful for providing some guidance to the deacidifcation pre-treatment of crude oil before refning.
2 Experimental
2.1 Samples
The crude oil sample was collected from the Liaohe oilfeld in northeast China, with the basic properties of the crude oil listed in Table 1 and the viscosity-temperature relationship of crude oil presented in Figure 1. It can be seen from Table 1 that the density and viscosity of Liaohe crude oil were very high, and its TAN value was measured to be as high as 3.61 mgKOH/g, indicating that the Liaohe crude oil could be classifed as a highly acidicextra-heavy oil. Figure 1 shows that the viscosity of crude oil decreased quickly with the increase of temperature in the range of 20 ℃—40 ℃, and this decreasing trend would slow down when the temperature increased continuously, indicating to the typical characteristics of non-Newtonian fuid.

Table 1 Properties of Liaohe crude oil and its esterified sample

Figure 1 Viscosity-temperature curves of Liaohe crude oil and its esteri fi ed crude oil
2.2 Experiments on esteri fi cation of naphthenic acids in crude oil
The esterifcation reaction of naphthenic acids in crude oil was investigated in a 500 mL three-necked round-bottom fask equipped with a magnetic stirrer for agitation of the sample. A total of 200 g of oil sample with a TAN value of 3.61 mgKOH/g, 4 g of glycol and 1 g of catalyst were placed into the reactor, respectively. The rotary speed of the magnetic stirrer was preset at 800 rpm, and the reactor was operated at 250 ℃ and under atmospheric pressure. The catalyst used in the experiments was the prepared ZnAl-HTlc. After a reaction time of 60 min, the catalyst was separated by filtration immediately, and the TAN value of esterifed oil was measured. The TAN value of the crude oil was decreased by 93.35% to 0.24 mgKOH/g. The density and viscosity of the esterifed crude oil were also measured, with the results listed in Table 1, which indicated that the density of crude oil slightly increased. Its viscosity also increased, and this gap between them would narrow with an increasing esterifcation temperature. So, the remarkable decrease of TAN value of crude oil was realized at the expense of the increase in density and viscosity.
2.3 FT-IR analysis
The FT-IR spectra of the crude oil and its esterifed sample were recorded by a Nicolet Magna-IR 560 ESP spectrometer. The FT-IR spectra were obtained in the range from 4 000 cm-1to 400 cm-1in the transmission mode with a resolution of 0.35 cm-1. Each spectrum was an average of 32 scans. The FT-IR analysis was performed on a liquid sample that was sandwiched between two potassium bromide (KBr) slices.
2.4 ESI FT-ICR MS analysis
The method for sample preparation prior to the analysis of acidic species in crude oil and its esterifed sample by the negative-ion ESI FT-ICR MS had been previously reported[5,13,17]. Ten mg of every sample were completely dissolved in 1 mL of toluene. And then, each mixture solution was then diluted to a concentration of 0.2 mg/mL with a mixed solvent consisting of toluene/methanol (1:3 v/v). Furthermore, 5 μL of 28% NH4OH were then added to each diluted solution to facilitate the deprotonation of acidic species to yield [M-H]-ions[2,13]. And then 5 μL of formic acid were added to the diluted solution to facilitate the protonation of basic compounds to yield [M-H]+ions to ensure effcient ionization for positive-ion ESI analysis[18-19]. The ESI FT-ICR MS analysis was performed using a Bruker Apex-ultra 9.4 T FT-ICR MS mass spectrometer. The sample solution was injected into the ESI source at a rate of 180 μL/h using a syringe pump. The operating conditions for negative-ion (or positive-ion) formation covered an emitter voltage of 4.0 kV (or -4.0 kV), a capillary column front end voltage of 4.5 kV (or -4.5 kV), and a capillary column end voltage of -320 V (or 320 V). Ions accumulated for 0.01 s in a hexapole. The delay was set to 1.2 ms to transfer ions to an ICR cell by the electrostatic focusing of transfer optics. The data size was set to 4 M words, and the time-domain data sets were co-added from 128 data acquisitions.
2.5 Mass calibration and data analysis
The mass spectra obtained thereby were calibrated internally according to the most abundant homologous series compounds. Peaks with a relative abundance of greater than 6 times the standard deviation of the baseline noise level were exported to a spreadsheet. Data analysis was implemented by using custom software which had been described by some references[5,13,18]. In general, the data analysis was performed by selecting a two-mass scale-expanded segment in the middle of the mass spectrum, followed by detailed identifcation of each peak. The peak of at least one of each heteroatom class species was arbitrarily selected as the reference. Species with the same heteroatom class and its isotopes with different values by DBE and carbon number were searched within a set of ±0.001 Kendrick mass defect (KMD) tolerance.
Compounds of a homologous series would be identifed according to the KMD, and the essence was that compounds should have the same heteroatom composition and number of rings plus the double-bond equivalence (DBE)[13,20]. DBE was defned as the number of rings plus double bonds involving carbon for a petrochemical product of the composition,

3 Results and Discussion
3.1 FT-IR analysis
Figure 2 shows the FTIR spectra of crude oil and the esterifed oil. The spectra exhibited characteristic bands for aliphatic functional groups at 2 920 cm-1, 2 850 cm-1, 1 460 cm-1, and 1 376 cm-1[21]. The spectral band at 1 706 cm-1was likely the stretching vibration of C=O in the carboxyl group of naphthenic acids in the crude oil, but it was not observed in the spectra of the esterifed oil sample. Meanwhile, the appearance of spectral band at 1 738 cm-1was associated with the stretching vibration of C=O in the ester group of naphthenic esters in the esterified crude oil, and this spectral band was not found in the spectra of crude oil. This demonstrated that the naphthenic esters were formed in crude oil following the esterifcation of naphthenic acids.

Figure 2 FT-IR spectra of fresh crude oil and the esteri fi ed crude oil
3.2 Mass distribution
Figure 3 shows negative-ion ESI FT-ICR MS broad-band spectra atm/z200—900 of crude oil and the esterified crude oil. The ions counted as peaks were 6-fold larger than the baseline noise. The MS spectra of crude oil and its esterifed oil sample were basically similar. Compared to the spectra of crude oil, the esterifed crude oil seems to have more intense signals at high molecular weight, which could be attributed to the esterifcation process. On the other hand, the ionization efficiency and the solvation energy of the analyte would also affect the ESI response[22].
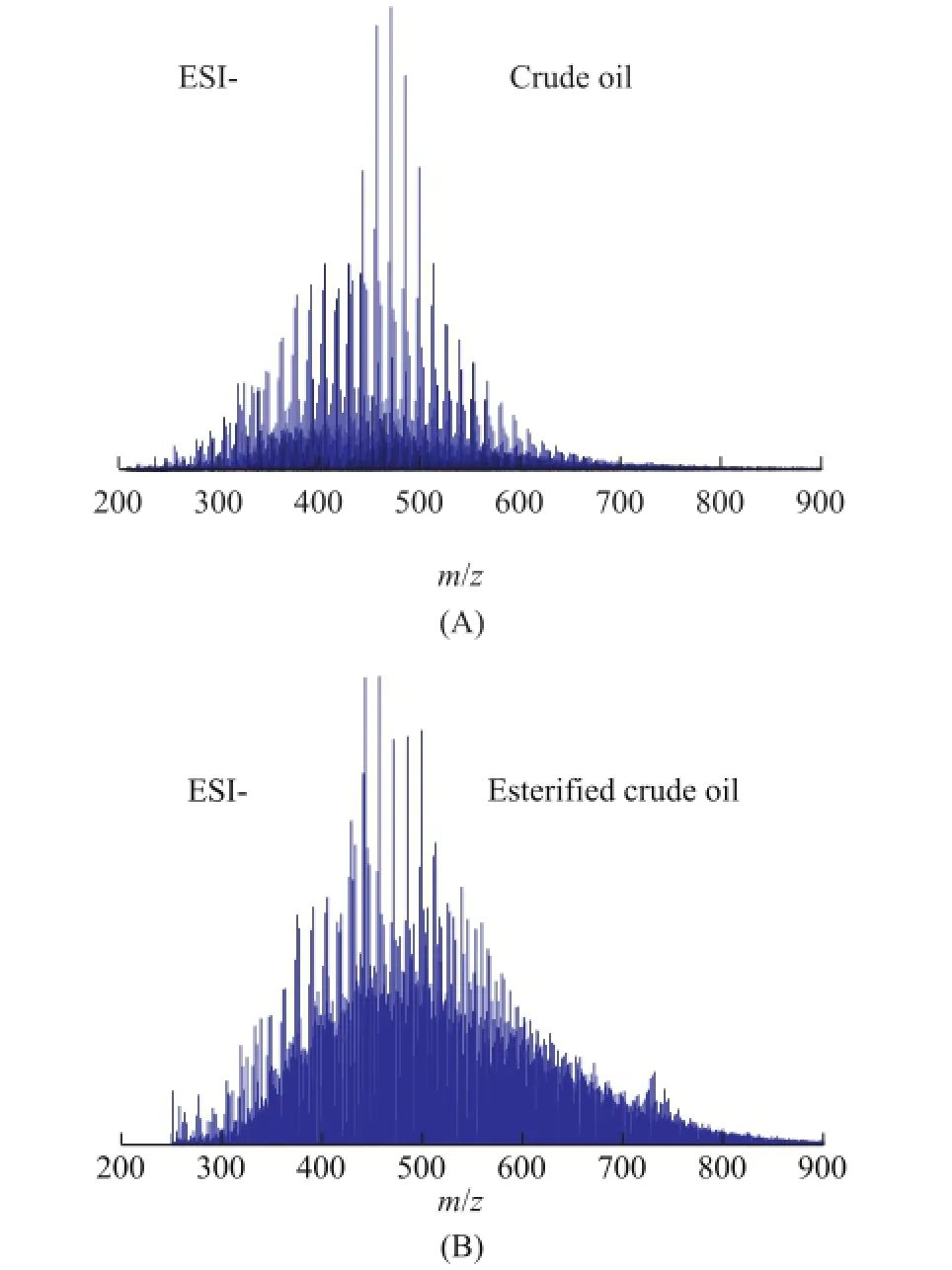
Figure 3 Broadband negative-ion ESI FT-ICR mass spectra of Liaohe crude oil(A) and its esteri fi ed oil sample (B)
3.3 Heteroatom class composition
The heteroatom class species identifed in the MS spectra are presented in Figure 4. N1, N1O1, N1O2, N2, O1and O2classes were identified both in crude oil and the esterifed sample by the negative-ion mass spectra. Shi, et al.[23]have utilized ESI FT-ICR MS to characterize Liaohe crude oil and its subfractions, and their results showedthat the neutral nitrogen and acidic heteroatom compounds in the crude oil contained the N1, N2, N1O1, N1O2, N1O3, N1O4, O1, and O2heteroatom classes. The distribution of heteroatomic classes was determined by dividing each class species with varied DBE values by using the sum of the relative abundance of all species identifed in the mass spectra. It can be seen that the O2class species in crude oil were dominant with a relative abundance of 53.9%, followed by the N1and O1class species. While the O2class species were also the most abundant ones in the esterifed crude oil, however, their relative abundance decreased to 27.8%, and meanwhile, the O1, N1, N2, N1O1and N1O2class species exhibited an enhanced relative abundance, which was attributed to the fact that esterifcation had transformed a considerable number of naphthenic acids into naphthenic acid esters. Moreover, it is noticed that the TAN value of the esterifed crude oil was decreased by 93.35% (Table 1); however, with regard to the O2class species as the major contributor to TAN, their relative abundance in the esterifed crude oil was only reduced by about 26.1%. This can be ascribed to the ionization effciency between different species, since carboxylic and naphthenic acids are the strongest acids among the polar acidic compounds in crude oil, and they can be ionized by deprotonation more effectively than other existing less acidic compounds[22], even though they are present at a low concentration.

Figure 4 Class species distribution of Liaohe crude oil (A) and its esteri fi ed oil (B) based on negative-ion ESI FT-ICR MS analyses
With the choice of some mass points with a better signalto-noise ratio (SNR), it can be seen that there were multiple mass peaks within one unit mass point when the mass spectra were unfolded further, while the mass points 371 Da and 457 Da for the crude oil and its esterifed oil sample could serve as an example (Figure 5). The importance of ultrahigh mass resolving power and mass accuracy in the determination of compositional differences is evident again. Figure 5 shows that the intensity of masspeaks of other class species became stronger after esterifcation owing to the decrease of the relative abundance of O2class species, which was in accordance with the above results.
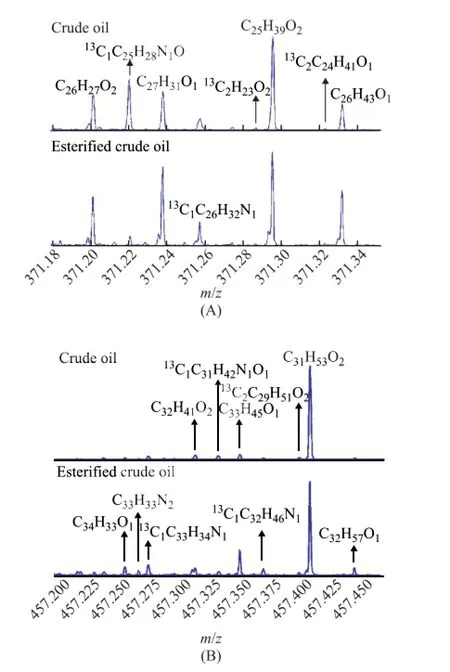
Figure 5 Mass scale expansion at a nominal mass of 371 Da (A) and 457 Da (B)
3.4 DBE versus carbon number distribution for O2class species
The isoabundance maps of DBE as a function of the carbon number for the O2class species in the crude oil and its esterified crude oil are shown in Figure 6. It can be observed that the O2class species in the crude oil spread over a wide range of DBE values (1—18) and carbon numbers (13—60), suggesting that the molecular structures of individual O2class species were significantly different. And they were mainly concentrated in defnite DBE values (3—6) and carbon numbers (25—35), in which the DBE values of 5 series with a range of carbon numbers (30—35) were the most abundant. Similar results were also found by Shi,et al.[23], although the fatty acids with a DBE value of 1 exhibited a high relative abundance at carbon numbers of between 16 and 18 that were contaminated by foreign matters. Basically, the appearance of DBE versus carbon number distribution for the O2class species in the esterifed crude oil was similar to that in crude oil. In detail, it seems that the gap of the abundance between each O2compound in a DBE range of 3—6 in the esterifed oil sample was relatively small as compared to the crude oil, presenting a slightly average distribution. This comparison implied that the naphthenic acids with different DBE values and carbon numbers in crude oil roughly exhibited the similar esterification effciency to form naphthenic esters.

Figure 6 Plots of DBE as a function of the carbon number for O2class species from negative-ion ESI FT-ICR mass spectra of Liaohe crude oil (A) and its esteri fi ed oil sample (B)
Naphthenic acid monoester, which could be formed when the –COOH in naphthenic acid molecule was esterifed with one -OH in glycol molecule, should be the O3class species. Mclntyre, et al.[16]considered that strongly acidic compounds, which could preferentially ionize to form negative ions, might do so to a lesser degree after esterifcation and should have a lower relative abundance. In contrast, the positive ion spectra might be artifically enhanced, as esters could be more likely to form positive ions. So, in order to identify the naphthenic acid esters derived from esterifcation in the esterifed crude oil, we employed the positive-ion ESI FT-ICR MS to characterize the two samples. The positive-ion ESI FT-ICR MS broadband spectra (m/z150—850) and the identified heteroatom class species (N1, N2, N1O1and N1O2) of the esterifed crude oil are shown in Figure 7, respectively. It can be found that the O3class species were not detected in the esterifed crude oil. This occurred because the heteroatom compounds in crude oil were composed of molecular species with predominately highly polar oxygen-containing compounds with carboxylic functionalities, which were most effciently ionized by negative ESI and thus could suppress the ionization of less acidic species[24]. So the O3class species as esters originating from the O2class species by esterifcation could not be identifed by the negative-ion ESI FT-ICR MS. On the other hand, the basic nitrogen species could be selectively ionized by the positive ESI; however, other less polar species could not be effciently ionized[24-25]. That is why naphthenic esters in the esterifed crude oil were not detected by the positiveion ESI FT-ICR MS.

Figure 7 Broadband positive-ion ESI FT-ICR mass spectra (A) and class species distribution (B) of the esteri fi ed crude oil
In summary, in the present work, we mainly employed the negative-ion ESI FT-ICR MS to study the esterification of naphthenic acids in crude oil with glycol preliminarily, resulting in some useful information. Also, we found that the formed naphthenic esters were not capable of being identifed by using the positive-ion ESI FT-ICR MS.
4 Conclusions
The negative-ion ESI coupled with the FT-ICR MS was used to characterize the acidic composition of a crude oil and its esterified product for tracking the catalytic esterifcation of naphthenic acids in the Liaohe crude oil of China. Glycol and Zn-Al hydrotalcite were used as the reactant and catalyst, respectively. Under the specifed reaction conditions, the TAN value of Liaohe crude oil signifcantly decreased from 3.61 mgKOH/g to 0.24 mgKOH/g after esterifcation. The characteristic bands for carboxyl group of naphthenic acids in crude oil, and the ester group of naphthenic esters in the esterifed crude oil, were identified in the FT-IR spectra of the two oil samples, respectively, indicating that the naphthenic acids in crude oil were converted to naphthenic acid esters during the esterifcation process. The results of negative-ion ESI FTICR MS analysis demonstrated that the acidic heteroatom compounds including the N1, N1O1, N1O2, N2, O1and O2class species were identifed both in the Liaohe crude oil and the esterified crude oil. The O2class species were dominant in the crude oil. After esterifcation, the O2class species were also the predominant compounds; however, they showed a decreased relative abundance as compared to the crude oil before esterifcation. The O2class species in fresh crude oil and the esterifed crude oil were characterized by the double-bond equivalence (DBE) values and carbon numbers. The DBE value versus carbon number distribution of O2class species were found to be without signifcant difference before and after esterifcation. The O2class species having carbon numbers in the range of 30-34 and a DBE value of 5 were the most abundant ones in the two oil samples. It can be concluded that the naphthenic acids with varied DBE values and carbon numbers demonstrated almost similar esterifcation effciency.
Acknowledgements: This work was financially supported by the National Natural Science Foundation of China (No. 21206194). The authors thank all the help from members of the Research Institute of Petroleum Processing of SINOPEC and the State Key Laboratory of Heavy Oil Processing, China University of Petroleum in Beijing, and thank the Liaohe oilfeld of CNPC for providing oil samples.
[1] Barrow M P, Headley J V, Peru K M, et al. Fourier transform ion cyclotron resonance mass spectrometry of principal components in oilsands naphthenic acids[J]. J Chromatogr A, 2004, 1058 (1/2): 51-59
[2] Qian K N, Robbins W K, Hughey C A, et al. Resolution and identifcation of elemental compositions for more than 3000 crude acids in heavy petroleum by negative-ion microelectrospray high-field Fourier transform ion cyclotron resonance mass spectrometry[J]. Energ Fuel, 2001, 15(6): 1505-1511
[3] Smith D F, Rahimi P, Teclemariam A, et al. Characterizationof Athabasca bitumen heavy vacuum gas oil distillation cuts by negative/positive electrospray ionization and automated liquid injection feld desorption ionization Fourier transform ion cyclotron resonance mass spectrometry[J]. Energ Fuel, 2008, 22(5): 3118-3125
[4] Li X H, Zhu J H, Wu B C, et al. Characterization of acidic compounds in vacuum gas oils and their dewaxed oils by Fourier transform ion cyclotron resonance mass spectrometry[J]. Energ Fuel, 2012, 26 (9): 5646-5654
[5] Stanford L A, Kim S, Rodgers R P, et al. Characterization of compositional changes in vacuum gas oil distillation cuts by electrospray ionization Fourier transform-ion cyclotron resonance (FT-ICR) mass spectrometry[J]. Energ Fuel, 2006, 20(4): 1664-1673
[6] Slavcheva E, Shone B, Turnbull A. Review of naphthenic acid corrosion in oil refning[J]. Br Corros J, 1999, 34(2): 125-131
[7] Turnbull A, Slavcheva E, Shone, B. Factors controlling naphthenic acid corrosion[J]. Corrosion, 1998, 54(11): 922-930
[8] Humblot F. Method for prevention of corrosion by naphthenic acids in refineries: The United States, US 10563549[P]. 2006-07-20
[9] Sartori G, Savage D W, Blum S C, et al. Metal compounds as accelerators for petroleum acid esterifcation: The United States, US 594238[P]. 1999-09-07
[10] Li X H, Zhu J H, Liu Q L, et al. The removal of naphthenic acids from dewaxed VGO via esterifcation catalyzed by Mg-Al hydrotalcite[J]. Fuel Process Technol, 2013, 111: 68-77
[11] Wang Y Z, Sun X Y, Liu Y P, et al. Removal of naphthenic acids from a diesel fuel by esterification[J]. Energ Fuel, 2007, 21(2): 941-943
[12] Barrow M P, McDonnell L A, Feng X D, et al. Determination of the nature of naphthenic acids present in crude oils using nanospray Fourier transform ion cyclotron resonance mass spectrometry: The continued battle against corrosion[J]. Anal Chem, 2003, 75(4): 860-866
[13] Mapolelo M M, Rodgers R P, Blakney G T, et al. Characterization of naphthenic acids in crude oils and naphthenates by electraspray ionization FT-ICR mass spectrometry[J]. Int J Mass Spectrom, 2011, 300(2/3): 149-157
[14] Vaz B G, Abdelnur P V, Rocha W F C, et al. Predictive petroleomics: Measurement of the total acid number by electrospray Fourier transform mass spectrometry and chemometric analysis[J]. Energ Fuel, 2013, 27(4): 1873-1880
[15] Colati K A P, Dalmaschio G P, De Castro E V R, et al. Monitoring the liquid/liquid extraction of naphthenic acids in Brazilian crude oil using electrospray ionization FT-ICR mass spectrometry (ESI FT-ICR MS)[J]. Fuel, 2013, 108: 647-655
[16] McIntyre C, McRae C, Jardine D, et al. Self-esterifcation of fulvic acid model compounds in methanolic solvents as observed by electrospray ionization mass spectrometry[J]. Rapid Commun Mass Spectrum, 2002, 16(8): 785-789
[17] Hughey C A, Rodgers R P, Marshall A G, et al. Identifcation of acidic NSO compounds in crude oils of different geochemical origins by negative ion electrospray Fourier transform ion cyclotron resonance mass spectrometry[J]. Org Geochem, 2002, 33(7): 743-759
[18] Zhu X C, Shi Q, Zhang Y H, et al. Characterization of nitrogen compounds in coker heavy gas oil and its subfractions by liquid chromatographic separation followed by Fourier transform ion cyclotron resonance mass spectrometry[J]. Energ Fuel, 2011, 25(1): 281-287
[19] Zhang Y H, Zhao H, Shi Q, et al. Molecular investigation of crude oil sludge from an electric dehydrator[J]. Energ Fuel, 2011, 25(7): 3116-3124
[20] Hughey C A, Galasso S A, Zumberge J E. Detailed compositional comparison of acidic NSO compounds in biodegraded reservoir and surface crude oils by negative ion electrospray Fourier transform ion cyclotron resonance mass spectrometry[J]. Fuel, 2007, 86(5/6): 758-768
[21] Muller H, Pauchard V O, Hajji A A. Role of naphthenic acids in emulsion tightness for a low total acid number (TAN)/high asphaltenes oil: Characterization of the interfacial chemistry[J]. Energ Fuel, 2009, 23(3): 1280-1288
[22] Teravainen M J, Pakarinen J M H, Wickstrom K, et al. Comparison of the composition of Russian and North Sea crude oils and their eight distillation fractions studied by negative-ion electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry: The effect of suppression[J]. Energ Fuel, 2007, 21(1): 266-273
[23] Shi Q, Hou D J, Chung K H, et al. Characterization of heteroatom compounds in a crude oil and its saturates, aromatics, resins, and asphaltenes (SARA) and non-basic nitrogen fractions analyzed by negative-ion electrosprayionization Fourier transform ion cyclotron resonance mass spectrometry[J]. Energ Fuel, 2010, 24(4): 2545-2553
[24] Podgorski D C, McKenna A M, Rodgers R P, et al. Selective ionization of dissolved organic nitrogen by positive ion atmospheric pressure photoionization coupled with Fourier transform ion cyclotron resonance mass spectrometry[J]. Anal Chem, 2012, 84(11): 5085-5090
[25] Wang Wei, Liu Yingrong, Liu Zelong, et al. Quantitative analysis using Fourier transform ion cyclotron resonance mass spectrometry and correlation between mass spectrometry data and sulfur content of crude oils[J]. China Petroleum Processing and Petrochemical Technology, 2015, 17(4): 71-80
Received date: 2016-05-10; Accepted date: 2016-07-20.
Dr. Li Xiaohui, Telephone: +86-29-88382701; E-mail: lixiaohui@xsyu.edu.cn.
杂志排行
中国炼油与石油化工的其它文章
- Removal of Nitrogen Compounds from Shale Diesel Fraction Using Ionic Liquid [C4mim]HSO4
- Improved Ti-containing Mesoporous Silica Catalyst Synthesized by Using Anionic Surfactant as Co-template
- Synthesis of Hierarchically Porous FAU/γ-Al2O3Composites with Different Morphologies via Directing Agent Induced Method
- Study on CO2Absorption by Aqueous Benzylamine and Its Formulations with Monoethanolamine as a Component for Post-Combustion Capture Process
- Effect of Magnetic Field on Tribological Properties of Lubricating Oils with and without Tricresyl Phosphate
- The Effect of Surfactant on Synthesis of ZSM-5 in a Super-Concentrated System
