Puri fi cation of Aromatics over a PromisingCatalyst
2016-03-22
(The State Key Laboratory of Chemical Engineering, East China University of Science and Technology, Shanghai 200237)
Puri fi cation of Aromatics over a PromisingCatalyst
Zhao Qing; Liu Jianfei; Yao Jiajia; Shi Li; Wang Xin
(The State Key Laboratory of Chemical Engineering, East China University of Science and Technology, Shanghai 200237)
A promising sulfated zirconia (SZ) was successfully synthesized to remove olefns from aromatics. The effects of type and quantity of precipitant, the concentration of impregnants and the calcination temperature were studied, and the synthesized samples were characterized by XRD, SEM, TG-DTG, FTIR, N2adsorption analysis and the FTIR pyridine adsorption (py-IR) techniques. This catalyst was found to be superiorly active as compared with the commercial ROC, which exhibited promising application prospects in industry. The optimum synthesis variables required that SZ was impregnated with 2.0 mol/L sulfuric acid and calcined at 650 ℃, and the precursor was precipitated by ammonium hydroxide to reach eventually a pH value of over 9.46. The addition of SO42-species increased the amount of catalytically active t-ZrO2, which was helpful to an increasing abundance of acid sites for enhancing the activity. Meanwhile, the calcination temperature had a great effect on the structure, composition and properties of sulfated zirconia.
aromatics; sulfated zirconia; purifcation; olefn conversion; catalyst
1 Introduction
Benzene, toluene and xylene (BTX) are mainly produced by the catalytic reforming and thermal cracking processes[1]. However, BTX streams derived from naphtha reforming are accompanied by undesirable sideproducts—olefns, which will lead to a series of adverse effects on further separation[2]. These detrimental olefns can be effectively removed via the acid-catalyzed alkylation of specific aromatics[3-4]. Conventionally, aromatics are refned by acid clay and zeolites in industrial plants. However, commercial clay has disadvantages such as poor regeneration performance, and rapid deactivation. Therefore, there is a room to improve the process for purifcation of aromatics particularly via developing easily synthesizable and recyclable catalysts.
In the past decades, there has been an increased interest in the application of solid superacids because of their ability to catalyze many reactions, such as esterification[5], alkylation[6], and isomerization[7]. Among them, sulfated oxides such as sulfated titania, zirconia and iron oxide, and especially sulfated zirconia have exhibited great application prospects in many reactions of industrial signifcance thanks to its greater thermal stability, better superacidic property, and higher catalytic activity and selectivity.
Despite some reports on the Friedel-Crafts alkylation studies over SZ catalysts[6,8], few reports have focused on the purifcation of aromatics over SZ catalysts so far. It is well known in the literature that the catalytic activity of SZ catalysts signifcantly depends on its synthesis procedure[9], such as the type and quantity of precipitants, the concentration of impregnant and the calcination temperature. In this context, the objective of this work is to deal with optimization of these synthesis variables on the fnal catalytic activity to achieve industrialization of solid superacids.
2 Experimental
2.1 Materials
Experimental raw materials, with a bromine index (BI) of 1068 mgBr/(100 g), were obtained from the PX combination units at the Sinopec Zhenhai Refining & Chemical Company, with their hydrocarbon components shownin Table 1. The commercial acid activated clay was produced in Anhui, China. All other practical reagents were commercially obtained from the reagent suppliers, and they were of the analytically pure (AR) grade without any further purifcation.
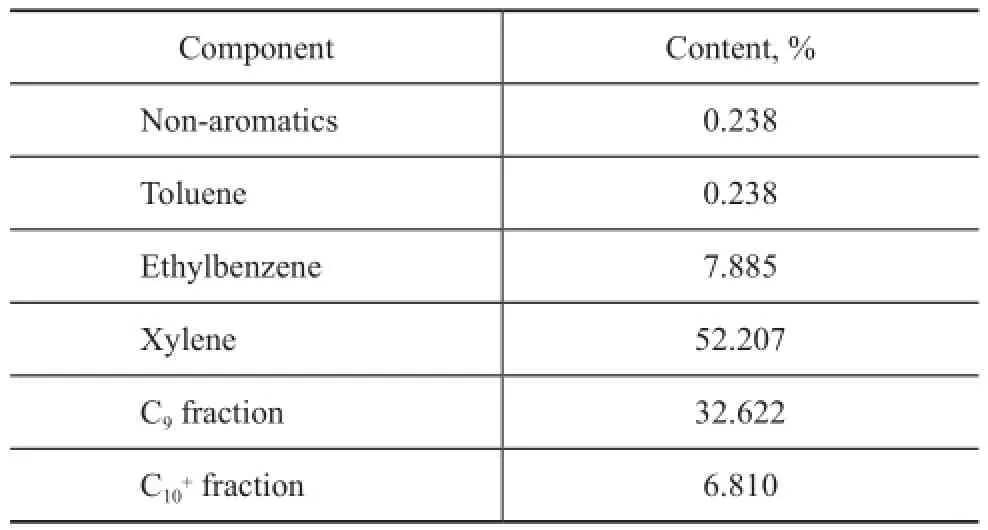
Table 1 Composition of the aromatic materials
2.2 Catalyst preparation & regeneration
Zirconium oxychloride (ZrOCl2·8H2O, 99%) was dissolved in distilled water, then a hydroxide solution (25%—28%) was dropwise added under vigorously stirring until a desired pH value of 9.5 was reached, and then the mixture was subject to ageing for 12 h at room temperature. The obtained precipitate was filtered, washed with distilled water until it was free from chloride ions, and was subject to forced-air drying at 120 ℃ for 24 h. Then the above dried zirconium hydroxide (Zr(OH)4) was sulfated by a sulfuric acid solution for 1 h. After fltration, the samples were subject to forced-air drying at 120 ℃ for 12 h prior to being activated at various temperatures in ambient air for 3 h. Hereafter the as-synthesized sulfated zirconia catalyst is denoted as SZ and the dried sulfated zirconia without calcination is just named SZ120. Correspondingly, the sulfated zirconia samples activated at 500 ℃, 550 ℃, 600 ℃, 650 ℃, 700 ℃ are labeled as SZ500, SZ550, SZ600, SZ650, SZ700, respectively. For comparison, the dried zirconium hydroxide (Zr(OH)4) prepared using the co-precipitation method was calcined at 650 ℃ to synthesize pure ZrO2. SZyM (y=0.5—6.0) denotes SZ impregnated by a certain molar concentration of sulfuric acid solution. The same procedure was followed for the preparation of(ST) and(SF). The titanium precursor and iron precursor consisted of titanium tetrachloride (treated in the ice-water bath) and ferric oxide, respectively.
2.3 Catalytic activity evaluation
The activity of catalyst samples was carried out in a fixed-bed tubular reactor, which was filled with 1.5 mL of quartz sand (40—60 mesh), 2 mL of catalyst (20—40 mesh) and 1.5 mL of quartz sand (40—60 mesh) in turn. The evaluation system was equipped with an advection pump to stabilize the flow rate at a liquid hourly space velocity (LHSV) of 3 h-1, a pressure-stabilizing valve to keep the pressure at 1.0 MPa and a temperature controller to maintain the specifed temperature. Finally, catalyst samples were collected every hour, and their bromine index (BI) was measured by a bromine index analyzer. BI is the number of milligrams of elemental bromine reacting with 100 g of hydrocarbon sample. The olefns removal conversion rateXis calculated by the following equation:X=[(ni-n0)/n0]×100%, wheren0is the BI of the feedstock andniis the BI after reaction atih.
2.4 Characterization of catalyst
X-ray diffraction (XRD) of the catalyst samples was carried out on a Bruker AXS D8 diffractometer with a monochromator using CuKα radiation (λ=0.154 nm) at a scan rate of 1 ℃/min in the 2θrange from 10° to 80°. The scanning electron microscopy (SEM) analysis was carried out using a JSM-6360LV scanning electron microscope. The TGA-DSC analysis was carried out using a SDT-Q600 synchronous thermal analyzer by heating the samples in the range of 0—900 ℃ at a temperature increase rate of 10 ℃/min under air fow (40 mL/min). The BET surface area, total pore volume, and micropore volume of the samples were calculated by the data of N2adsorption/desorption isotherms at -196 ℃ (ASAP 2010N, Micromeritics, USA). The surface acidity of different catalyst samples was examined by py-FTIR spectroscopy. The Fourier transform infrared (FTIR) spectra were recorded using a FT-IR (Nicolet-6700) spectrometer on sample wafers in the range from 400 cm-1to 4 000 cm-1.
3 Results and Discussion
3.1 Catalytic activity of SZ for aromatics re fi ning
3.1.1 Comparison of different catalysts on removing ole fi ns from aromatics
The catalytic performance of various materials for re-moval of olefins from aromatics is shown in Figure 1. ROC is the most common industrially-used material for removal of olefns. As presented in Figure 1(I), the olefns conversion of ROC was high at the beginning of the reaction, but it lost activity fast. Although the activity of pure ZrO2is not as good as expected, the sulfated ZrO2showed superiority over ROC, indicating that the interaction of sulfur species and ZrO2greatly improved the catalytic activity for removal of olefns from aromatics. Besides SZ, the sulfated TiO2(ST) and sulfated Fe2O3(SF) are also common solid superacids, with their performance shown in Figure 1(II). However, in comparison with SZ, the catalytic activity of ST and SF was far lower than expected. The above results indicated that SZ could be considered as a promising superior material for the purification of aromatics. In order to optimize the catalytic properties of SZ in detail, the effects of preparation and reaction conditions on olefin conversion were systematically investigated.
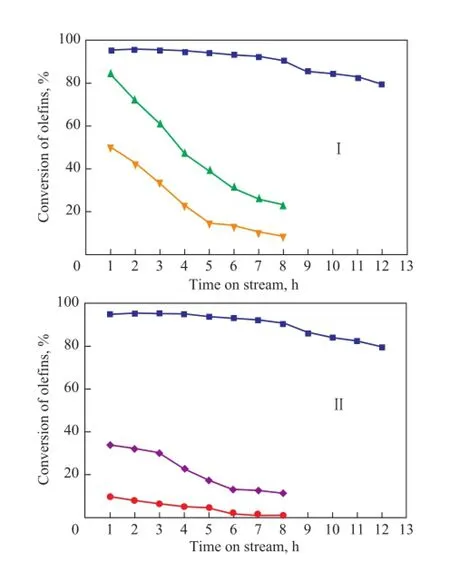
Figure 1 Effects of different catalysts: different types of materials for aromatics re fi ning (I) and different types of solid superacids (II) tested at a reaction temperature of 175 ℃
3.1.2 Effects of calcination temperature and sulfuric acid concentration
The effects of calcination temperature and sulfuric acid concentration are presented in Figure 2. As for sulfated ZrO2, the variation in catalytic performance for olefin conversion was comparatively better correlated with the increase of activation temperature (Figure 2(I)) than sulfuric acid concentration (Figure 2(II)). As it can be seen in Figure 2(I), SZ exhibited its maximum catalytic activity at a calcination temperature of 650 ℃. Practically total olefns were catalytically removed at this calcination temperature even after operation for 12 h of time-on-stream (TOS). It is worth noting that the performance of SZ550 and SZ500 was even poorer than that of unmodified ZrO2as illustrated in Figure 1(I). This fact indicated that the control of calcination temperature was of great significance in enhancing the olefn removal activity.
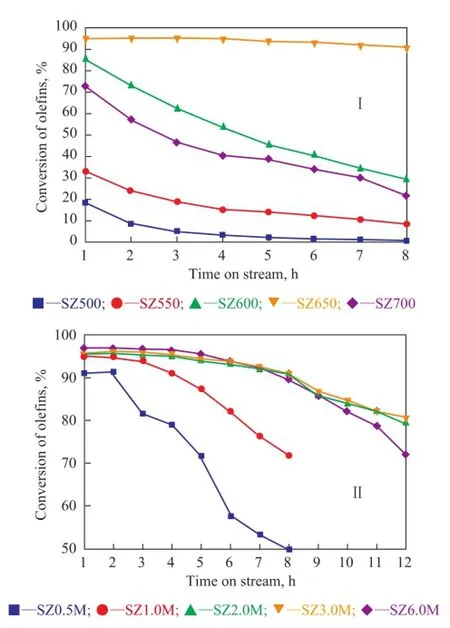
Figure 2 Effects of (I) calcination temperature and (II) sulfuric acid solution concentration on removal of ole fi ns at a reaction temperature of 175 ℃
The infuence of sulfuric acid concentration on olefn conversion is shown in Figure 2(II). As the increase in sulfu-ric acid concentration could improve the loading of sulfate on SZ, olefn conversion increased with an increasing sulfuric acid concentration and reached a maximum at 2.0 mol/L, but beyond that concentration the conversion decreased. An excessive concentration could salify a certain proportion of SZ, which would lead to the decrease of super acidity and catalytic activity. With regard to SZ6.0M, the initial activity was extremely high, and the conversion of olefins in the first five hours almost reached 100%. However, the deactivation rate was relatively sharper, which meant that an excessive concentration of sulfuric acid solution could lead to the decrease of effective running time.
3.1.3 Effect of precursor preparation condition
Catalytic properties of catalysts prepared from different zirconium hydroxide precursors are shown in Figure 3. It is clear that the method of precipitation for synthesizing SZ played a vital role in the fnal catalytic performance of the catalyst (Figure 3(I)). Precipitation with ammonium hydroxide yielded SZ with a remarkably higher activity than the cases using urea and sodium hydroxide as precipitants. SZ obtained by the homogenous precipitation method using urea as the precipitant possessed a higher activity than that using the strong base—sodium hydroxide.
The fnal pH value of the solution during precipitation is another major factor that affects the fnal catalyst performance (Figure 3(II)). The insuffciency of added ammonium hydroxide NH3·H2O led to incomplete precipitation of precursor, and thus the conversion of olefins was reduced drastically. A fnal pH value of 9.46 was close to an optimum level for the removal of olefns, as an excessive amount of added NH3·H2O did not play a further promotional effect on the catalyst activity.
3.1.4 Effect of reaction temperature
Figure 4 shows the effect of temperature on the removal of olefns. Even at a lower reaction temperature (125 ℃), the performance of SZ was still considerably acceptable. The most favorable olefn removal rates were obtained at 175 ℃. Remarkably, this optimal reaction temperature is of great industrial application value, as the conventional temperature in industrial operation is in the range of 160—180 ℃.
Figure 3 Effects of precursor preparation condition relating to: (I) different precipitants and (II) fi nal pH value (the quantity of added alkali) on removal of ole fi ns at a reaction temperature of 175 ℃
3.2 Morphology and crystallization analysis
The XRD patterns of unmodifed ZrO2and SZ activated at different temperatures (500—700 ℃) are given in Figure 5. It can be seen from Figure 5 that pure ZrO2consisted of peaks arising from both monoclinic (M) and tetragonal(T) phases[10]. But when ZrO2was incorporated with sulfate ions, the intensity of both tetragonal and monoclinic phases increased greatly. SZ500 exhibited poor crystallinity with broad diffraction peaks of the tetragonal phases. The peak intensity of tetragonal and monoclinic phases grew steadily with the increase in calcination temperature from 500 ℃ to 650 ℃. However, when the calcination temperature increased from 650 ℃ to 700 ℃, the intensity of tetragonal phase decreased along with an increase in the intensity of the peak corresponding to monoclinic phase, indicating that a further calcination could lead to the transition of tetragonal phase to monoclinic phase. SZ exhibited a prominent tetragonal phase at a calcination temperature of 650 ℃, which was the optimal calcination temperature for sulfated zirconia to obtain a superb catalytic activity. It showed that the calcination temperature demonstrated a strong influence on the phase modifcation of zirconia from thermodynamically more stable monoclinic phase to the metastable tetragonal phase.
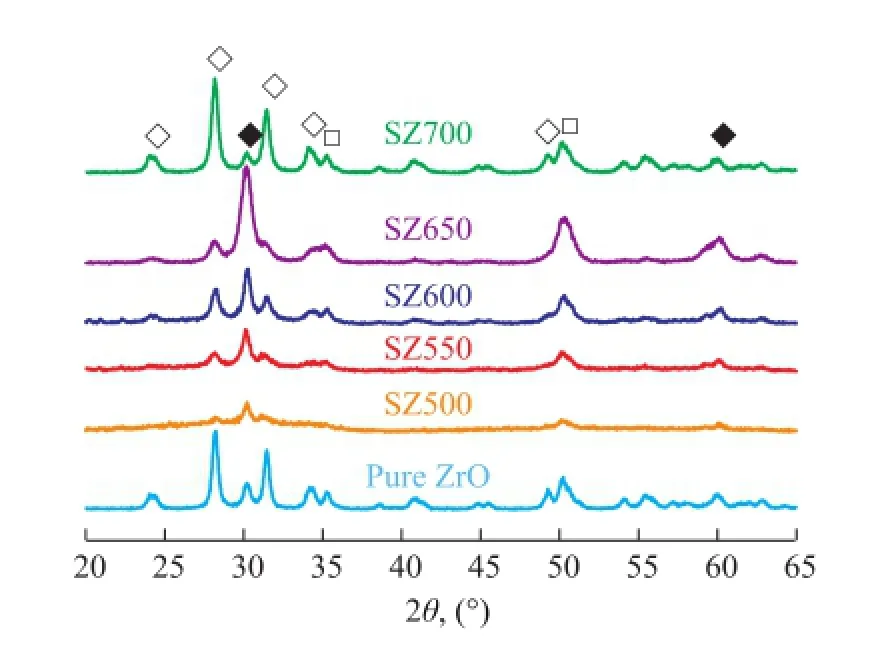
Figure 5 X-ray powder diffraction patterns of unmodi fi ed and sulphate-modi fi ed zirconia samples calcined at different temperatures
The SEM micrographs of different samples at 300Х magnifcation are reported in Figure 6. The image of unmodifed ZrO2showed large irregular agglomerated particles. The uncalcined SZ containing worm-like particles showed a relatively amorphous state. After the sulfdation and calcination procedure, SZ650 consisted of smaller and dispersive glass-like particles which could be clearly observed in Figure 6.
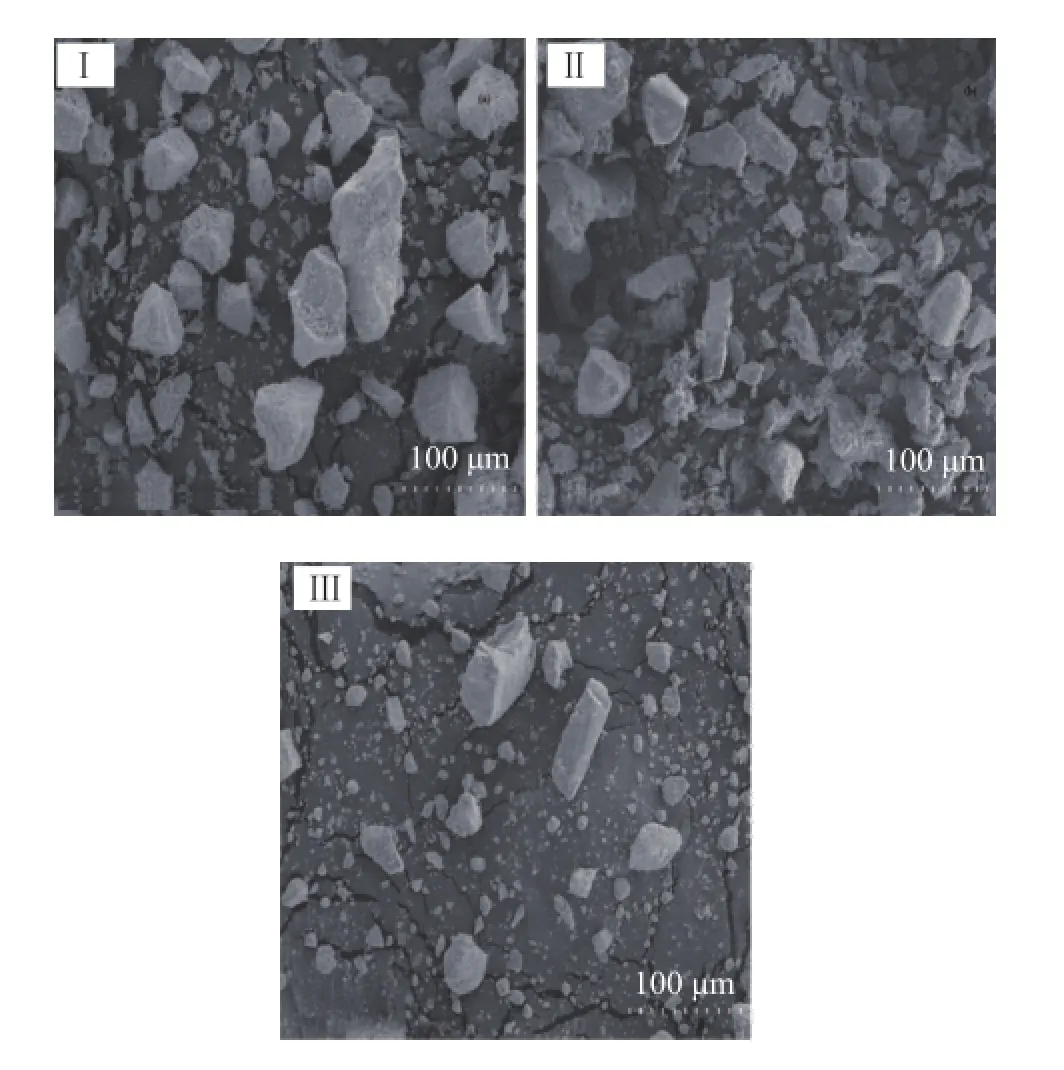
Figure 6 SEM images of unmodi fi ed and sulphate-modi fi ed zirconia samples: (I) pure ZrO2, (II) SZ120, (III) SZ650
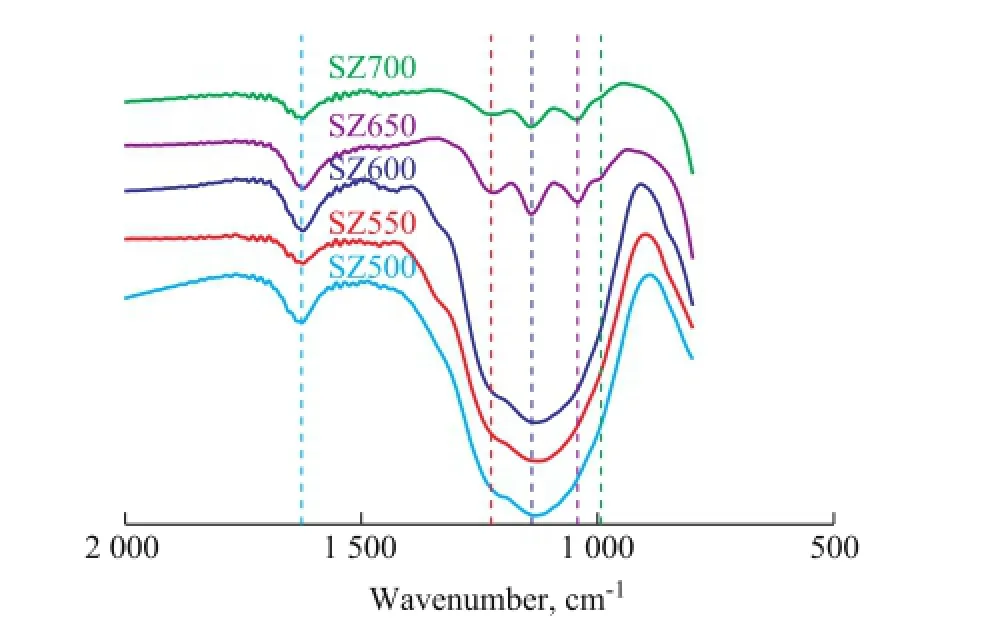
Figure 7 FTIR spectra of sulphated zirconia samples calcined at different temperatures
3.3 FT-IR & in-situ FT-IR analysis
The FI-IR spectroscopy (Fig. 7) of SZ samples shows broad bands in the range of 800—3800 cm-1confirming the effect of calcination temperature on the chemical property of the material. The band appearing at 991 cm-1can be attributed to the symmetric vibrations of O—S—O bonds while the bands at 1 057 cm-1, 1 140 cm-1and 1 212 cm-1are assigned to asymmetric stretching frequencies of antisymmetric O=S=O bonds[10]. All of the spectrums exhibited the appearance of dominant shoulder peaks at around 1 140 cm-1when the calcination tempera-ture was lower than 650 ℃, which occurred because the sulphate ions on ZrO2were in a chelating bidentate state. As for SZ650, the existence of new bands at 991 cm-1, 1 057 cm-1and 1 212 cm-1indicated that the bidentate sulphate ion had been successfully coordinated to the surface of ZrO2. However, the intensity of these peaks decreased with an increasing calcination temperature due to decomposition of sulfate groups upon calcination. Meanwhile, SZ700 showed a shoulder peak at 1 140 cm-1, implying the formation of labile sulphate species in the form of a polynuclear complex type such as
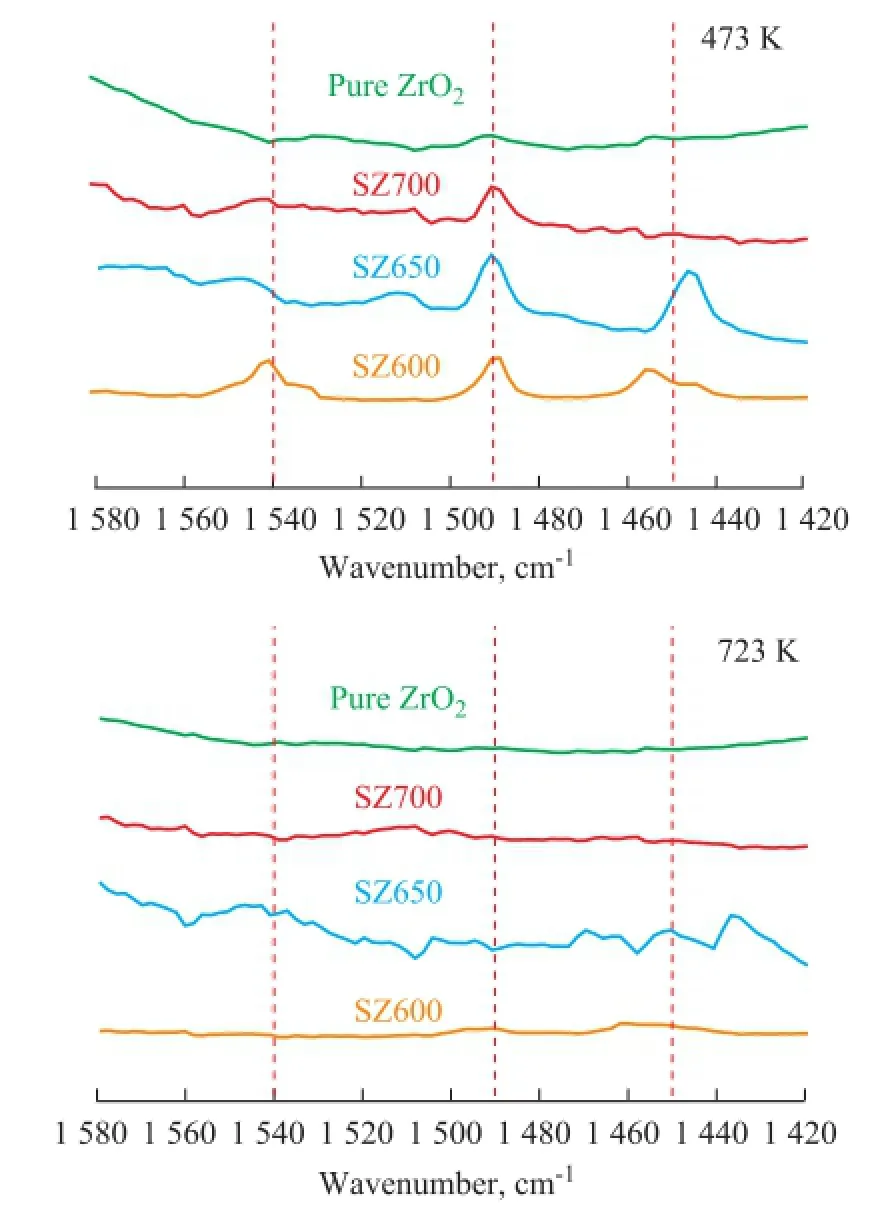
Figure 8 FTIR spectra of pyridine-ZrO2and pyridine-SZ
To investigate the acidity of samples, the in-situ FTIR analysis was applied. The FTIR spectra (Figure 8) of pyridine adsorbed on unmodified ZrO2and SZ samples calcined at different temperatures represented the characteristic peaks for pyridine molecules (Brnsted acid sites, B) at 1 545 cm-1and covalently bonded pyridine (Lewis acid sites, L) at around 1 450 cm-1. The acid amount calculated by the peak area desorbed at 473 K and 723 K was related to the total acid and strong acid, respectively, and their difference value was the amount of weak acids[12-13]. According to the Lambert-Beer Law, the quantitative analysis results (Table 2) can be calculated by means of the relevant empirical equations[14].
Pure ZrO2only contained a small number of acid sites, which led to its low catalytic activity from the beginning. As for SZ600, both Lewis and Brnsted acid sites were observed to be strong enough even after calcination at 600 ℃, indicating to the generation of acidity during the sulfdation treatment. Compared to SZ650, the relatively lower acidity of SZ600, especially in terms of the Lewis acidity, which played a promotional effect on the catalytic activity for removal of trace olefns[4], was caused by insuffcient calcination and the content of the complex O=S=O species. Particularly, in this work SZ650 exhibited a maximum amount of Lewis acids, which were reasonably helpful for acid-catalytic purifcation of aromatics[15], although the intensity of the value decreased by over one order of magnitude after successive calcination at 700 ℃. This result was ascribed to the thermal decomposition of sulfate. Upon combining the FTIR results with XRD analysis, it could be found that the surface acidity could be well correlated with tetragonal ZrO2.
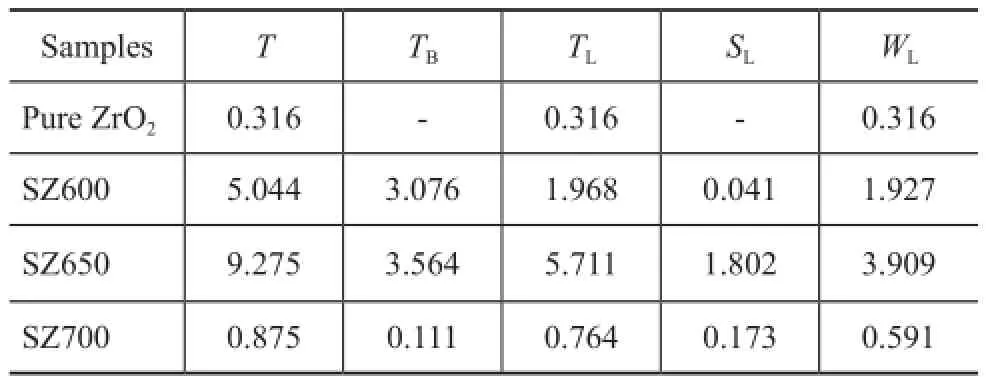
Table 2 Amount of Lewis and Brønsted sites (×10-4mol/g)
3.4 DSC/DSG analysis
Thermal analysis experiments were carried out in order to explore the effects of calcination temperature. The TG/ DTA curves of dried SZ (SZ120) are given in Figure 9 at temperatures in the range of 0—900 ℃. There are two weight loss stages, with one ranging from 65—400 ℃ and the other ranging from 600—850 ℃, respectively. According to DTG curve, the former stage was mainly caused by the elimination of physically absorbed water and crystalline water, and the highest point of this peak was at 120 ℃, while the change in temperature profle was caused by the desorption of chemically bound water, with the peak point identified at 200 ℃. The second endothermic peak at 740 ℃ indicated that the sulphate began to decomposestarting from 650 ℃. Notably, there was a small endothermic peak at 590—605 ℃, while no weight loss was observed in the TGA curve, hence the peak was attributed to the transition from an amorphous phase to a tetragonal metastable phase of zirconia, which was in good agreement with the XRD data. The DTG results indicated that the optimum calcination temperature of S2Z sample was about 650 ℃, which was in accordance with the earlier reports.
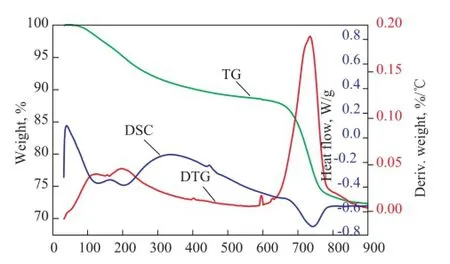
Figure 9 TGA-DSC curves of uncalcined SZ
3.5 BET surface area & pore size analysis
The pore size distribution (BJH model) curves of samples obtained under different synthesis conditions are displayed in Figure 10. There were two pore types observed for Zr(OH)4, and the first average pore diameter was centered at around 2.0 nm and the second one was over 3.0 nm in pore diameter. However, after doping with, only the second pore type remained and it even shifted to a slightly larger pore diameter for SZ120, indicating that the porosity decreased. This shift can be explained by the presence of closed porosity during the sulfidation process. It can be observed that there was a stronger peak at around 5.0 nm after calcination at 650 ℃, which demonstrated that some pores were enlarged during the heat treatment. However, SZ700 exhibited a relatively wider pore size distribution, which showed that additional calcination could transform the smaller tetragonal phase into the monoclinic phase.
Table 3 demonstrates the textural properties of samples under different preparation conditions. The comparison between Zr(OH)4precursor and SZ120 revealed that the sulfate impregnation decreased the BET surface area sharply, especially with regard to the micropore area. After modifcation at 650 ℃, the specifc surface area increased signifcantly from 32.1 m2/g to 86.3 m2/g, but showed a subsequent decrease to 52.8 m2/g after being subject to calcination at 700 ℃.
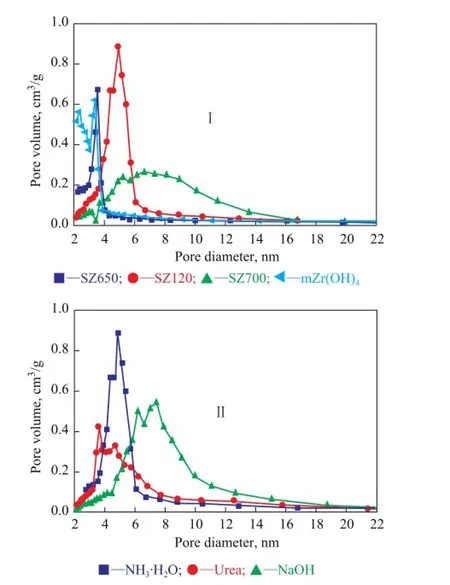
Figure 10 Pore size distribution of samples under different preparation conditions
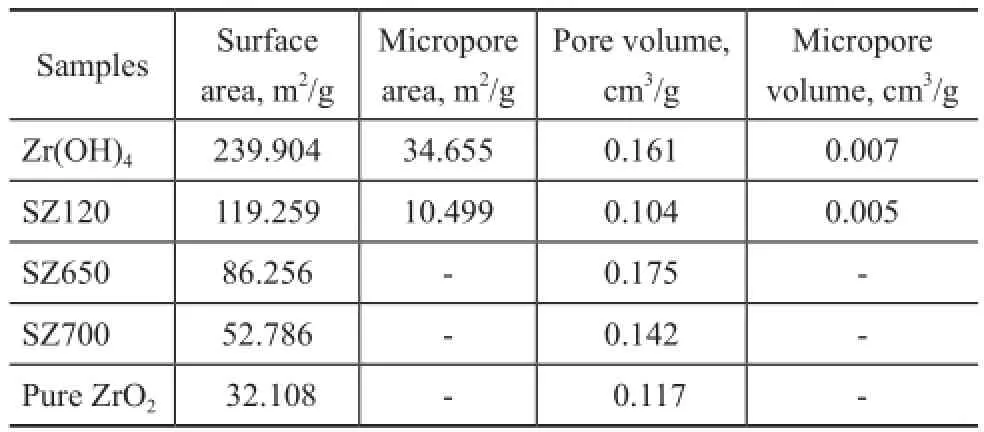
Table 3 Specific surface area and pore volume of different samples
Figure 10(II) shows the pore size distribution for the fnal SZ catalysts synthesized by different precipitants. It can be found that precipitation with NH3·H2O yielded SZ with narrow and uniform mesopore size distribution. Urea and NaOH led to wide and unordered pore size distribution, and especially the SZ catalyst treated by the strong base —sodium hydroxide—even had an average pore size of exceeding 7.5 nm (Table 4). As illustrated in Table 4, the highest surface area was obtained by using ammonia as the precipitating agent.
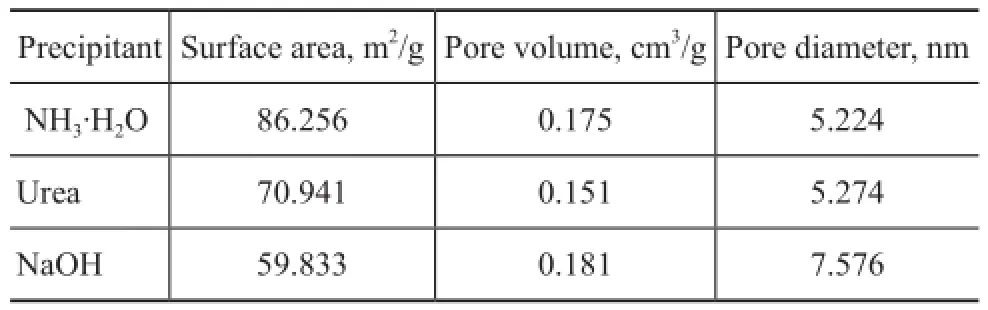
Table 4 BET surface area analysis of catalysts synthesized by different precipitants
4 Conclusions
Sulfated zirconia with variable preparation parameters was synthesized by the precipitation and impregnation method, and showed its superiority over commercial ROC in the purification of aromatics. The type and quantity of precipitants, the concentration of impregnant and the calcination temperature were varied and found to be the vital influencing factors in the synthesis of the catalyst. The optimum precursor for synthesizing SZ was precipitated by ammonium hydroxide with a final pH value of over 9.46. The fnal catalyst, which was impregnated with 2.0 mol/L sulfuric acid and calcined at 650 ℃, performed satisfactorily for the purifcation of aromatics. The characterization results revealed that properties of the final catalyst were interconnected closely with these preparation variables. The high catalytic activity as well as the long lifetime showed SZ was a prospective material in industry for olefns removal.
[1] Pu X, Shi L. Commercial test of the catalyst for removal of trace olefns from aromatics and its mechanism[J]. Catalysis Today, 2013, 212: 115-119
[2] Luan J, Li G, Shi L. Study of modifed clay and its industrial testing in aromatics refning[J]. Ind Eng Chem Res, 2011, 50(12): 7150-7154
[3] Liu N, Pu X, Shi L. Direct syntheses of a promising industrial organic–inorganic hybrid silica containing methanesulphonate[J]. Chemical Engineering Science, 2014, 119: 114-123
[4] Pu X, Liu N, Shi L. Acid properties and catalysis of USY zeolite with different extra-framework aluminum concentration[J]. Microporous and Mesoporous Materials, 2015, 201: 17-23
[5] Yadav G D, Krishnan M S. Etherification of β-naphthol with alkanols using modifed clays and sulfated zirconia[J]. Ind & Eng Chem Res, 1998, 37(8): 3358-3365
[6] Smirnova M Y, Toktarev A V, Ayupov A B, et al. Sulfated alumina and zirconia in isobutane/butene alkylation and npentane isomerization: Catalysis, acidity, and surface sulfate species[J]. Catalysis Today, 2010, 152(1): 17-23
[7] Tatsumi T, Matsuhashi H, Arata K. A study of the preparation procedures of sulfated zirconia prepared from zirconia gel. The effect of the pH of the mother solution on the isomerization activity ofn-pentane[J]. Bulletin of the Chemical Society of Japan, 1996, 69(5): 1191-1194
[8] Yadav G D, Murkute A D. Novel effcient mesoporous solid acid catalyst UDCaT-4: dehydration of 2-propanol and alkylation of mesitylene[J]. Langmuir, 2004, 20(26): 11607-11619
[9] Corma A, Fornes V, Juan-Rajadell M I, et al. Infuence of preparation conditions on the structure and catalytic properties of SO42−/ZrO2superacid catalysts[J]. Applied Catalysis A: General, 1994, 116(1/2): 151-163
[10] Garcia C M, Teixeira S, Marciniuk L L, et al. Transesterification of soybean oil catalyzed by sulfated zirconia[J]. Bioresource Technology, 2008, 99(14): 6608-6613
[11] Li X, Nagaoka K, Simon L J, et al. Infuence of calcination procedure on the catalytic property of sulfated zirconia[J]. Catalysis Letters, 2007, 113(1/2): 34-40
[12] Volkova G G, Reshetnikov S I, Shkuratova L N, et al.n-Hexane skeletal isomerization over sulfated zirconia catalysts with different Lewis acidity[J]. Chemical Engineering Journal, 2007, 134(1): 106-110
[13] Yadav G D, Yadav A R. Insight into esterifcation of eugenol to eugenol benzoate using a solid super acidic modifed zirconia catalyst UDCaT-5[J]. Chemical Engineering Journal, 2012, 192: 146-155
[14] Parry E P. An infrared study of pyridine adsorbed on acidic solids: Characterization of surface acidity[J]. Journal of Catalysis, 1963, 2(5): 371-379
[15] Yao J, Liu N, Shi L, et al. Sulfated zirconia as a novel and recyclable catalyst for removal of olefins from aromatics[J]. Catalysis Communications, 2015, 66:126-129
Received date: 2016-05-23; Accepted date: 2016-07-24.
Prof. Wang Xin, wangxin890@ecust. edu.cn.
杂志排行
中国炼油与石油化工的其它文章
- Removal of Nitrogen Compounds from Shale Diesel Fraction Using Ionic Liquid [C4mim]HSO4
- Improved Ti-containing Mesoporous Silica Catalyst Synthesized by Using Anionic Surfactant as Co-template
- Synthesis of Hierarchically Porous FAU/γ-Al2O3Composites with Different Morphologies via Directing Agent Induced Method
- Study on CO2Absorption by Aqueous Benzylamine and Its Formulations with Monoethanolamine as a Component for Post-Combustion Capture Process
- Effect of Magnetic Field on Tribological Properties of Lubricating Oils with and without Tricresyl Phosphate
- The Effect of Surfactant on Synthesis of ZSM-5 in a Super-Concentrated System
