NOX4蛋白在乳腺癌中的表达及与18F-FDG摄取值的相关性研究
2016-03-22李雪娜尹雅芙杜补林李亚明
李雪娜,尹雅芙,杜补林,李亚明
(中国医科大学附属第一医院核医学科,辽宁 沈阳 110001)
NOX4蛋白在乳腺癌中的表达及与18F-FDG摄取值的相关性研究
李雪娜,尹雅芙,杜补林,李亚明
(中国医科大学附属第一医院核医学科,辽宁 沈阳 110001)
目的:烟酰胺腺嘌呤二核苷酸磷酸氧化酶4(NOX4)在乳腺癌中的表达与糖代谢的相关性尚不清楚,本研究拟探讨乳腺癌18F-FDG摄取值与NOX4的表达水平的相关性及其临床意义。资料和方法:本研究入组乳腺癌患者术前行氟-18代脱氧葡萄糖正电子发射断层显像(18-fluorodeoxyglucose positron emission tomography,18F-FDG PET)。应用免疫组织化学方法检测乳腺癌患者病灶病理标本NOX4表达。收集患者的淋巴结转移、组织分型和组织学分级病理资料,分析乳腺癌NOX4表达与18F-FDG摄取值(最大标准摄取值,SUVmax)的相关性,及与肿瘤临床病理参数的关系。结果:56例患者符合入选标准而纳入研究,共56个乳腺癌灶。病灶SUVmax为4.78±2.96。NOX4表达阳性33例,阴性23例。NOX4表达阳性组SUVmax为6.42±3.23,NOX4表达阴性组SUVmax为3.22±1.57,NOX4表达阳性组的SUVmax高于表达阴性组,差异具有统计学意义 (t=4.83,P<0.05)。淋巴结转移阳性组的NOX4表达阳性率高于淋巴结转移阴性组(χ2=4.52,P<0.05)。NOX4表达与组织学类型无显著相关性(χ2=2.88,P>0.05)。组织学分级Ⅲ级组的NOX4表达阳性率高于Ⅰ+Ⅱ级组(χ2=6.32,P<0.01)。浸润性导管癌组SUVmax高于小叶癌组(t=2.97,P<0.05)。淋巴结转移组SUVmax高于非转移组(t=2.04,P=0.04)。组织学分级Ⅲ级组SUVmax高于Ⅰ+Ⅱ组(t=-2.5,P<0.05)。结论:乳腺癌NOX4表达水平与18F-FDG摄取值具有相关性;NOX4表达、18F-FDG摄取值与淋巴结转移、组织学分级具有相关性。
乳腺肿瘤;氟脱氧葡萄糖F18;正电子发射断层显像术
乳腺癌转移是导致乳腺癌患者死亡的重要原因。因此研究乳腺癌转移的机制尤为重要[1]。肿瘤细胞活性氧(ROS)的增加对肿瘤转移的影响越来越受到重视[2-3],NAPDH氧化酶和线粒体是细胞内ROS产生的主要来源[4],NAPDH氧化酶在一些肿瘤中高表达的mRNA水平[5-6],NADPH氧化酶是细胞可逆调控ROS产生的重要机制[7-8]。烟酰胺腺嘌呤二核苷酸磷酸氧化酶4(NOX4)主要分布在肾脏,在成骨细胞、内皮细胞、造血干细胞及神经细胞中有表达[9-10],NOX4在乳腺癌细胞和乳腺癌组织中有表达[11],但与能量代谢和乳腺癌转移的相关性尚不清楚。基于以上背景,本研究拟探讨乳腺癌18F-FDG摄取值与NOX4的表达水平的相关性及其临床意义。
1 资料与方法
1.1 一般资料
本研究收集2005年5月—2008年1月在中国医科大学附属第一医院核医学科行18F-FDG PET显像的乳腺癌患者。入选标准:①显像后约2周内在外科进行乳腺癌手术切除。②术前未进行放疗和化疗。③所有患者签署知情同意书。收集并记录患者的一般临床资料,如年龄、病变大小。
1.2 显像方法
采用GE Discovery LS PET/CT仪扫描。18FFDG由GE Minitrace回旋加速器生产,通过合成模块自动合成,放化纯>95%。受检者空腹4 h以上,空腹血糖<7 mmol/L。平静状态下按5.55 MBq/kg静脉注射18F-FDG。平卧50~60 min后行PET/CT显像。CT为4排螺旋CT,层厚4.25 mm,140 kV,80 mA。PET(18环)扫描采用二维(2D)模式采集,每个床位采集3 min,图像重建采用有序子集最大期望值迭代法,矩阵128×128。18F-FDG PET/CT图像半定量分析。逐层阅读PET/CT影像,选择病灶摄取最显著的层面,应用感兴趣区技术测定病灶最大标准摄取值(SUVmax)[12]。
1.3 乳腺组织病理学分析
本研究选取乳腺癌组织标本,标本均经4%中性甲醛固定、石蜡包埋、HE常规染色后明确诊断。分别由2名病理医师根据WTO乳腺肿瘤分类标准(2003)和TNM分期系统(2003),确定肿瘤的组织学类型、淋巴结转移情况和临床病理分期。
1.4 免疫组织化学染色及结果判定
组织标本经中性福尔马林溶液固定、石蜡包埋,制成4 μm切片,切片经二甲苯脱蜡、梯度酒精脱苯水化后,采用链霉素抗生物素蛋白-过氧化物酶(SP法)检测NOX4蛋白表达,具体方法如下:①切片经二甲苯、梯度酒精脱蜡至水;②高温高压抗原修复95 s;③3%过氧化氢37℃浸泡20 min;④磷酸盐缓冲液(PBS)冲洗3次,每次5 min;⑤37℃条件下,湿盒内非免疫血清封闭30 min;⑥滴加山兔抗人多克隆NOX4抗体(1∶100 Abcam公司),湿盒内4℃孵育过夜;⑦PBS冲洗3次,每次5 min;⑧滴加生物素标记二抗,湿盒内37℃孵育40 min;⑨PBS冲洗3次,每次5min;⑩滴加链霉菌抗生物素蛋白——过氧化物酶,湿盒内37℃,40min;⑾PBS冲洗3次,每次5min;⑿DAB显色;⒀苏木素复染;⒁梯度酒精脱水,二甲苯透明;⒂中性树脂封片;⒃显微镜下观察染色效果,于400倍镜下拍照。
结果判定:多克隆抗体NOX4以细胞核中出现棕黄色颗粒为阳性显色[13]。高倍视野(400倍)下选阳性信号最强区域计数200个肿瘤细胞中阳性细胞数,按表达百分率分为以下4个等级:0%为0分,>0%~50%为1分,>50%~75%为2分,>75%为3分。根据免疫组化染色强度分为3个等级:浅黄色计为1分,棕黄色计为2分,黄褐色计为3分。以阳性细胞率和染色强度的分值乘积作为每1例的积分,积分<4者判定为阴性,积分≥4为阳性。
1.5 统计学分析
采用SPSS 16.0软件进行统计学分析。正态分布的连续性变量以均数±标准差(±s)表示,组间比较采用独立样本t检验;组间比较应用卡方检验;P<0.05为差异具有统计学意义。
2 结果
研究期间共56例患者符合入选标准而纳入研究,年龄(55.6±9.5)岁。病变大小(2.1±0.5)cm。其中包括45例乳腺浸润性导管癌,11例乳腺小叶癌。肿瘤直径<3 cm者50例,≥3 cm者6例;淋巴结转移33例,无淋巴结转移23例。组织学分级Ⅰ+Ⅱ期25例,Ⅲ期31例。
56例乳腺癌患者,共 56个乳腺癌灶。病灶SUVmax为4.78±2.96。NOX4表达阳性33例,阴性23例。NOX4表达阳性组SUVmax为6.42±3.23,NOX4表达阴性组SUVmax为3.22±1.57,NOX4表达阳性组的乳腺癌糖代谢高于表达阴性组,差异具有统计学意义(t=4.83,P<0.05)(图1,2)。
在56例乳腺癌患者中,NOX4表达水平与年龄无显著性相关(χ2=3.61,P=0.06),NOX4表达与淋巴结转移显著性相关性(χ2=4.52,P=0.03),淋巴结转移阳性组的NOX4表达阳性率高于淋巴结转移阴性组。NOX4表达与组织学类型无显著相关性 (χ2= 2.88,P=0.09)。NOX4表达与组织学分级具有显著相关性(χ2=6.32,P=0.00),组织学分级Ⅲ级组NOX4表达的阳性率高于Ⅰ+Ⅱ级组。
年龄≥55岁组和<55岁组乳腺癌灶的SUVmax分别为4.2±2.32和5.3±3.5,两者无统计学差异(t=-1.42,P=0.15)。浸润性导管癌组和小叶癌组的SUVmax分别为 5.75±3.15和 3.50±2.1 (t=2.97,P= 0.01),浸润性导管癌组SUVmax高于小叶癌组。淋巴结转移组与非转移组的SUVmax分别为6.68± 5.19和4.04±3.77,淋巴结转移组的SUVmax高于非转移组(t=2.04,P=0.04)。组织学分级Ⅰ+Ⅱ组和Ⅲ级组的SUVmax分别为3.75±2.47和7.46±7.75,组织学分级Ⅲ级组SUVmax高于Ⅰ+Ⅱ组(t=-2.5,P=0.01)。

表1 56例乳腺癌肿瘤NOX4表达、18F-FDG摄取率与临床病理学参数的相关性分析
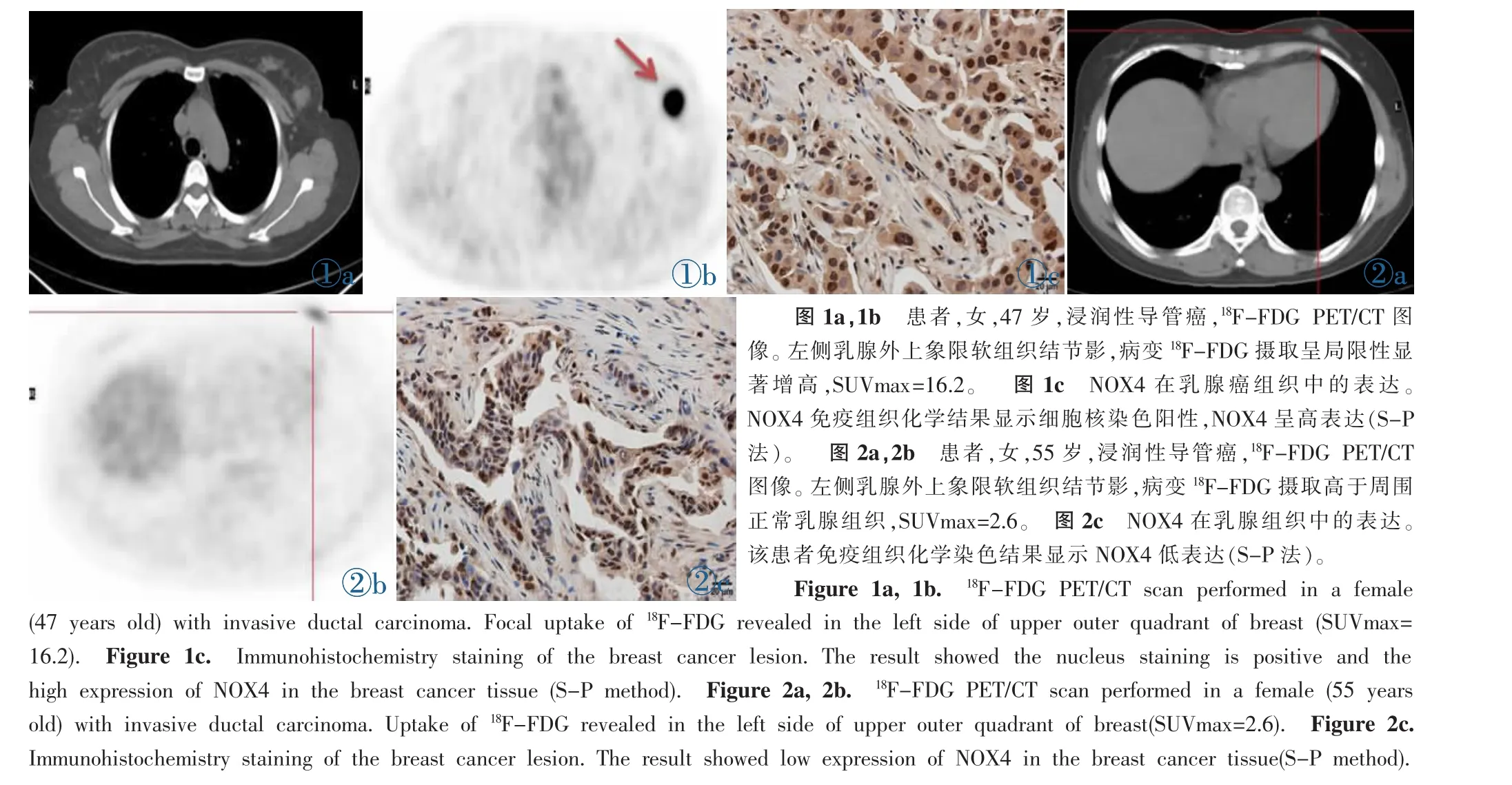
图1a,1b 患者,女,47岁,浸润性导管癌,18F-FDG PET/CT图像。左侧乳腺外上象限软组织结节影,病变18F-FDG摄取呈局限性显著增高,SUVmax=16.2。 图 1c NOX4在乳腺癌组织中的表达。NOX4免疫组织化学结果显示细胞核染色阳性,NOX4呈高表达(S-P法)。 图2a,2b 患者,女,55岁,浸润性导管癌,18F-FDG PET/CT图像。左侧乳腺外上象限软组织结节影,病变18F-FDG摄取高于周围正常乳腺组织,SUVmax=2.6。 图2c NOX4在乳腺组织中的表达。该患者免疫组织化学染色结果显示NOX4低表达(S-P法)。Figure 1a,1b.18F-FDG PET/CT scan performed in a female (47 years old)with invasive ductal carcinoma.Focal uptake of18F-FDG revealed in the left side of upper outer quadrant of breast(SUVmax= 16.2). Figure 1c. Immunohistochemistry staining of the breast cancer lesion.The result showed the nucleus staining is positive and the high expression of NOX4 in the breast cancer tissue(S-P method). Figure 2a,2b.18F-FDG PET/CT scan performed in a female(55 years old)with invasive ductal carcinoma.Uptake of18F-FDG revealed in the left side of upper outer quadrant of breast(SUVmax=2.6). Figure 2c. Immunohistochemistry staining of the breast cancer lesion.The result showed low expression of NOX4 in the breast cancer tissue(S-P method).
3 讨论
NADPH氧化酶最早认为存在于吞噬细胞内,氧化酶产生的ROS清除入侵的微生物。后来研究发现NADPH氧化酶不仅参与宿主防御,且与非吞噬细胞内和细胞间的信息传递有关。与吞噬细胞所产生的ROS不同,NOX家族所产生的活性氧不参与细胞防御功能,而参与细胞分化、增殖等的调节,NOX家族在肿瘤中的作用还处于研究的起始阶段,有研究[14]显示NOX在肿瘤细胞和正常组织中表达不同,提示NOX异常表达和调节可能与肿瘤的发生有关。NOX4酶复合体不需要RAC蛋白的活化,NOX4是一个一直处于活化状态的NADPH氧化酶。NOX4在肿瘤细胞中表达,在不同的肿瘤中表达具有异质性[15-16],而且它的生物学功能尚不清楚。肿瘤患者NOX4表达与能量代谢的相关性尚无相关报道。
本研究中,我们对乳腺癌患者NOX4表达水平与肿瘤的糖酵解水平进行了相关性分析。将NOX4表达阳性组与NOX4表达阴性组乳腺癌灶18F-FDG摄取值SUVmax进行比较分析,结果显示,两组间SUVmax具有显著性差异,NOX4表达阳性组SUVmax高于阴性组,提示乳腺癌糖酵解率增高,NOX4表达的阳性率增高。既往的体外细胞实验研究结果也显示人为干预T-Rex 293细胞的线粒体功能,使细胞线粒体功能发生障碍,细胞的能量代谢转换为有氧糖酵解,同时细胞的NOX1表达增高,糖酵解的重要底物NAD+增高,提示NOX在细胞内能量代谢中具有重要的作用[17]。本研究首次报道人的乳腺癌组织中的NOX4蛋白表达与18F-FDG摄取值具有相关性。恶性肿瘤细胞表面葡萄糖转运体(Glucose transport,Glut)多呈高表达,Glut介导葡萄糖摄取到细胞内,有研究发现乳腺癌18F-FDG摄取与组织的Glut表达具有相关性[18-19]。因此,乳腺癌中NOX4表达与Glut表达的相关性如何,有待进一步深入研究。
有研究显示乳腺癌的18F-FDG摄取值与乳腺癌的预后、恶性程度具有相关性[20],本研究进一步分析了乳腺癌NOX4表达与临床病理学参数的相关性,结果提示NOX4表达与术中淋巴结转移具有相关性。淋巴结转移组的NOX4表达阳性率高于无淋巴结转移组。因此,提示NOX4表达与乳腺癌的转移具有相关性。本研究结果显示乳腺癌NOX4的表达与乳腺癌组织学分级具有相关性,组织学分级越高,NOX4的阳性表达率越高。最新的研究[21]报道,细胞实验和动物实验结果显示,通过抑制NOX4的表达可降低鼠乳腺癌细胞4T1的侵袭转移能力。最近的体外人乳腺癌细胞研究[22]也显示,NOX4是ROS的重要来源,在乏氧下通过TGF-β通路进行调解,而TGF-β和乏氧是肿瘤转移的重要因素,提示NOX4表达与乳腺癌转移具有相关性。但在人乳腺癌的标本中的表达与转移、组织学分级的相关性还未见相关报道。本研究结果显示乳腺癌的18F-FDG摄取值与肿瘤的淋巴结转移和组织学分级具有相关性,既往有研究报道乳腺癌的18F-FDG摄取值与肿瘤分级和预后相关[23-24],和我们的研究结果一致。 综上所述,本研究表明乳腺癌NOX4表达水平与糖酵解具有相关性;NOX4表达与乳腺癌转移相关。
本研究仍存在一定的局限性。首先,本研究的样本量较少,使多因素回归分析受限,其次,单中心研究,不能除外选择性偏倚。对于NOX4影响乳腺癌能量代谢、转移的分子机制还需要进一步研究。
[1]O’Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer[J].Oncologist,2005,10(suppl 3):20-29.
[2]Ferraro D,Corso S,Fasano E,et al.Pro-metastatic signaling by C-Met through RAC-1 and reactive oxygen species(ROS)[J]. Oncogene,2006,25(26):3689-3698.
[3]Suh YA,Arnold RS,Lassegue B,et al.Cell transformation by the superoxide-generating oxidase Mox1[J].Nature,1999,401 (6748):79-82.
[4]Chen K,Craige SE,Keaney JF.Downstream targets and intracellular compartmentalization in Nox signaling[J].Antioxid Redox Signal,2009,11(10):2467-2480.
[5]Fukuyama M,Rokutan K,Sano T,et al.Overexpression of a novel superoxide-producing enzyme,NADPH oxidase 1,in adenoma and well differentiated adenocarcinoma of the human colon [J].Cancer Lett,2005,221(1):97-104.
[6]Lim SD,Sun C,Lambeth JD,et al.Increased Nox1 and hydrogen peroxide in prostate cancer[J].Prostate,2005,62(2):200-207.
[7]Fleming I,Michaelis UR,Bredenkotter D,et al.Endothelium-derived hyperpolarizing factor synthase(Cytochrome P450 2C9)is a functionally significant source of reactive oxygen species in coronary arteries[J].Circ Res,2001,88(1):44-51.
[8]Martyn KD,Frederick LM,von Loehneysen K,et al.Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases[J].Cell Signal,2006,18(1):69-82.
[9]Kuroda J,Ago T,Matsushima S,et al.NADPH oxidase 4(Nox4) is a major source of oxidative stress in the failing heart[J].Proc Natl Acad Sci USA,2010,107(35):15565-15570.
[10]Piccoli C,Ria R,Scrima R,et al.Characterization of mitochondrial and extra-mitochondrial oxygen consuming reactions in human hematopoietic stem cells.Novel evidence of the occurrence of NAD(P)H oxidase activity[J].J Biol Chem,2005,280(28): 26467-26476.
[11]Graham KA,Kulawiec M,Owens KM,et al.NADPH oxidase 4 is an oncoprotein localized to mitochondria[J].Cancer Biol Ther, 2010,10(3):223-231.
[12]De Cicco C,Gilardi L,Botteri E,et al.Is[18F]fluorodeoxyglucose uptake by the primary tumor a prognostic factor in breast cancer?[J].Breast,2013,22(1):39-43.
[13]Shono T,Yokoyama N,Uesaka T,et al.Enhanced expression of NADPH oxidase Nox4 in human gliomas and its roles in cell proliferation and survival[J].Int J Cancer,2008,123(4):787-792.
[14]Edderkaoui M,Nitsche C,Zheng L,et al.NADPH oxidase activation in pancreatic cancer cells is mediated through Akt-dependent up-regulation of p22phox[J].J Biol Chem,2013,288 (51):36259.
[15]Yamaura M,Mitsushita J,Furuta S,et al.NADPH oxidase 4 contributes to transformation phenotype of melanoma cells by regulating G2-M cell cycle progression[J].Cancer Res,2009,69 (6):2647-2654.
[16]Weyemi U,Caillou B,Talbot M,et al.Intracellular expression of reactive oxygen species-generating NADPH oxidase NOX4 in normal and cancer thyroid tissues[J].Endocr Relat Cancer, 2010,17(1):27-37.
[17]Lu W,Hu Y,Chen G,et al.Novel Role of NOX in supporting aerobic glycolysis in cancer cells with mitochondrial dysfunction and as potential target for cancer therapy[J].Plos One,2012,10 (5):e1001326.
[18]Bos R,van Der Hoeven JJ,van Der Wall E,et al.Biologic correlatesof (18)fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography[J].J Clin Oncol,2002,20(2):379-387.
[19]Brown RS,Goodman TM,Zasadny KR,et al.Expression of hexokinaseⅡ and Glut-1 in untreated human breast cancer[J]. Nucl Med Biol,2002,29(4):443-453.
[20]Cochet A,Dygai-Cochet I,Riedinger JM,et al.18F-FDG PET/ CT provides powerfulprognosticstratification in theprimary staging of large breast cancer when compared with conventional explorations[J].Eur J Nucl Med Mol Imaging,2014,41(3):428-437.
[21]Zhang B,Liu Z,Hu X.Inhibiting cancer metastasis via targeting NAPDH oxidase[J].Biochem Pharmacol,2013,86(2):253-266.
[22]Boudreau HE,Casterline BW,Rada B,et al.Nox4 involvement in TGF-beta and SMAD3-driven induction of the epithelial-tomesenchymal transition and migration of breast epithelial cells[J]. Free Radic Biol Med,2012,53(7):1489-1499.
[23]Groheux D,Giacchetti S,Moretti JL,et al.Correlation of high18F-FDG uptake to clinical,pathological and biological prognostic factors in breast cancer[J].Eur J Nucl Med Mol Imaging, 2011,38(3):426-435.
[24]Sanli Y,Kuyumcu S,Ozkan ZG,et al.Increased FDG uptake in breast cancer is associated with prognostic factors[J].Ann Nucl Med,2012,26(4):345-350.
Relationships between NOX4 expression and18F-FDG uptake in patients with breast cancer
LI Xue-na,YIN Ya-fu,DU Bu-lin,LI Ya-ming
(Department of Nuclear Medicine,the First Hospital of China Medical University,Shenyang 110001,China)
Objective:The relationship between the NOX4(NADPH oxidase 4)expression and glucose metabolism in breast cancer is unclear.This present study intends to study the expression levels of NOX4 and18F-FDG uptake.And to further explore the clinical significance.Methods:The patients diagnosed as breast cancer who underwent preoperative18F-FDG PET were enrolled.We examined the maximum standardized uptake values(SUVmax)of lesions.The levels of NOX4 were measured with immunohistochemical analysis.The clinical,histopathology of breast cancer patients were collected and recorded,and the correlation between NOX4 and clinical and histological predictors and metastasis were analyzed.Results:Fifty-six patients met the inclusion criteria and were included in the study,a total of 56 breast lesions.The SUVmax of lesions were 4.78±2.96.Thirty-three cases had positive expression of NOX4,23 cases had negative expression.The SUVmax in the positive expression of NOX4 group was 6.42±3.23,the negative NOX expression group was 3.22±1.57.The SUVmax was higher in the NOX4 positive expression group than negative expression group,the difference was statistically significant(t=4.83,P<0.05).NOX4 expression levels were significantly correlated with lymph node metastasis(χ2=4.52,P<0.05).The expression of NOX4 and histological types have no significant correlation(χ2=2.88,P>0.05).The expression of NOX4 and histological grading were significantly related to histological grade,the positive rate of NOX4 expression in the groupⅢ was higher thanⅠ+Ⅱ Group(χ2= 6.32,P<0.01).The SUVmax in infiltrating ductal carcinoma was higher than lobular carcinoma(t=2.97,P<0.05).The SUVmax in lymph node metastasis was higher than no lymph node metastasis(t=2.04,P=0.04).The SUVmax in the groupⅢ was higher thanⅠ+Ⅱ Group(t=-2.5,P<0.05).Conclusions:The levels of NOX4 expression were correlated with the18F-FDG uptake.The expression of NOX4 and the18F-FDG uptake are associated with lymph node metastasis and histological grade.
Breast neoplasms;Fluorodeoxyglucose F18;Positron-emission tomography
R737.9;R817.4
A
1008-1062(2016)08-0547-04
2015-12-31;
2016-01-15
李雪娜(1980-),女,辽宁盘锦人,讲师。E-mail:lixuenacmunm@163.com
李亚明,中国医科大学附属第一医院核医学科,110001。E-mail:ymli2001@163.com
国家自然科学基金项目(81271605);国家自然科学基金青年科学基金项目(81301249);辽宁省科学技术计划项目(2012225013)。
