黄芩中4种黄酮的抗肿瘤细胞株活性比较*
2016-02-14何耀昌
何耀昌,薛 红
(香港科技大学生命科学部及应用基因组中心 香港 999077)
黄芩中4种黄酮的抗肿瘤细胞株活性比较*
何耀昌,薛 红**
(香港科技大学生命科学部及应用基因组中心 香港 999077)
目的:汉黄芩苷、汉黄芩素、黄芩苷及黄芩素是黄芩的主要成分。对前三种黄酮的抗癌活性文献有不同的报导。方法: 本研究比较了4种黄酮在相同浓度下对4个肿瘤细胞株24 h及48 h存活率的影响。结果:实验结果显示,汉黄芩素对4种细胞株都有明显的抑制作用,48 h半抑制浓度分别为97.9 μM(肺腺癌A549)、15.3 μM(子宫颈癌HeLa)、147 μM(肝细胞癌HepG2)及104 μM(乳腺癌MCF-7)。其相应的糖化物,汉黄芩苷对4种细胞均未显示抑制。黄芩素抑制HeLa细胞,而糖化的黄芩苷抑制HepG2,且对MCF-7显示刺激作用。结论: 以上结果显示,汉黄芩素在4种主要黄芩黄酮中具有最强的抑制肿瘤细胞生长效能,而其他3种黄酮的抗肿瘤作用并不一致,黄芩苷甚至促进个别肿瘤细胞株的生长。因此,黄酮抗癌作用的研究及可能的临床应用更应在单体水平进行。
黄芩 汉黄芩素 汉黄芩苷 黄芩素 黄芩苷 肿瘤细胞株
In 2015, there were estimated 1.6 million new cancer cases and 590 000 deaths from cancer in the U.S. Lung cancer was the leading cancer of all populations, while breast cancer the leading cancer in women[1]. In addition to the cancer disease itself, cancer patients also suffered from anxiety or depression[2-4]. Therefore, there is a need to develop medications that treat both cancer and anxiety.
Plants of the genus Scutellaria, commonly known as skullcaps, are widely distributed with about 100 species in China and 17 species in Japan. The dry root of S. baicalensis Georgi, or Huang Qin, is one of the most frequently employed medicinal herbs in traditional Chinese medicine, possessing anti-bacterial, antiinflammatory and sedative effects[5]. It is widely applied to the treatments of common cold, artherosclerosis, hypertension, dysentery, hyperlipemia, inflammation and weight reduction[6,7].
Three major constituents in S. baicalensis, namely baicalin, baicalein and wogonin, have been tested on a variety of cancer cell lines including those of lung, cervical, liver and breast cancers. Their efficacies observed varied between different types of cancer cells and different studies without clear indication of the most active anti-cancer agent in S. baicalensis. Moreover, contradictory results were reported: in one study, a baicalin-enriched fraction of S. baicalensis promoted the proliferation of cancer cells over certain concentrations[8]. In view of this, in the present study, the anti-cancer effects of baicalin, baicalein, wogonoside and wogonin from S. baicalensis were tested on the human cancer cell line, A549 lung adenocarcinoma, HeLa cervical carcinoma, HepG2 hepatocellular carcinoma and MCF-7 breast adenocarcinoma in order to assess their comparative effectiveness as anti-cancer agents.
1 Materials and Methods
1.1 Materials
Baicalin, baicalein and wogonoside were obtained from Indofine Chemical (Hillsborough, USA, Lot No.: 040212) and wogonin from Wako Pure Chemical Industries Ltd. (Osaka, Japan, Lot No.: SEL 7669). Each compound (Fig. 1) was dissolved in dimethyl sulfoxide (DMSO) from Sigma-Aldrich (St. Louis, USA, Lot No.:RNBD4545) in a 0.25 M stock solution and stored at -20°C. Thiazolyl blue tetrazolium bromide was also obtained from Sigma-Aldrich (St. Louis, USA, Lot No.:MKBR4419V).
1.2 Cell culture
Human A549 lung adenocarcinomas, HeLa cervical carcinoma cells, HepG2 hepatocellular carcinomas and MCF-7 breast adenocarcinoma cells obtained from American Type Culture Collection (Manassas, USA) were cultivated in a growth medium comprising Dulbecco’s Modified Eagle Medium adjusted to pH=7.4 with addition of 10% fetal bovine serum (Gibco, Grand Island, USA, Lot No.: 1791921). The cells were incubated in a humidified atmosphere of 95%-5% CO2at 37°C.

Fig.1 Structures of the four major flavonoids in S. baicalensis
1.3 Assay for viable cells
Cells were seeded at a density of 2×103cells per well in 96-well plates and incubated overnight. Upon the drug treatment, baicalin, baicalein, wogonoside and wogonin stock solutions were separately diluted with DMSO to 1 000 times their test concentrations, followed by 1:1 000 dilution (v/v) with the growth mediums. Wells containing the growth mediums with 0.1% DMSO were served as control. The growth medium in each test well was replaced with 100 μL of 0, 10, 30, 50, 100, 150 or 200 μM of one of the flavonoids, and the cells were incubated for another 24 or 48 hrs. At the end of the incubation, 20 μL of 5 mg·mL-1thiazolyl blue tetrazolium bromide dissolved in growth medium was added to the individual wells, including blank wells containing only a growth medium without cells, andincubated for another 4 hrs at 37°C. The supernatant was removed and 150 μL DMSO was adopted to extract the dye from the cells. Absorbance was measured in a 96-well plate reader at 570 nm. The average absorbance reading of the blank wells was subtracted from the reading of each well. Viable cells were estimated by the equation: viable cells = (mean test absorbance / mean control absorbance) × 100%, where the mean test absorbance represented the absorbance of wells containing a specified flavonoid concentration and mean control absorbance, the absorbance of well containing no flavonoid. The 50% inhibition concentration (IC50) of each flavonoid was estimated at the end of the 24 and 48 hrs incubation using the Logit method.
1.4 Statistical analysis
Data in the different groups were expressed in Mean ± S.E.M. All data were obtained from at least three independent experiments, and analyzed by one-way ANOVA with Dunnett’s t-test as post-hoc test using the GraphPad Prism 5.0 software.
2 Results
2.1 Effects on A549 lung cancer cell lines
A549 cells multiplied 2.40 and 4.74 folds in culture to the initial count after 24 and 48 hrs’ incubation respectively (Fig. 2). Administered by the test flavonoids to the cells, baicalin and baicalein at 50, 100 or 200 μM induced no loss of cell viability after 24 or 48 hrs’incubation. Wogonoside at 200 μM decreased cell viability to 82% after the 48 hrs’ incubation (P < 0.01). Wogonin decreased cell viability to 88% at 50 μM after 48 hrs(P < 0.001), and to 76% and 6% (both P< 0.001) at 100 and 200 μM after 24 hrs’ incubation, while to 49% and 11% at 100 and 200 μM after 48 hrs’incubation (both P < 0.001) , respectively (Fig. 3).
2.2 Effects on HeLa cervical cancer cell lines
HeLa cells multiplied 2.97 and 6.84 folds in culture to the initial count after 24 and 48 hrs’ incubation, respectively (Fig. 2). Administered by the test flavonoids, baicalin and wogonoside induced no loss of cell viability after the 24 or 48 hrs’ incubation. Baicalein decreased cell viability to 80% (P < 0.05), 66% (P < 0.001) and 57% (P < 0.001) at 10, 30 and 50 μM after 24 hrs, respectively; while to 87% (P < 0.05), 30% (P < 0.001) and 19% (P < 0.001) at 10, 30 and 50 μM after the 48 hrs’ incubation. Wogonin decreased cell viability to 49% (P < 0.01) at 50 μM after 24 hrs’ incubation, and to 71% (P < 0.001), 18% (P < 0.001) and 13% (P < 0.001) at 10, 30 and 50 μM after 48 hrs’ incubation (Fig. 4).
2.3 Effects on HepG2 liver cancer cell lines
HepG2 cells multiplied 1.81 and 2.94 folds in culture to the initial count after 24 and 48 hrs’incubation, respectively (Fig. 2). When the test flavonoids were administered to these cells, baicalin decreased cell viability to 64% (P < 0.01) and 46% (P<0.001) at 150 and 200 μM after 24 hrs’ incubation, while to 82% (P < 0.05), 56% (P < 0.001) and 34% (P <0.001) at 100, 150 and 200 μM after 48 hrs’ incubation. Baicalein decreased cell viability to 79% (P < 0.05) and 58% (P < 0.001) at 150 and 200 μM after 24 hrs’ incubation; and to 64% (P < 0.001) at 200 μM after 48 hrs’ incubation, respectively. Wogonoside at 200 μM decreased cell viability to 78% (P < 0.05) after the 24 hrs’ incubation and 81% (P < 0.001) after the 48 hrs’ incubation. Wogonin decreased cell viability to 79% (P < 0.05) and 58% (P < 0.001) at 150 and 200 μM after 24 hrs’incubation, and to 82% (P < 0.001), 50% (P < 0.001) and 21% (P < 0.001) at 100, 150 and 200 μM after the 48 hrs’ incubation (Fig. 5).
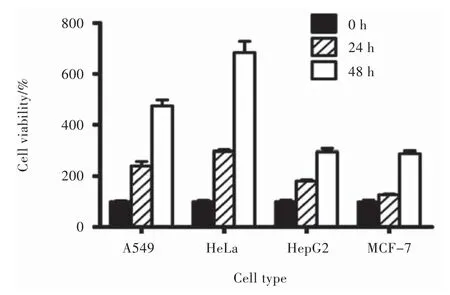
Fig. 2 Proliferation of different cell lines in the absence of flavonoids (n = 3)

Fig. 3 Effects of different flavonoids on the growth of human lung adenocarcinoma cell line A549 (n = 3)
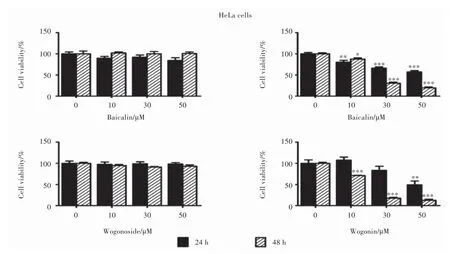
Fig. 4 Effects of different flavonoids on the growth of human cervical carcinoma HeLa cell line (n = 3)

Fig. 5 Effects of different flavonoids on the growth of human hepatocellular carcinoma cell line HepG2 (n = 3)
2.4 Effects on MCF-7 breast cancer cell lines
MCF-7 cells multiplied 1.26 and 2.88 folds in culture to the initial count after 24 and 48 hrs’incubation, respectively (Fig. 2). Administered by the test flavonoids to the cells, baicalein induced no loss of cell viability after 24 or 48 hrs’ incubation. Baicalin increased cell viability to 122% (P < 0.05), 125% (P <0.01) and 119% (P < 0.05) at 100, 150 and 200 μM after 48 hrs’ incubation, respectively. Wogonoside at 200 μM decreased cell viability to 77% (P < 0.05) after 48 hrs’incubation. Wogonin at 150 and 200 μM decreased cell viability to 60% (P < 0.05) and 48% (P < 0.05) after 24 hrs’incubation; and to 52% (P < 0.01), 32% (P < 0.001) and 23% (P < 0.001) at 100, 150 and 200 μM after 48 hrs’incubation (Fig. 6).
3 Discussion
The pharmacological effects of baicalin, baicalein, wogonoside and wogonin have been widely explored in recent years[6,9-22]. Similar pharmacological effects were exerted including anti-inflammation, anti-cancer and anti-viral effects. Though different flavonoids or cancer cells were involved in different studies for various purposes, it was difficult to evaluate their relative anti-cancer activities. The reported efficacies of the flavonoids towards different cancer cell lines were presented in Table 1. In the present study, it was found that different human cancer cell lines showed markedly different sensitivities towards the flavonoids. The IC50values of each flavonoid towards the four tested cancer cell lines were summarized in Table 2. Baicalin was effective on inhibiting HepG2 cells, ineffective against A549 and HeLa cells, and even stimulated the growth of MCF-7 cells. Baicalein was effective against HeLa cells, moderately effective against HepG2 cells, but ineffective against A549 and MCF-7 cells. Wogonoside was only slightly effective against A549, HepG2 and MCF-7 cells, and ineffective against HeLa cells. In contrast tothe variable effects of baicalin, baicalein and wogonoside against the different cancer cells lines, wogonin was strongly inhibitory against all the four human cancer cell lines tested in this study. As indicated in Table 1, previous studies have demonstrated the inhibitory effects of wogonin against A549 and HeLa cells, the SK-LU-1 and SK-MES-1 human lung cancer cells[18,19].

Fig. 6 Effects of different flavonoids on growth of human breast adenocarcinoma cell line MCF-7. (n = 3)

Table 1 Previously reported IC50against human lung, cervical, liver and breast cancer cell lines
Besides its anti-cancer effect in vitro againstcancer cell lines, wogonin had also been found to be inhibitory against the human cancer xenografts A549 lung adenocarcinoma, T-47D ductal carcinoma and MDA-MB-231 breast adenocarcinoma on nude mice in vivo[13,16,17]. Moreover, wogonin was known to display the four different types of anti-cancer actions:cytocidal action, anti-metastatic action, inhibition of drug resistance and cancer prevention[6]. Specifically, the beneficial effects of wogonin included its antiviral effects against hepatitis B virus (HBV)[25-27]and suppression of oncogenes in Human papillomavirus (HPV)-infected cervical cancer cells[28], conferring potential protection on HBV and HPV carriers against cancers, and its enhancement of Bcl-2/Bax ratio[18,29]and the upregulation of p53 gene and caspase-3, -8 and -9[18,29,30], thereby triggering the apoptosis of tumor cells. In addition, wogonin downregulated IL-6, TNF-α and the PI3k/ AKt/NF-κB pathway[16,29,31]and inhibited Cox-2[32], thus reducing inflammation which may promoted the initiation of cancer[33]. Wogonin downregulated matrix metalloproteinase to reduce the invasiveness and metastasis of human lung adenocarcinoma A549 and human breast carcinoma MCF-7 cells[16,34], and inhibited angiogenesis required for the growth of cancer[35]; and downregulated survivin and growth factors, such as VEGF signaling and IGF-1, probably facilitating the initiation and the growth of cancer[29,35,36].
In addition, numerous cancers developed drug resistance in the course of chemotherapy, while wogonin was found to reduce cancer drug resistance mediated by the MRP-1 and BCRP proteins[37-39]. Furthermore, cancer patients were often susceptible to anxiety disorders[40-42], and wogonin exerted anxiolytic effects through the benzodiazepine site of GABAA receptors, unaccompanied by the sedative and muscle-weakness side effects induced by benzodiazepine anxiolytics[43]. Wogonin performed the tightest binding to GABAA receptors compared with baicalin and baicalein, being suggested that it be exert the best anxiolytic effect[44-46].
Although baicalin presented the similar growth inhibitory effect as wogonin on HepG2 cells, while stimulated the growth of MCF-7 cells. Baicalin was the most abundant flavonoids in the root of S. baicalensis[47], revising a safety issue upon consumption of crude S. baicalensis extract, which can be prepared conveniently[48], by cancer patients. Also, this finding suggested that anticancer studies on flavonoids and their potential clinical application should be carried out with more pure compounds.
In conclusion, wogonin was found in the present study to be the most potent anti-cancer flavonoid in S. baicalensis. Given its unique combination of anticancer and anxiolytic actions, wogonin represented an exceptional candidate drug for the treatment of anxiety disorders in cancer patients, patients in cancer remission and high cancer-risk individuals.
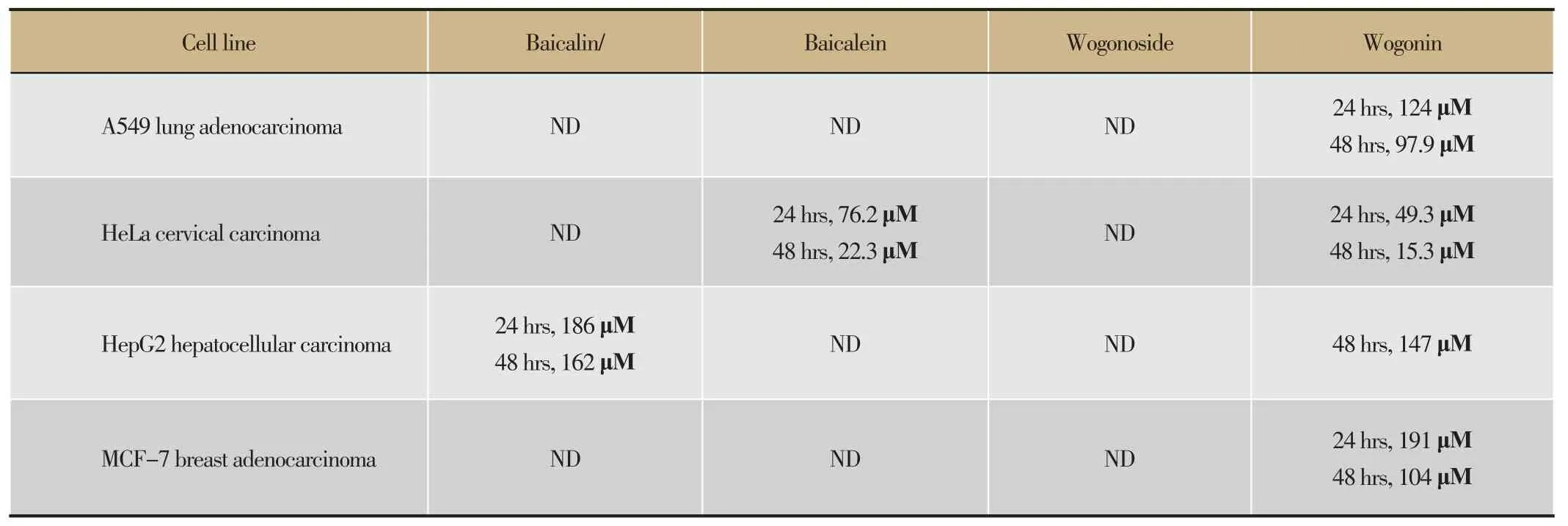
Table 2 Comparison of the action of flavonoids against human lung, cervical, liver and breast cancer cell lines
参考文献
1 Siegel R L, Miller K D, Jemal A. Cancer statistics, 2015. CA: Cancer J Clin, 2015, 65(1):5-29.
2 Schellekens M P, van den Hurk D G, Prins J B, et al. The suitability of the hospital anxiety and depression scale, distress thermometer and other instruments to screen for psychiatric disorders in both lung cancer patients and their partners. J Affect Disord, 2016, 203:176-183.
3 Tang S T, Chen J S, Chou W C, et al. Longitudinal Analysis of Severe Anxiety Symptoms in the Last Year of Life Among Patients With Advanced Cancer: Relationships With Proximity to Death, Burden, and Social Support. J Natl Compr Canc Netw, 2016, 14(6):727-734.
4 王霞,杨宇飞. 肿瘤康复的研究进展. 世界科学技术-中医药现代化,2015,17(12):2490-2496.
5 Kubo M, Kimura M, Odani T, et al. Studies on Scutellariae radix. Part II: The antibacterial substance. Planta Med, 1981, 43(10):194-201.
6 Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents wogonin, baicalein and baicalin. Cancer Treat Rev, 2009, 35(1):57-68.
7 崔琳,路玲玲,李强,等. 黄芩水提液对3T3-L1脂肪细胞增殖、诱导分化及脂联素启动子荧光素酶活性的影响. 世界科学技术-中医药现代化, 2015, 17(11):2360-2366.
8 Wang C Z, Li X L, Wang Q F, et al. Selective fraction of Scutellaria baicalensis and its chemopreventive effects on MCF-7 human breast cancer cells. Phytomedicine, 2010, 17(1):63-68.
9 Ikemoto S, Sugimura K, Yoshida N, et al. Antitumor effects of Scutellariae radix and its components baicalein, baicalin, and wogonin on bladder cancer cell lines. Urology, 2000, 55(6):951-955.
10 Chang W H, Chen C H, Lu F J. Different effects of baicalein, baicalin and wogonin on mitochondrial function, glutathione content and cell cycle progression in human hepatoma cell lines. Planta Med, 2002, 68(2):128-132.
11 Martin J, Dusek J. The Baikal scullcap (Scutellaria baicalensis Georgi)--a potential source of new drugs. Ceska Slov Farm, 2002, 51(6):277-283.
12 Wang T, Gao J, Yu J, et al. Synergistic inhibitory effect of wogonin and low-dose paclitaxel on gastric cancer cells and tumor xenografts. Chin J Cancer Res, 2013, 25(5):505-513.
13 Yang L, Wang Q, Li D, et al. Wogonin enhances antitumor activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo through ROS-mediated downregulation of cFLIPL and IAP proteins. Apoptosis, 2013, 18(5):618-626.
14 Peng Y, Fu Z Z, Guo CS, et al. Effects and mechanism of baicalin on apoptosis of cervical cancer HeLa cells in-vitro. Iran J Pharm Res, 2015, 14(1):251-261.
15 Wang Y, Yin R F, Teng J S. Wogonoside induces cell cycle arrest and mitochondrial mediated apoptosis by modulation of Bcl-2 and Bax in osteosarcoma cancer cells. Int J Clin Exp Pathol, 2015, 8(1):63-72.
16 Zhao Y, Yao J, Wu X P, et al. Wogonin suppresses human alveolar adenocarcinoma cell A549 migration in inflammatory microenvironment by modulating the IL-6/STAT3 signaling pathway. Mol Carcinog, 2015, 54:81-93.
17 Chung H, Jung Y M, Shin D H, et al. Anticancer effects of wogonin in both estrogen receptor-positive and -negative human breast cancer cell lines in vitro and in nude mice xenografts. Int J Cancer, 2008, 122(4):816-822.
18 Gao J, Morgan W A, Sanchez-Medina A, et al. The ethanol extract of Scutellaria baicalensis and the active compounds induce cell cycle arrest and apoptosis including upregulation of p53 and Bax in human lung cancer cells. Toxicol Appl Pharmacol, 2011, 254(3):221-228.
19 Cao X D, Ding Z S, Jiang F S, et al. Antitumor constituents from the leaves of Carya cathayensis. Natural Product Research, 2012, 26(22):2089-2094.
20 Ding D, Zhang B, Meng T, et al. Novel synthetic baicalein derivatives caused apoptosis and activated AMP-activated protein kinase in human tumor cells. Org Biomol Chem, 2011, 9(21):7287-7291.
21 Ma X, Yan W, Dai Z, et al. Baicalein suppresses metastasis of breast cancer cells by inhibiting EMT via downregulation of SATB1 and Wnt/ β-catenin pathway. Drug Des Devel Ther, 2016, 10:1419-1441.
22 喻小兰,卢科莲,夏纪毅,等. 黄芩素通过抑制ERK通路下调HeLa细胞中基质金属蛋白酶的表达. 细胞与分子免疫学杂志,2014,30(8):798-801.
23 Chen C H, Huang T S, Wong C H, et al. Synergistic anti-cancer effect of baicalein and silymarin on human hepatoma HepG2 Cells. Food Chem Toxicol, 2009, 47(3):638-644.
24 Zhou Q M, Wang S, Zhang H, et al. The combination of baicalin and baicalein enhances apoptosis via the ERK/p38 MAPK pathway in human breast cancer cells. Acta Pharmacol Sin, 2009, 30(12):1648-1658.
25 Huang R L, Chen C C, Huang H L, et al. Anti-hepatitis B virus effects of wogonin isolated from Scutellaria baicalensis. Planta Med, 2000, 66(8):694-698.
26 Guo Q, Zhao L, You Q, et al. Anti-hepatitis B virus activity of wogonin in vitro and in vivo. Antiviral Res, 2007, 74(1):16-24.
27 Chen Y, Zhu J. Anti-HBV effect of individual traditional Chinese herbal medicine in vitro and in vivo: an analytic review. J Viral Hepat, 2013, 20(7):445-452.
28 Kim M S, Bak Y, Park Y S, et al. Wogonin induces apoptosis bysuppressing E6 and E7 expressions and activating intrinsic signaling pathways in HPV-16 cervical cancer cells. Cell Biol Toxicol, 2013, 29(4):259-272.
29 Huang K F, Zhang G D, Huang Y Q, et al. Wogonin induces apoptosis and down-regulates survivin in human breast cancer MCF-7 cells by modulating PI3K-AKT pathway. Int Immunopharmacol, 2012, 12(2):334-341.
30 Yang L, Zhang H W, Hu R, et al. Wogonin induces G1 phase arrest through inhibiting Cdk4 and cyclin D1 concomitant with an elevation in p21Cip1 in human cervical carcinoma HeLa cells. Biochem Cell Biol, 2009, 87(6):933-942.
31 Zhao K, Song X, Huang Y, et al. Wogonin inhibits LPS-induced tumor angiogenesis via suppressing PI3K/Akt/NF-κB signaling. Eur J Pharmacol, 2014, 737:57-69.
32 Chen L G, Hung L Y, Tsai K W, et al. Wogonin, a bioactive flavonoid in herbal tea, inhibits inflammatory cyclooxygenase-2 gene expression in human lung epithelial cancer cells. Mol Nutr Food Res, 2008, 52(11):1349-1357.
33 Pesic M, Greten FR. Inflammation and cancer: tissue regeneration gone awry. Curr Opin Cell Biol, 2016, 43:55-61.
34 Chen P, Lu N, Ling Y, et al. Inhibitory effects of wogonin on the invasion of human breast carcinoma cells by downregulating the expression and activity of matrix metalloproteinase-9. Toxicology, 2011, 282(3):122-128.
35 马兴,谢鲲鹏,尚斐,等. 汉黄芩素抑制胰岛素样生长因子-1对乳腺癌细胞增殖与雌激素受体α表达的促进作用和鸡胚尿囊膜的血管生成. 生理学报,2012,64(2):207-212.
36 Song X, Yao J, Wang F, et al. Wogonin inhibits tumor angiogenesis via degradation of HIF-1α protein. Toxicol Appl Pharmacol, 2013, 271(2):144-155.
37 Cheng J, Cheng L, Chen B, et al. Effect of magnetic nanoparticles of Fe3O4 and wogonin on the reversal of multidrug resistance in K562/ A02 cell line. Int J Nanomedicine, 2012, 7:2843-2852.
38 Xu X, Zhang Y, Li W, et al. Wogonin reverses multi-drug resistance of human myelogenous leukemia K562/A02 cells via downregulation of MRP1 expression by inhibiting Nrf2/ARE signaling pathway. Biochem Pharmacol, 2014, 92(2):220-234.
39 Abdallah H M, Al-Abd A M, El-Dine R S, et al. P-glycoprotein inhibitors of natural origin as potential tumor chemo-sensitizers: A review. J Adv Res, 2015, 6(1):45-62.
40 van Oostrom I, Meijers-Heijboer H, Duivenvoorden H J, et al. Prognostic factors for hereditary cancer distress six months after BRCA1/2 or HNPCC genetic susceptibility testing. Eur J Cancer, 2007, 43(1):71-77.
41 Linden W, Vodermaier A, Mackenzie R, et al. Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age. J Affect Disord, 2012, 141(2-3):343-351.
42 Mehnert A, Brähler E, Faller H, et al. Four-week prevalence of mental disorders in patients with cancer across major tumor entities. J Clin Oncol, 2014, 32(31):3540-3546.
43 Hui K M, Huen M S, Wang H Y, et al. Anxiolytic effect of wogonin, a benzodiazepine receptor ligand isolated from Scutellaria baicalensis Georgi. Biochem Pharmacol, 2002, 64(9):1415-1424.
44 Hui K M, Wang X H, Xue H. Interaction of flavones from the roots of Scutellaria baicalensis with the benzodiazepine site. Planta Med, 2000, 66(1):91-93.
45 Wang H, Hui K M, Chen Y, et al. Structure-activity relationships of flavonoids, isolated from Scutellaria baicalensis, binding to benzodiazepine site of GABA(A) receptor complex. Planta Med, 2002, 68(12):1059-1062.
46 Xu Z, Wang F, Tsang S Y, et al. Anxiolytic-Like Effect of baicalin and its additivity with other anxiolytics. Planta Med, 2006, 72(2):189-192.
47 杨立新, 刘岱, 冯学锋,等. 高效液相色谱法测定不同产地黄芩中黄酮化合物的含量. 中国中药杂志,2002,27(3):166-170.
48 李锐华, 李英, 王永香,等. Box-Behnken响应面法优化疏风定喘颗粒黄芩组的提取工艺研究. 世界科学技术-中医药现代化,2015,17(11):2290-2295.
Effects of 4 Flavonoids from Scutellaria Baicalensis on Different Cancer Cell Lines
Ho Timothy Yiu Cheong, Xue Hong
(Division of Life Science and Applied Genomic Center, The Hong Kong University of Science and Technology, Hong Kong 999077, China)
Wogonoside, wogonin, baicalin and baicalein are major chemical constituents of S. baicalensis. Baicalin, baicalein and wogonin have been reported previously to exert anti-cancer effects. The present study compared the anti-cancer effects of the four flavonoids individually towards four human cancer cell lines after24- and 48-hour treatment based on cell viability assay. As a result, wogonin inhibited the growth of the four cell lines. The IC50values after 48 hours of incubation were 97.9 μM for A549 lung adenocarcinoma cells, while 15.3 μM for HeLa cervical carcinoma cells, 147 μM for HepG2 hepatocellular carcinoma cells and 104 μM for MCF-7 breast adenocarcinoma cells. In contrast, wogonoside, the glycoside of wogonin, showed no inhibitory effect against any one of the four cell lines. Baicalein inhibited the growth of HeLa cells, while baicalin inhibited the growth of HepG2 cells, both with higher IC50values and less potent than wogonin. These findings established wogonin as the most active anti-cancer agent among the four major flavonoids of S. baicalensis. Since baicalin, the most abundant flavonoid in the herb , was found to enhance the growth of MCF-7 cells. Clinical applications of ingredients from S. baicalensis to the treatment of cancer should be carried out with purified compounds.
Scutellaria baicalensis, wogonin, wogonoside, baicalein, baicalin, cancer cell lines
10.11842/wst.2016.11.003
R965.1
A
(责任编辑:马雅静,责任译审:朱黎婷)
2016-11-08
修回日期:2016-11-20
* 香港科技大学深圳研究院研究项目(P1203):应用基因组学新技术在预防医学中的应用,负责人:薛红。
** 通讯作者:薛红,本刊编委,教授,博士生导师,主要研究方向:中药及基因组医学研究。
