悬浮固化液相微萃取-高效液相色谱法测定人血浆和尿样中的卡巴咪嗪
2015-12-26MohammadASADI,AliMohammadHAJISHABANI,ShayesstehDADFARNIA等
Carbamazepine (5H-dibenz[b,f]azepine-5-carboxamide,CBZ),as an antiepileptic drug (AED)is a tricyclic drug for the treatment of epilepsy,trigeminal neuralgia and schizophrenia[1-3]. It has been the first-choice antiepileptic drug for the wide range of seizure disorders in both adults and children due to its efficacy and acceptable safety profile. The therapeutic range of CBZ in human serum is about 4-12 mg/L[4]. Research findings confirm that CBZ could have some side effects on central nervous system including diplopia,dizziness,headache,nausea and incoordination[5].Thus,it is important to establish a reliable and sensitive method for the determination of CBZ in biological fluids to study its pharmacokinetics and metabolism[6].
Several publications deal with the determination of CBZ in human plasma. High performance liquid chromatography (HPLC)in conjunction with UV[7],electrochemical [8]or mass spectroscopy(MS)[9-11]detector is considered as an accepted method for drug analysis. However,as the matrix of biological fluid is too complex and the amounts of drug are too low,a sample preparation step with the aim of matrix removal and preconcentration of analyte to a suitable level is required prior to drug determination by HPLC[12].Typically,this would require an extraction step such as liquid-liquid extraction (LLE)or solid phase extraction (SPE). However,conventional LLE consumes large amounts of expensive and potentially hazardous organic solvents. In addition,in trace analysis,a large volume of sample is often required which its handling can be extremely time consuming besides being tedious.SPE uses much less solvent and is less time consuming than LLE but requires column conditioning and is relatively expensive[13]. To overcome these problems Liu and Dasgupta [14,15]and Jeannot and Cantwell[16]tried to miniaturize the conventional LLE and a technique termed liquid-phase microextraction (LPME)was introduced. Since then,various types of LPME including single drop microextraction (SDME)[17],hollow fiber LPME[18,19],homogeneous liquidliquid extraction (HLLE)[20,21],dispersive liquid-liquid microextraction (DLLME)[22]and solidified floating organic drop microextraction(SFODME)[23]have been developed. It should be noted that in most of the LPME the density of the extraction solvent should be higher than water;but the high-density extraction solvents,being mostly halogenated,are generally hazardous to laboratory personnel and environment. Attempt has been made to use less toxic solvents (alcohols,alkanes,etc.)with a density lower than aqueous samples. In these cases,collection and separation of organic phase for determination of analytes are not as simple as that with the highdensity extraction solvents. Different techniques such as use of a capillary tube[24]or specialized extraction vessel [25]were reported for collection of light extraction solvents. However,complete collection of the separated phase is difficult or impossible in most cases. Alternatively,SFODME was developed [26]in which a small volume of an organic solvent with a melting point near room temperature (10-30 ℃)is floated on the surface of an aqueous sample. The aqueous phase is stirred for a prescribed period of time until equilibrium is reached and then the sample is transferred to an ice bath. When the organic solvent is solidified,it is simply transferred to a small conical vial,and the melted organic solvent is used for analyte determination. The performance of SFODME was illustrated by extraction of different organic and inorganic compounds from different matrices[27-30].
In this study,the SFODME combined with high performance liquid chromatography (HPLC)has been used for the separation/preconcentration and determination of CBZ in human plasma and urine samples. This method is simple,fast,and efficient,consumes low-toxic organic solvents,and can be directly used for the determination of CBZ in biological samples (human plasma and urine). Several factors such as the extraction time,temperature,type and volume of organic solvent,sample volume,ionic strength,stirring rate,and sodium hydroxide concentration were optimized. Then,the applicability of the developed method for the extraction and determination of low level of CBZ in biological samples was considered.
1 Experimental
1.1 Chemicals and apparatus
Carbamazepine,primidone,phenytoin,phenobarbital,1-undecanol,2-undecanol and 1-decanol were purchased from Sigma-Aldrich (St,Louis,MO,USA). Methanol and water (HPLC grade),sodium hydroxide and sodium chloride (analytical grade)were purchased from Merck (Darmstadt,Germany). All other chemicals used were of analytical grade and distilled deionized water was used throughout the sample preparation. All solutions were stored in the clean polypropylene containers (Nalgene,Lima,OH,USA). The stock standard solution of CBZ (1 000 mg/L)was prepared by dissolving appropriate amount of CBZ in methanol. The stock standard solution was stored at -4 ℃in the dark place and it was stable for 6 months. Working standard solutions were prepared daily by appropriate dilution of the stock standard solution with HPLC grade water prior to use.
Knauer HPLC system (Berlin,Germany)and 100 μL HPLC microsyringe (Knauer,Berlin,Germany)was applied for chromatographic performance. A heater-stirrer (Heidolph,Germany)was used for heating and stirring the sample solutions.
1.2 Chromatographic procedure
The chromatographic analysis was performed on Knauer HPLC system (Berlin,Germany)equipped with a LC-pump 1000,20 μL sample loop and a UV detector 2600. A personal computer equipped with a ChromGate program for LC was used to process chromatographic data. The analyte was separated on Nucleosil-C18column(250 mm×4.6 mm i. d.,5 μm)with pre column. The mobile phase was a mixture of methanol-water (65 ∶35,v/v)and the flow rate was 0.9 mL/min. The column temperature was 40 ℃and the detection wavelength was 210 nm.
1.3 Extraction procedure
Standard or sample solution (8 mL)was transferred into the 10 mL sample vial containing a 8 mm×4 mm magnetic stirring bar,the NaCl and sodium hydroxide concentrations were adjusted to 3% (w/v)and 1 mol/L,respectively. Then,40 μL of organic solvent was placed on the surface of the solution using a 100 μL microsyringe,the sample vial was put on a hot plate stirrer and was stirred for 60 min at 50 ℃. After the extraction was complete,the sample vial was transferred into an ice bath until the organic solvent was solidified. The solidified solvent was then transferred into a conical vial where it melted immediately.Finally,20 μL of the melted solvent was injected into the HPLC for the quantification of analyte.
1.4 Sample preparation
Two milliliters of the biological fluids (plasma or urine)were mixed with acetonitrile at 1 ∶1 volume ratio. The solution was afterwards stirred for 10 min at 1 200 r/min and centrifuged for 15 min at 5 000 r/min[31]. The transparent solution was transferred to a sample vial and diluted to 8 mL with deionized water. The ionic strength and sodium hydroxide concentration of the solution were adjusted to 3% (w/v)and 1 mol/L,respectively. The resulting solution was treated according to the given procedure.
2 Results and discussion
2.1 Effect of sodium hydroxide concentration
The pH of sample solution is a key factor in the extraction of acidic or basic analytes as it determines the ionic state of the analytes. Thus,in order to extract the CBZ into organic phase,the pH of the sample solution should be properly adjusted to convert it into neutral form. So,as the CBZ is a basic drug (pKa13.9±0.1),the effect of the sodium hydroxide concentration on the extraction of CBZ in the range of 0.1-2.0 mol/L was investigated. According to the results (Fig.1),the extraction efficiency of CBZ increased by an increase in sodium hydroxide concentration and reached a maximum in the concentration range of 1.0 - 1.8 mol/L. Consequently,a 1.0 mol/L of sodium hydroxide was chosen as the optimal concentration.

Fig.1 Effect of the sodium hydroxide concentration on the extraction efficiency (n=3)
2.2 Effect of stirring rate
In liquid phase microextraction techniques,it is well-known that stirring is an effective way to enhance the mass transfer between the aqueous solution and the extraction solvent. Stirring of the sample reduces the time required to reach the equilibrium between the aqueous and extractant phase by enhancing the diffusion of the analyte towards the organic phase. Furthermore,by stirring the aqueous phase,the convection is induced in the organic drop. For this purpose,experiments were carried out by varying the stirring rate in the range of 100-500 r/min. The results (Fig.2)indicated that the analytical signal increased with increasing stirring rate from 100 to 400 r/min,and then remained constant with further increase in the stirring rate up to 500 r/min.Thus,400 r/min was selected as the optimum stirring rate.
2.3 Effect of temperature

Fig.2 Effect of stirring rate on the extraction efficiency (n=3)
Temperature is one of the parameters affecting the kinetics of extraction and extraction efficiency at a fixed extraction time. In most LPME works,a temperature raise led to higher enrichment factors [32]. This is because it facilitates mass transfer of the analyte from sample to the organic solvent and thus increases the extraction efficiency in a constant extraction time. The effect of sample solution temperature on the extraction efficiency was studied in the range of 30-55 ℃. Experimental results showed that the extraction efficiency of CBZ was improved by increasing the temperature up to 50 ℃and then remained constant. Therefore,in further experiments the sample vial temperature was held at 50 ℃.
2.4 Effect of extraction time
In SFODME method,the extraction time is defined as the time at which the sample is stirred after the organic solvent is placed on the surface of the solution. The effect of the extraction time was examined in the range of 20-70 min under other constant experimental conditions. The results (Fig.3)demonstrated that after 60 min the extraction efficiency reaches a maximum and no significant change was observed by further increase in extraction time. Therefore,extraction time period of 60 min was chosen to obtain a reasonable sensitivity.
2.5 Effect of ionic strength

Fig.3 Effect of extraction time on the extraction efficiency (n=3)
An increase in ionic strength usually decreases the solubility of organic compound in water and enhances the extraction efficiency. For investigating the effect of the ionic strength on the extraction of CBZ by SFODME method,various experiments were performed in presence of different amounts of sodium chloride (0-4%,w/v). The results showed that the extraction efficiency of CBZ increased slightly with the increase in salt concentrations from 0 to 3% (w/v)and no significant effect was observed when higher amount of sodium chloride (4%,w/v)was added (Fig.4).Based on these results,3% (w/v)of NaCl was chosen for the subsequent studies.

Fig.4 Effect of salt content on the extraction efficiency (n=3)
2.6 Selection of nature and volume of extractant
The extraction solvent should possess the following criteria:(1)immiscibility with aqueous solution,(2)high affinity for the analyte,(3)high boiling point,so that its loss during extraction is avoided,(4)melting point near room temperature (10-30 ℃),(5)a density lower than water and (6)a suitable chromatographic behavior. In this study,three organic solvents,1-undecanol,2-undecanol and 1-decanol which satisfy the above criteria were selected,and their extraction efficiency for CBZ was examined. The results of this study (Fig.5)showed that the extraction efficiency and repeatability were the highest with 1-undecanol,so it was selected as the extraction solvent in the further studies.

Fig.5 Effect of type of extractant on the extraction efficiency (n=3)
An important aspect of the SFODME method development is to show its preconcentration capability. A decrease in the ratio of the volume of organic phase to the aqueous phase will increase the preconcentration factor,but it may reduce the extraction efficiency in a given extraction time. For this purpose,the effect of 1-undecanol volume on the extraction efficiency of CBZ was investigated.Experiments were performed with different volumes of 1-undecanol (40,60,80 and 100 μL),and the results revealed that the peak area of the CBZ decreased proportional to the increase in extractant volume. The use of 1-undecanol volume less than 40 μL led to higher enrichment factor,but the collection of solvent after the solidification was difficult. Consequently,40 μL 1-undecanol was selected as the extraction solvent. Furthermore,the volume of solvent after the extraction remained at (30±1)μL.
2.7 Effect of sample volume
In order to explore the possibility of achieving high preconcentration factor for CBZ,the effect of sample volume on extraction efficiency was considered. An increase in sample volume would enhance the amount of CBZ transferred into the organic solvent,which improves the sensitivity[33]. For this purpose five different volumes (4,6,8,10 and 15 mL)of spiked sample containing 0.3 μg of CBZ were subjected to the extraction procedure under optimum conditions in proper vial size. The results showed that the peak area and extraction quantity were constant up to the sample volume of 8 mL and then decreased by further increase in sample volume (Fig.6). Thus,based on the organic phase volume (40 μL)and the maximum sample volume (8 mL)a preconcentration factor of 200 was determined.

Fig.6 Effect of sample volume on the extraction efficiency (n=3)
2.8 Analytical performance and application of the method
The figures of merit of the developed method including the corresponding regression equation,correlation coefficient (r2),linear dynamic range(LDR),limit of detection (LOD),relative standard deviation (RSD),enhancement factor (EF)and extraction recovery (ER)were investigated under the optimized conditions for 8 mL sample.Calibration curve was linear in the range of 0.4-700.0 μg/L of CBZ with a r2value of 0.999 (Y =81 798 X +37 123,where Y is peak areas,X is mass concentration (μg/L)). The RSD for six replicate extraction and determination of CBZ at 100 μg/L level was found to be 4.1%. The LOD based on a signal-to-noise ratio (S/N)of 3 was 0.1 μg/L. The ER and EF were calculated using the following equations:

where Coand Ciare the concentrations of analyte in the final extract and the initial concentration in the sample,and Voand Vaqare the volumes of the organic phase and sample solution,respectively.The EF and ER were found to be 166.5 and 83.2%,respectively.
The chromatograms of the real samples (plasma and urine)after SFODME extraction under the optimum conditions are shown in Fig.7. The chromatograms are characterized by symmetrical peak shape and the retention times of the analyte are constant during the experiment. In addition,the method was tested for possible interferences from co-prescribed AEDs,phenytoin,primidone and phenobarbital at 10 000 μg/L levels (Fig.8),and no interferences were found. Thus,the present method has a great potential in monitoring and measurement of low level of CBZ in real samples.
The accuracy of the developed method was further studied by analysis of the blank samples of urine and plasma spiked at two concentration levels (5 and 200 μg/L)of CBZ. The results of this investigation (Table 1)illustrate that the recovery of added analytes is good (90%-98%). Thus the method is capable of measurement of CBZ in the sample type examined.
2. 9 Comparison of the developed method with other LPME-HPLC methods

Fig.7 Chromatograms of blank and spiked human plasma and urine samples

Fig.8 Chromatogram of extracted mixture of CBZ (200 μg/L), phenytoin (10000 μg/L), primidone(10000 μg/L)and phenobarbital (10000 μg/L)under the optimum conditions
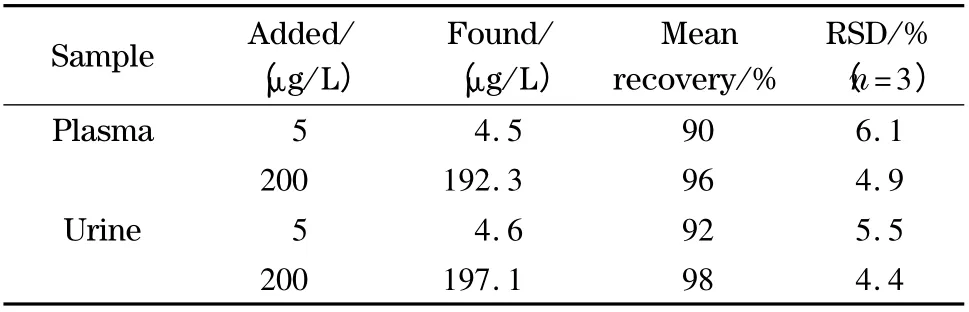
Table 1 Determination of CBZ in human plasma and urine samples
The analytical performance of the developed SFODME-HPLC method is compared with other reported LPME-HPLC methods for the determination of CBZ. The results are summarized in Table 2 and reveal that the LOD of the developed method is lower and it has good RSDs compared with other microextraction methods coupled with HPLC for the extraction and determination of CBZ in various biological samples. Furthermore,the dynamic range of the method is wider than most of other reported methods[34-39].
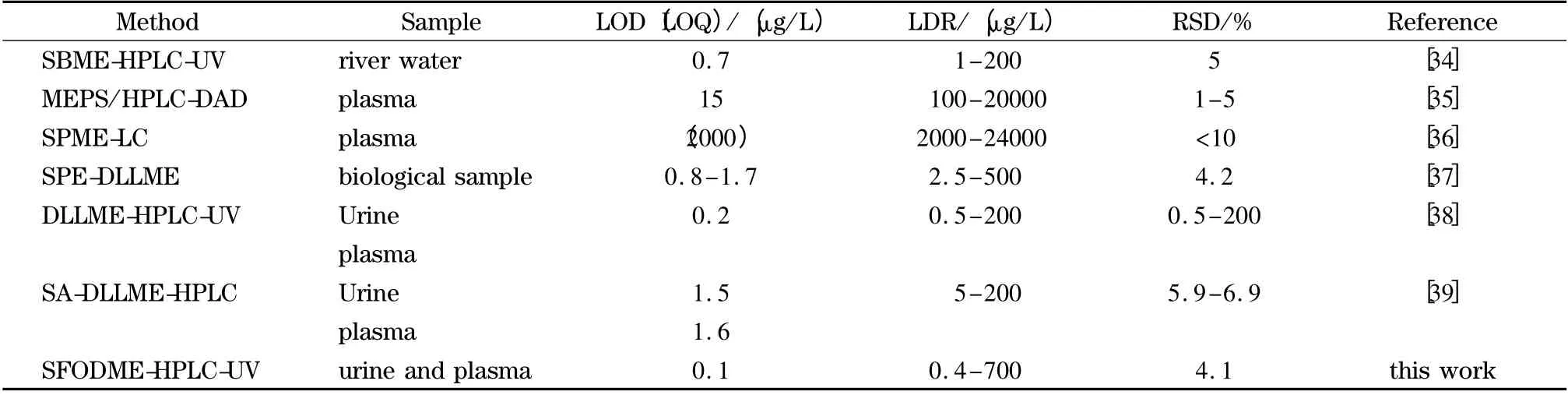
Table 2 Comparison of SFOME-HPLC-UV with other microextraction methods for determination of CBZ
3 Conclusion
In this work,a SFODME method was developed for preconcentration of CBZ prior to its determination with HPLC. The method provided low detection limit,good precision,efficient recoveries and good preconcentration factor without using organic dispersive solvent and hazardous extraction solvent. In addition,this method is easy,green and suitable for microextraction technique in the separation and determination of CBZ from various biological samples in clinical laboratories.
[1] Bernus I,Dickinson R G,Hooper W D,et al. Epilepsy Res,1996,24:163
[2] Deleu D,Aarons L,Ahmed I A. Eur J Clin Pharmacol,2001,57(3):243
[3] Albani F,Riva R,Baruzzi A. Pharmacopsychiatry,1995,28:235
[4] Yoshida T,Imai K,Motohashi S,et al. J Pharm Biomed Anal,2006,41(4):1386
[5] Duzova A,Baskin E,Usta Y,et al. Hum Exp Toxicol,2001,20(4):175
[6] Alexishvili M M,Rukhadze M D,Okujava V M. Biomed Chromatogr,1997,11(1):36
[7] Fortuna A,Bicker J,Alves G,et al. J Sep Sci,2011,34(12):1391
[8] Messiha F S. Alcohol,1986,3(2):135
[9] Miao X S,Metcalfe C D. Anal Chem,2003,75(15):3731
[10] Kim K B,Seo K A,Kim S E,et al. J Pharm Biomed Anal,2011,56(4):771
[11] Zhu Y X,Chiang H,Wulster-Radcliffe M,et al. J Pharm Biomed Anal,2005,38(1):119
[12] Wu J,Xiang B,Xia J. Microchim Acta,2009,166(1/2):157
[13] Junk G A,Richard J. Anal Chem,1988,60(5):451
[14] Liu S,Dasgupta P K. Anal Chem,1995,67(13):2042
[15] Liu S,Dasgupta P K. Anal Chem,1996,68(11):1817
[16] Jeannot M A,Cantwell F F. Anal Chem,1996,68(13):2236
[17] He Y,Lee H K. Anal Chem,1997,69(22):4634
[18] Piroozi F,Ghasemi E,Qomi M,et al. J Liq Chromatogr Relat Technol,2014,37(5):760
[19] Tian J,Chen X,Bai X H. Chinese Journal of Chromatography,2012,30(5):507
[20] Farajzadeh M A,Bahram M,Zorita S,et al. J Hazard Mater,2009,161(2/3):1535
[21] Rezaee M,Mashayekhi H A,Mohammad Hosseini M,et al. J Liq Chromatogr Relat Technol,2014,37(18):2559
[22] Xiaohuan Z,Guijiang Z,Chun W,et al. Chinese Journal of Chromatography,2015,33(2):103
[23] Wang P,Qiu X,Yang Y. J Liq Chromatogr Relat Technol,2015,38(5):640
[24] Farajzadeh M A,Djozan D J,Bakhtiyari R F. Talanta,2010,81(4/5):1360
[25] Farajzadeh M R,Seyedi S E,Safi Shalamzari M,et al. J Sep Sci,2009,32(18):3191
[26] Leong M I,Huang S D. J Chromatogr A,2008,1211(1/2):8
[27] Dadfarnia S,HajiShabani A M. Anal Chim Acta,2010,658:107
[28] Rohani Moghadam M,Dadfarnia S,Haji Shabani A M. J Hazard Mater,2011,186(1):169
[29] Dadfarnia S,Haji Shabani A M,Kamranzadeh E. Talanta,2009,79(4):1061
[30] Suh J H,Lee Y Y,Lee H J,et al. J Pharm Biomed Anal,2013,75:214
[31] Adlnasab L,Ebrahimzadeh H,Yamini Y,et al. Talanta,2010,83:370
[32] Bagheri H,Saber A,Mousavi S R. J Chromatogr A,2004,1046(1/2):27
[33] Besharati-Seidani A,Jabbari A,Yamini Y. Anal Chim Acta,2005,530(1):155
[34] Al-Hadithi N,Saad B,Grote M. Microchim Acta,2011,172:31
[35] Ferreira A,Rodrigues M,Oliveira P,et al. J Chromatogr B,2014,971:20
[36] Cantú M D,Toso D R,Lacerda C A,et al. Bioanal Chem,2006,386:256
[37] Rezaee M,Mashayekhi H A. Anal Methods,2012,4:2887
[38] Mashayekhi H A,Abroomand-Azar P,Saber-Tehrani M,et al. Chromatographia,2010,71:517
[39] Behbahani M,Najafi F,Bagheri S,et al. J Chromatogr A,2013,1308:25
