3种阴离子交换色谱固定相捕获细胞培养上清液中红细胞生成素的效果比较
2015-12-26LourdesHERNNDEZ,DiobelSTEWART,LourdesZUMALACRREGUI等
Erythropoietin (EPO)is a well-known cytokine responsible for the production of red blood cells.However,since the last decade,multiple functions outside of the bone marrow have been recognized. The potential market for this glycoprotein is supposed to increase,owing to new discoveries in novel applications[1-4].
Affinity matrices were generally employed to capture the EPO presented in the supernatant(SN);appropriate results were obtained[5-7].Several media,with specific EPO ligands,have been used for laboratory-scale purification,but these are unfeasible alternatives for large scale production[8]. Moreover,other processes have been reported where ion exchange chromatography was employed to capture EPO[9-11].
Ion exchangers are commonly used for downstream processing of pharmaceutical proteins and peptides. Industrial process purification usually comprises one or more ion exchange steps. Ion exchange adsorbents proved to be one of the most versatile stationary phases in liquid chromatography. Applications have been reported for capture step,intermediate purification and polishing of biotechnological processes [12,13]. However,only a limited number of matrices were tested for specific applications,and the results were not often published by the manufacturers.
On the other hand,the chromatographic matrices suppliers frequently publish comparisons of matrices in application notes and scientific papers[14]. In these studies,protein models are used to show the characteristics and properties of the stationary phases. But,the adsorption parameters vary depending on the strength of the adsorbentbiomolecule interactions. Therefore,the extrapolation of the published results is not so simple,since they do not guarantee a good result to any other applications.
A fast and efficient chromatographic process is required. Hence,new stationary phases must be developed for separating molecules by their charge. According to the configuration,there are three types of chromatographic adsorbents:beads,monoliths and membranes[15].
Currently,the penetration of chromatography perfusion technologies is taking place,as new matrices types,chromatography membranes and monolithic chromatographic columns [16-18].The structural conformations of these new technologies enable mass transfer occurs primarily by convection,thereby reducing the effects of mass transfer diffusion that affects the conventional chromatographic separation matrices. As a result,the adsorption capacity is not reduced when velocity is increased. The fundamental purpose of the new trends is to increase productivity and the flexibility of the biotechnological process.
This work aims to evaluate the application of new technologies as anion exchange step to capture recombinant human erythropoietin (r-HuEPO).
1 Experimental conditions
1.1 Materials and equipment
Eshmuno Q (resin slurry)was supplied by Merck (Germany). Sartobind Q membranes were provided by Sartorius (Germany). Anion exchange monoliths CIMmultus QA (1 mL column)was provided by Bia Separations (Slovenia).Some information about functional groups and basic matrix for each chromatographic support are presented in Table 1.
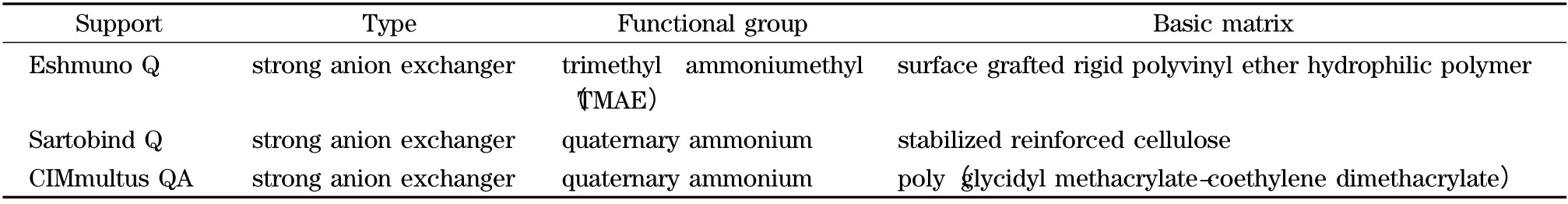
Table 1 Structural characteristic of the three chromatographic supports
All reagents were purchased from Spectrum(USA). Chromatography runs were carried out in Akta Purifier (GE,Sweden).
1.2 Experimental design
Ion exchange chromatography in a gel,a membrane and a monolithic column were evaluated as a capture step of the r-HuEPO purification process. A factorial experimental design was developed for each technology,separately. The ion exchange adsorbents evaluated were:Eshmuno Q(a gel),Sartobind Q (a membrane)and CIMmultus QA (a monolithic column). Each experimental design was performed under the same operating conditions,equipment and scale,in order to compare the results. A 22experimental design with replica was selected. Independent variables were:load (EPO g/L adsorbent)and flow rate(mL/min). The load and flow rate levels were fixed in common with those reported for the three technologies[19].
1.3 EPO capture by anion exchange chromatography
Erythropoietin was captured from the supernatant obtained by fermentation in a stirred tank bioreactor. The supernatant was filtered to ensure that it was sterile and free of cells. The pH (6.8-7.2)and conductivity (12-16 mS/cm)of the supernatant are not suitable for the adsorption of the protein to the ion exchange matrix. Therefore,the concentration step and buffer exchange by ultrafiltration-diafiltration process (UF/DF)should be performed. The membrane filter used was Hydrosart (cut off 10 kDa and 0.02 m2). The diafiltration buffer solution was Na2HPO41.81 mmol/L,NaH2PO418.18 mmol/L,pH 6,conductivity 1.9 mS/cm. The process started with 2.5 L of SN with a concentration of 30 mg/L approximately,to obtain a final volume of 250 mL,concentrated for 10 times. Then each technology was equilibrated with Na2HPO41.81 mmol/L,NaH2PO418.18 mmol/L,pH 6. Later,the application of the diafiltered supernatant was executed,and the erythropoietin was adsorbed on the anion exchangers. Protein was recovered from the adsorbents by increasing ionic strength. Finally the adsorbents were regenerated and sanitized,ready for the subsequent experimental run. Eluates from each technology were analyzed by reversed-phase high performance liquid chromatography (RP-HPLC)for purity and concentration calculations. The yield was determined by the percentage of the mass of protein purified from the total processed mass in each experimental run.
2 Results and discussion
2.1 Influence of different factors on the capture process performance using three anion exchange technologies
The effect of flow rate and load were studied on the purified protein recovered. Table 2 shows the results of the experimental design developed for each technology.
The experimental data shown were coded and entered in Statgraphics Plus for Windows 5.1. A Pareto chart for each technology was performed in order to determine the variables and its interactions that affect recovery (Fig.1).
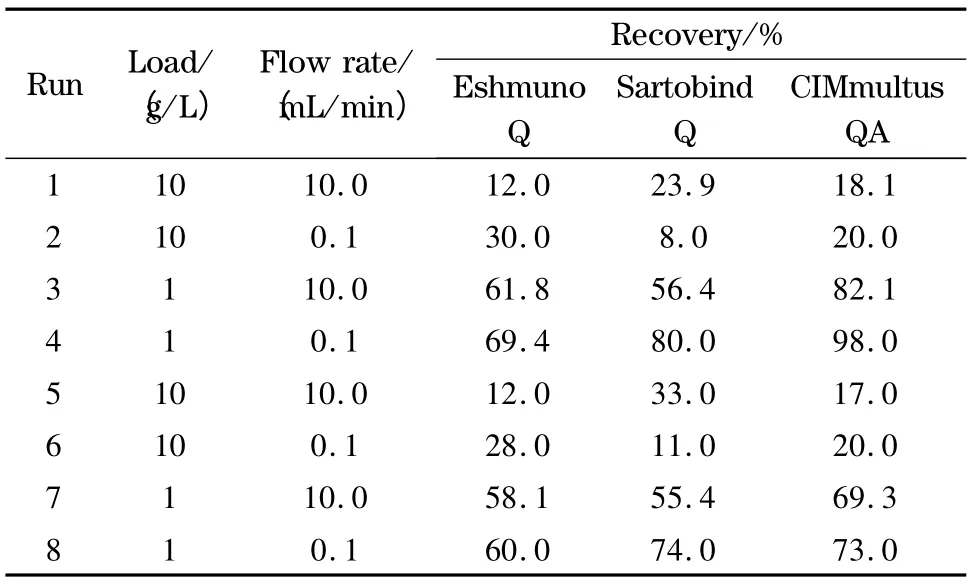
Table 2 Influences of load and flow rate on recovery
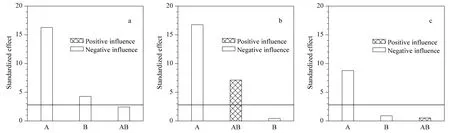
Fig.1 Pareto charts showing the influence of the load and flow rate on the recovery of the chromatography steps for (a)the Eshmuno Q matrix,(b)the membrane and (c)the monolithic column
Fig.1 shows the effect of three factors:load(A),flow rate (B)and their interaction(AB)on the recovery. From this analysis,interaction between the variables has no significant influence on the recovered protein for a 95% confidence level,except for the chromatographic membrane. The highest influence is load,showing a negative sign indicating that an increase of this variable represents a significant reduction in the process recovery. This result indicates that the adsorptive capacity of the adsorbents is exceeded in the experiments with the largest load value of 10 g/L. This means that the adsorption sites were saturated causing the protein loaded in excess and passing through the column. Although some studies sustain that it is possible to operate at high loads,dynamic adsorption capacity for a particular case should be checked[18].
The fact that the flow rate has a smaller influence on the process recovery for the Eshmuno Q matrix is in correspondence with the characteristics of the matrix. The tentacles existing in its structure provide many adsorption sites steric available for binding to biomolecules. This means that in this case,the flow rate increment and the consequent reduction of the contact time have less influence on matrix-biomolecule binding. Experiments verify that the chromatographic process through Eshmuno Q is able to withstand high flow rate without affecting their properties [8]. The results show that increments in load and flow rate significantly affect the binding of r-HuEPO to the matrix under the evaluated conditions.
For the membrane and the monolithic column,the flow rate has no significant influence on protein recovery for a 95% confidence level. This is due to the high operating flow rate with shorter residence time because of the convective channels that conforms the matrix structure;for that reason,the protein has an easier access to the adsorption sites. Some researches[19,20]affirmed that mass transfer occurs by convection within the pore for membrane and monolithic column;the flow rate is independent of capacity and resolution thus they can operate at greater flow rate,without affecting the process efficiency. Thus,the purification time can be significantly reduced,leading to a considerable reduction in purification costs.
2.2 Estimation of load capacity
From the experimental data models were developed to estimate the recovery for each support.The following equations were obtained:

These models can be modified,obtaining equations to estimate load as a function of flow rate and recovery. If a suitable value of recovery(70%)is fixed,we can estimate the appropriate load as the flow rate increases. Estimations of the load capacities by increasing the flow rate for each stationary phase are shown in Fig.2.
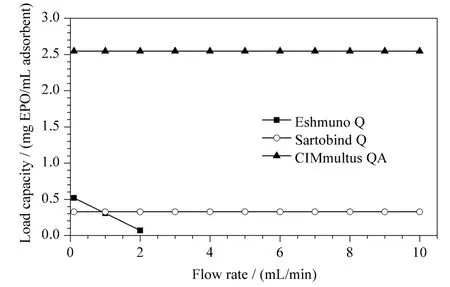
Fig.2 Estimation of the adsorption binding capacity with flow rate for each support
According to these results,the maximum flow rate in Eshmuno Q is 2 mL/min to ensure 70% of recovery. The membrane and the monolithic column may be operated at higher flow rates than Eshmuno Q matrix,without a significant decrease in the adsorption capacity for EPO in supernatant.
For the evaluated technologies dynamic capabilities higher than 10 g/L have been reported for other pure molecules [19,20]. From Fig.2,it can be concluded that the new technologies have lower adsorption capacities. This could be related with the sample used in this study:EPO with other impurities that are generated in the fermentation process. So the existence of other negatively charged contaminants in the cell culture supernatant decreases the possibility of adsorption of the target molecules. The dynamic binding capacities(DBC)reported in the literature[14]provided guidance for conducting the studies. So only the DBC studies performed with the target molecules reveals the true adsorption capacity that characterizes the system. The results indicate that the monolithic column has greater adsorption capacity of EPO from supernatant than the other technologies.
In Fig.2 ,the best operating conditions are also identified by establishing a compromise between adsorption capacity and flow rate. Note that the membrane and the monolithic column may be operated at higher flow rates with less operating time.
2.3 Analysis of the purity of the EPO captured
To evaluate the purity obtained at capture step exit,RP-HPLC analysis was done. Fig.3 shows the chromatograms obtained from each elution solution,when capture step was performed under the best operating conditions for each matrix. It also includes the analysis of the elution obtained from each technology including capture by an affinity matrix,showing the mean values for purity obtained in each case.
According to the elution profiles,a major peak is observed at about 15.5 min showing the presence of EPO. This indicates that the hydrophobic properties of EPO captured by different supports weren’t changed. Statistically it was demonstrated that the purity values obtained by Sartobind Q were similar to those obtained by affinity chromatography. The purity values obtained by the Eshmuno Q matrix and CIMmultus QA were superior to that obtained by the affinity matrix.
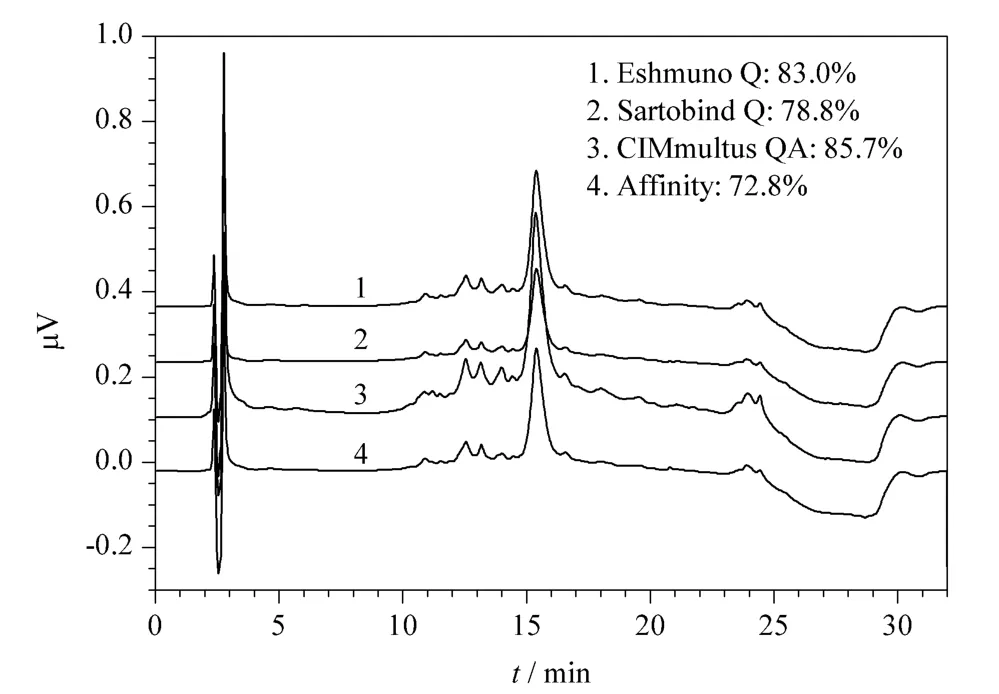
Fig.3 Superposition of the chromatograms obtained by RP-HPLC of the elution solution for each technology
2.4 Productivity analysis of the capture step
The new technologies have shown the possibility to improve the productivity of the capture step and satisfy product’s demand in less time. The quantitative analysis for productivity is shown in Table 3,knowing the amount of purified product and the operating time for each technology.
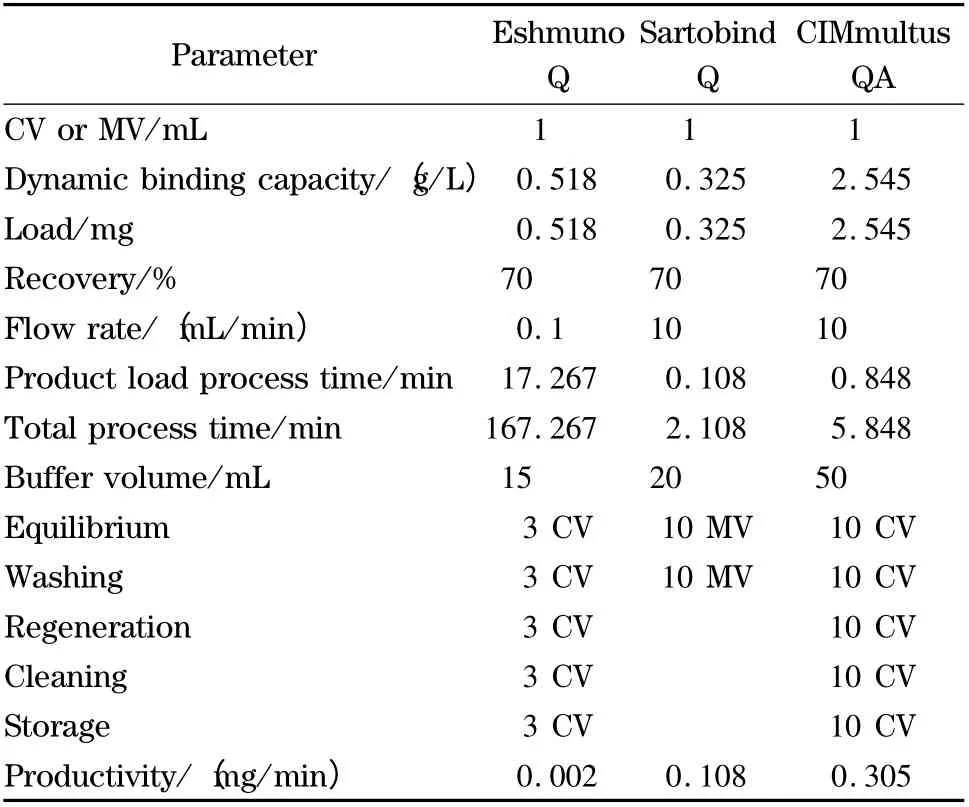
Table 3 Productivity analysis for each support capturing EPO from supernatant at a concentration of 0.3 g/L at laboratory scale
The results showed that the technology with the highest productivity is the monolithic column,nearly three times the productivity of the membrane and 152 times that of Eshmuno Q.
3 Conclusions
According to the results,Fractogel matrix,chromatographic membrane and monolithic anion exchange column are technically suitable to capture r-HuEPO. They show good performance and adequate purity profile. However,monolithic column is the option that provides the best operational benefits and superior productivity.
Acknowledgements:The authors are grateful to co-development link between the Center for Molecular Immunology and Higher Polytechnic Institute José Antonio Echeverría (the Technical Sciences University of Havana). BIA Separations,Slovenia is grateful for providing CIMmultus QA(1 mL)column.
[1] Joyeux-Faure M. J Pharmacol Exp Ther,2007,323(3):759
[2] Ng T,Marx G,Littlewood T,et al. Postgrad Med J,2003,79(933):367
[3] Arcasoy M O. Br J Haematol,2008,141(1):14
[4] Sharples E J,Thiemermann C,Yaqoob M M. Curr Opin Pharmacol,2006,6(2):184
[5] Surabattula R,Sambasiva R,Ratnagiri P. Research in Biotechnology,2011,2(3):58
[6] Wardiana A,Santoso A. Makara Sains,2011,1(15):75
[7] Zanette D,Soffientini A,Sottani C,et al. J Biotechnol,2003,101:275
[8] Khoury E,Wang Y,Wang D,et al. Biotechnol Bioeng,2013,110 (11):3063
[9] Nahrgang S. [PhD Dissertation]. Lausanne:École Polytechnique Fédérale de Lausanne,2002:125
[10] Rodney M,Jasbir S. US Patent,4677195. 1987-06-30
[11] Bavand M,Blasey H. EP 1428878 B1.2008-08-20
[12] Cote S,Gebski C. BioPharm Int,2011,24(4):s16
[13] Liu H F,Ma J,Winter C,et al. MAbs,2010,2(5):480
[14] Staby A,Jensen I H,Mollerup I. J Chromatogr A,2000,897:99
[15] Jungbauer A. J Chromatogr A,2005,1065:3
[16] Corbett R,Carta G,Iskra T,et al. J Chromatogr A,2013,1278:116
[17] Thomas H,de Neuville B C,Storti G,et al. J Chromatogr A,2013,1285:48
[18] Ribeiro D,Passos D F,Ferraz H C,et al. J Chromatogr B,2013,938:111
[19] Podgornik A,Jancar J,Merhar M,et al. J Biochem Biophys Methods,2004,60:179
[20] Staby A,Holm J I,Mollerup I. J Chromatogr A,2000,897:99
