MicroRNA调控动物脂肪细胞分化研究进展
2015-10-29张进威罗毅王宇豪何刘军李明洲王讯
张进威,罗毅,王宇豪,何刘军,李明洲,王讯
MicroRNA调控动物脂肪细胞分化研究进展
张进威,罗毅,王宇豪,何刘军,李明洲,王讯
四川农业大学动物科技学院,动物遗传育种研究所,成都611130
脂肪组织不仅在维持机体能量代谢和稳态上发挥重要作用,同时也是重要的内分泌器官。脂肪细胞分化是由间充质干细胞(Mesenchymal stem cells, MSC)向成熟脂肪细胞分化的复杂生理过程,该过程由大量转录因子、激素、信号通路分子协同调控。miRNA作为内源性非编码RNA,主要通过抑制转录后翻译等机制来调控基因表达。近年来越来越多的证据表明miRNA通过调控脂肪细胞分化相关的转录因子和重要信号分子进而影响动物脂肪细胞的分化和脂肪形成。本文对miRNA影响动物白色、棕色和米色脂肪细胞分化的作用机制及其相关调控通路和关键因子进行了归纳总结,以期为肥胖等代谢性疾病的治疗提供一定的理论指导和新的治疗思路。
miRNA;白色脂肪细胞;棕色脂肪细胞;米色脂肪细胞;分化
肥胖是由能量摄入和代谢不平衡使得身体脂肪异常增多而引起的。随着人们生活水平的提高和生活方式的改变,肥胖的比例正在逐年增加[1]。肥胖会导致一系列脂肪代谢紊乱引起的并发症,如Ⅱ型糖尿病、高血压、冠心病等[2]。脂肪组织在储存能量、调节体温、缓冲机械冲击等方面具有非常重要的作用,当糖代谢或脂代谢异常时,脂肪细胞体积增大(肥大)和数量增多(增生),机体就会出现肥胖[3]。脂肪细胞有3种类型:白色脂肪细胞、棕色脂肪细胞和米色脂肪细胞。白色脂肪细胞的主要功能是以甘油三酯的形式储存机体内多余能量。棕色脂肪细胞胞质中有许多线粒体,可以氧化脂类并以热能的形式释放。米色脂肪细胞是近年来新发现的一种脂肪细胞,在一定条件下由白色脂肪细胞转化而来,并代谢产热[4]。越来越多的研究表明,miRNA可以作为一种重要的调控因子参与调控脂肪细胞分化。随着相关研究的不断深入,miRNA调控脂肪细胞分化网络正在逐步完善[5, 6]。本文基于miRNA的生物学功能,结合脂肪细胞分化过程,对近年来miRNA调控3种脂肪细胞分化的最新研究进展进行了综述,为肥胖等代谢性疾病的治疗提供一定的理论指导和新的治疗思路。
1 miRNA的产生与生物学功能
miRNA是真核生物体内广泛存在的一类长度为19~22nt的单链非编码RNA,在转录后水平调控靶基因表达,从而调控细胞增殖、分化、凋亡等多种生物学过程[7]。最早发现的miRNA是lin-4和let-7,它们可以调节秀丽隐杆线虫()幼虫阶段性发育,从而拉开了miRNA研究的序幕[8]。经典的miRNA产生途径如下:miRNA基因在RNA聚合酶Ⅱ作用下转录形成几百个核苷酸长度的初级转录物pri-miRNA;pri-miRNA在RNaseⅢ家族的Drosha酶和伴侣蛋白DGCR8组成的复合物作用下被加工成长度约60nt并具有茎环结构的miRNA前体/pre-miRNA;pre-miRNA由转运蛋白Exportin-5转运到细胞质,然后被RNaseⅢ家族的Dicer酶加工形成约20nt的双链miRNA,最后解链形成成熟的单链miRNA[3, 9]。近年来在无脊椎动物和哺乳动物中发现了“mirtron途径”,这一途径不依赖Drosha酶而产生miRNA[10]。
在动物体内,成熟的miRNA可以与特定的核糖核蛋白AGO(Argonaute)结合生成沉默复合体 (RNA-induced silencing complex, RISC),RISC通过miRNA种子序列与mRNA 3′UTR或ORF区互补结合来识别靶基因,介导mRNA降解或抑制翻译,从而调控靶基因表达[7, 8]。miRNA还可以与mRNA竞争结合RNA结合蛋白,从而调控mRNA表达,如在肿瘤中miR-29与RNA结合蛋白HuR(Human antigen R protein)结合,最终使未结合HuR的mRNA表达升高[11]。此外,有研究发现某些miRNA(如let-7、miR-125b[12])可以加速mRNA脱腺苷,降低细胞内mRNA有效丰度,从而下调基因表达[13]。在某些植物中,miRNA可以介导自身基因座或靶基因甲基化,在表观水平调控基因表达[14, 15]。一个miRNA可以调节多个靶基因,多个miRNA也能同时作用于一个基因,因此在机体内就形成了复杂精细的miRNA调控网络[8]。
2 脂肪细胞起源与分化的分子机制
哺乳动物有两种脂肪组织:白色脂肪组织(White adipose tissue, WAT)和棕色脂肪组织(Brown adipose tissue, BAT)。WAT由大量白色脂肪细胞组成,成熟白色脂肪细胞含有一个大的脂滴,可以分泌一些脂肪细胞因子(如瘦素、脂联素等)[16]。BAT中含有棕色脂肪细胞,经典的棕色脂肪细胞中有许多小脂滴,胞质中有许多线粒体,高表达解偶联蛋白(Uncoupling protein-1, UCP-1),UCP-1可以破坏线粒体内膜两侧的跨膜质子梯度,阻碍ATP形成,增加产热。另外,米色脂肪细胞是近年来发现的新型脂肪细胞,它在一定条件下(长期冷刺激或β3-肾上腺素受体激动剂处理)由WAT中的白色脂肪细胞转化而来[17],通常米色脂肪细胞UCP-1表达较低,但经米色化信号刺激后,胞内UCP-1表达量显著升高,从而发挥产热功能[18, 19]。3种脂肪细胞均起源于间充质干细胞(Mesenchymal stem cells, MSCs),在不同调控元件共同作用下向每一种特定的细胞类型分化[16](图1)。
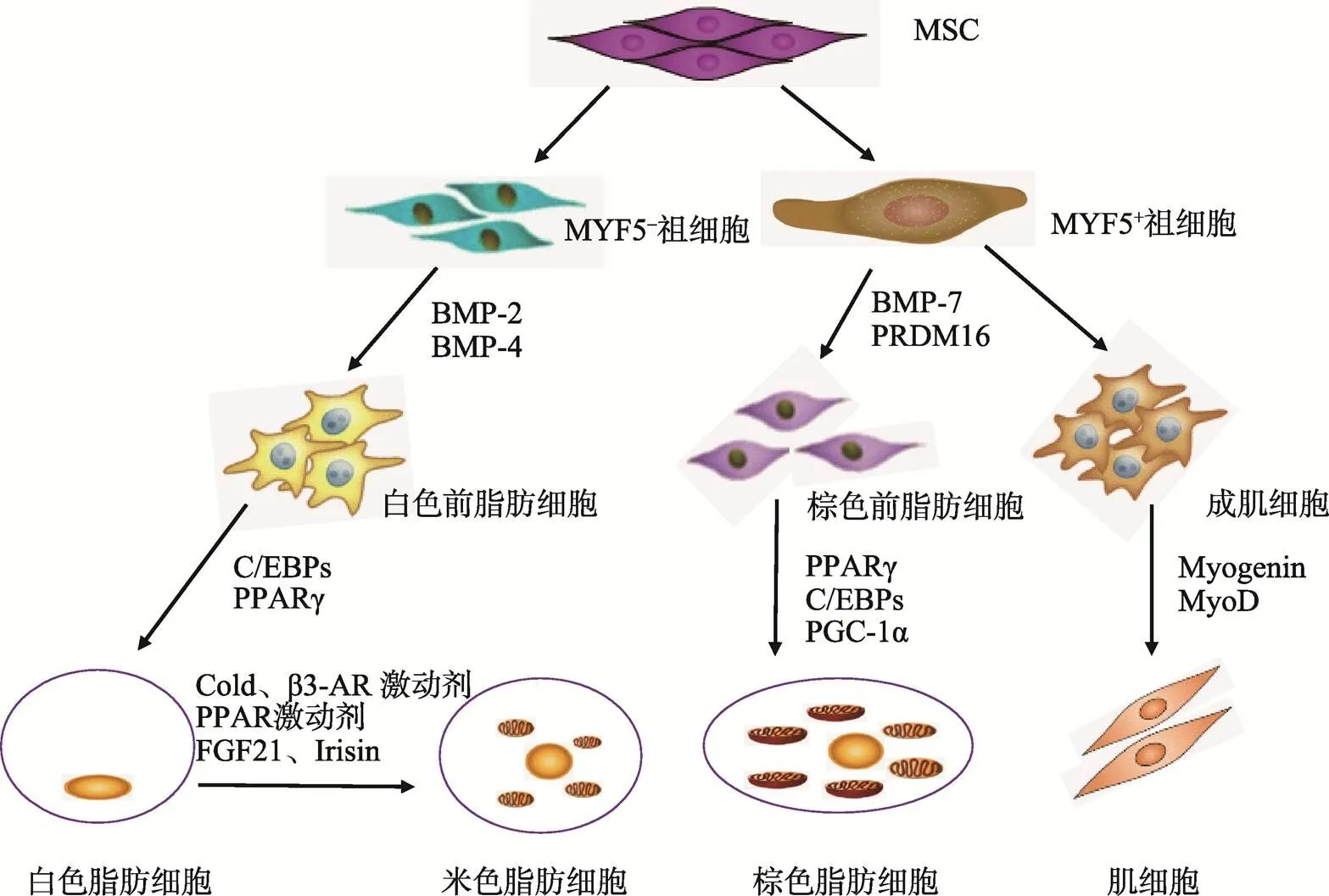
图1 3种脂肪细胞起源
MSC:间充质干细胞;MYF5:成肌因子5;BMP:骨形成蛋白;FGF21:成纤维细胞生长因子21;Irisin:鸢尾素;Myogenin:肌细胞生成素;MyoD:肌分化因子。
2.1 白色脂肪细胞
在多种转录因子和信号通路共同调控下,前体脂肪细胞向成熟脂肪细胞分化,并伴随一系列成脂基因和脂肪代谢相关酶基因的时序性表达。前体脂肪细胞分化经历3个阶段:接触抑制、克隆扩增、终末分化。第一阶段前体脂肪细胞长满进入细胞分裂停滞的G0期,细胞开始表达早期分化标志基因—前脂肪细胞因子()基因和脂蛋白酯酶(Lipoprotein lipase,)基因[20]。经历接触抑制后,在促有丝分裂和促脂肪生成信号刺激(如胰岛素、糖皮质激素)下,前体脂肪细胞再次进入细胞周期并经历两轮有丝分裂,此时有许多重要的转录因子开始表达,如过氧化物酶体增殖物激活受体γ(Peroxisome proliferator activated receptorγ, PPARγ)、CCAAT增强子结合蛋白家族(CCAAT/enhancer-binding proteins, C/EBPs)、固醇调节元件结合蛋白(Sterol regulatory element binding protein-1c, SREBP-1c)等,这些转录因子促进许多启动脂肪合成和聚集的相关基因表达,如乙酰辅酶A脱羧酶(Acetyl-CoA carboxylase, ACC)、脂肪酸结合蛋白(Adipocyte fatty acid binding proteins, ap2)、硬脂酰辅酶A去饱和酶(Stearoyl-CoA desaturase, SCD)等。这一时期细胞数目明显增多,形态发生显著改变,由梭形逐渐变为椭圆形,并伴随着许多小脂肪滴形成[21]。随后细胞进入终末分化阶段,此时与甘油三酯代谢相关酶活性显著升高,如ATP柠檬酸裂解酶、ACC、脂肪酸合成酶(Fatty acid synthetase, FAS)和3-磷酸甘油醛脱氢酶(Glyceraldehyde- 3-phosphate dehydrogenase, GAPDH)等。细胞胞质中小脂肪滴逐渐汇聚成单一的大脂滴,并占据大部分胞质,胞核被挤到靠近细胞膜的边缘部分[22]。
2.2 棕色脂肪细胞
棕色脂肪细胞是高表达UCP-1的生热型脂肪细胞,棕色脂肪细胞与肌细胞有共同的祖细胞,称为MYF5(Myogenic factor 5)/PAX7(Paired-box 7)祖细胞,当和两个基因表达时,间充质干细胞向MYF5/PAX7祖细胞分化。棕色脂肪细胞与肌细胞的前体细胞具有相似的基因表达模式和线粒体蛋白表达谱[17, 23]。MYF5/PAX7祖细胞在骨形态发生蛋白-7(Bone morphogenetic protein-7, BMP-7)、PPARγ、C/EBPs、过氧化物酶体增殖物激活受体α辅激活因子(Peroxisome proliferator activated receptor γ coactivator-1α, PGC-1α)和具有锌指结构的转录辅助调节因子(PR domain-containing 16, PRDM16)存在时分化为棕色脂肪细胞[16],其中PRDM16是决定MYF5/PAX7祖细胞向棕色脂肪细胞分化的开关[24]。成熟的棕色脂肪细胞胞质中有许多线粒体和微小脂滴。
2.3 米色脂肪细胞
米色脂肪细胞也是表达UCP-1的产热脂肪细胞,最初发现于成年动物皮下白色脂肪组织(Subcutaneous WAT, sWAT)。sWAT在长期冷刺激、β3-肾上腺素受体激活剂、PPARγ激动剂等刺激下,一些细胞在形态学和生理学上表现出与棕色脂肪相似的特征,如出现多室微小脂滴、线粒体增多、UCP-1表达上升等[23]。尽管米色脂肪细胞在形成 过程中有许多基因与经典棕色脂肪细胞表达相似,如和(Cell death- inducing Dff45 like effector),但其也有自身的标志基因,如(Cbp/p300-interacting transactivator, with Glu/Asp-rich carboxy-terminal domain 1)(Transmembrane protein 26)和(T-box 1)[25]。
3 miRNA调控脂肪细胞分化
越来越多的研究表明,miRNA可以与脂肪细胞分化相关的转录因子和重要信号分子相互作用来调控脂肪形成[3]。近年来已有许多调控脂肪细胞分化的miRNA和靶基因被发现(表1),miRNA调控脂肪细胞分化的网络正在逐步完善(图2)。
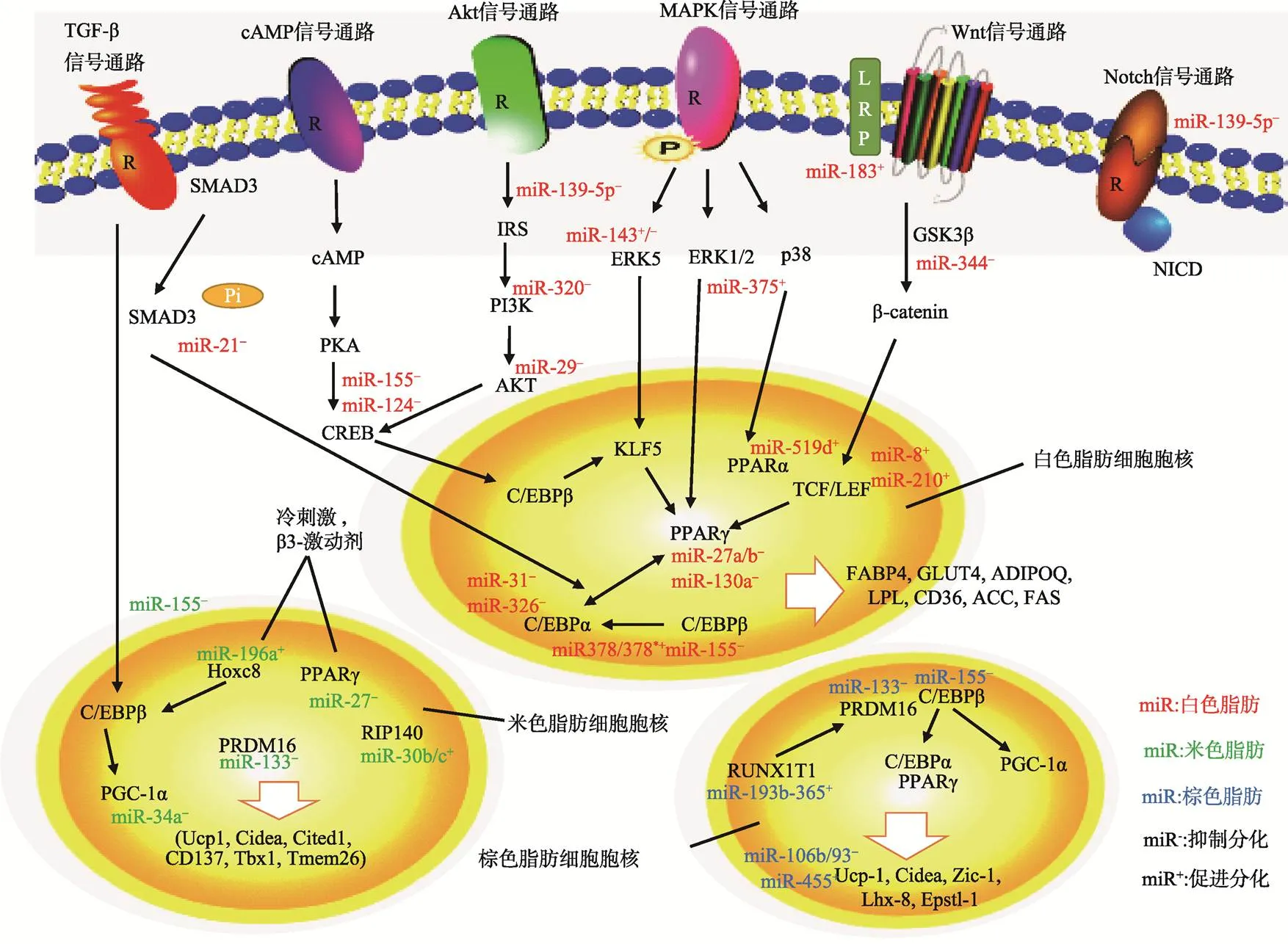
图2 脂肪细胞分化miRNA调控网络
FABP4:脂肪酸结合蛋白4;GLUT4:葡萄糖转运体;ADIPOQ:脂联素;LPL:脂蛋白酯酶;ACC:乙酰辅酶A脱羧酶;FAS:脂肪酸合成酶;Tbx1:T-box基因1;Tmem26:跨膜蛋白26;Zic-1:小脑锌指蛋白1;Lhx-8:LIM同源盒基因8;LEF:淋巴增强子结合因子1;PGC-1α:过氧化物酶体增殖物激活受体γ辅激活因子1α;NICD:Notch胞内结构域;SMAD3:Sma和Mad相关蛋白3。
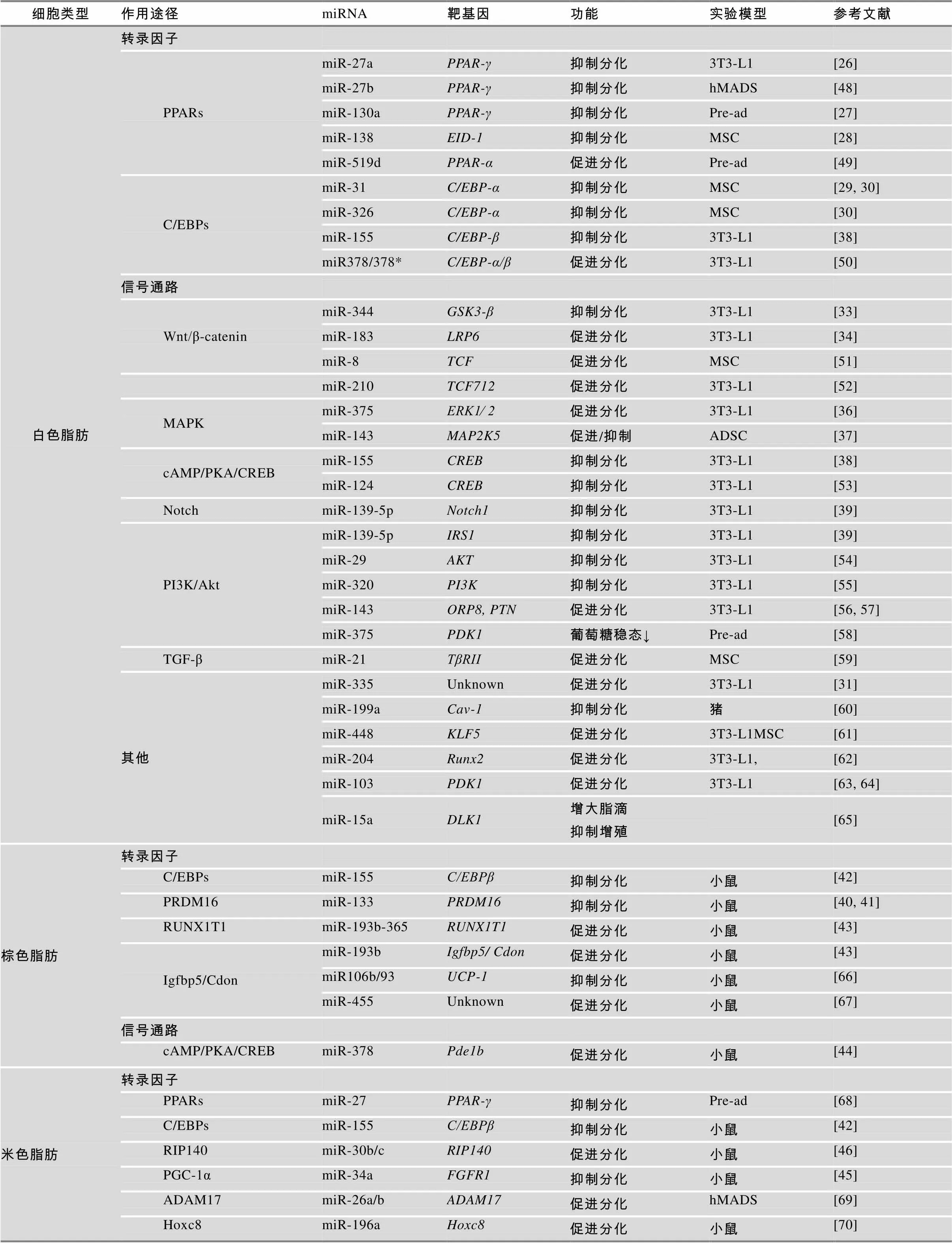
表1 miRNA调控脂肪细胞分化
注:PPARs:过氧化氢酶体增殖物激活受体;C/EBPs:CCAAT增强子结合蛋白;EID-1:EP300相互作用分化抑制因子1;Wnt/β-catenin:Wnt/β-连环蛋白;GSK3-β:糖原合成酶激酶3β;LRP6:低密度脂蛋白受体相关蛋白6;TCF:T细胞因子;MAPK:促分裂原活化蛋白激酶;ERK1⁄ 2:细胞外调节蛋白激酶1/2;cAMP/PKA/CREB:腺苷酸环化酶/蛋白激酶A/环磷腺苷效应元件结合蛋白;PI3K/Akt:磷脂酰肌醇3激酶/蛋白激酶B;IRS1:胰岛素受体底物;ORP8:氧类固醇结合蛋白相关蛋白;PTN:多效生长因子;PDK1:3-磷酸肌醇依赖性蛋白激酶1;TGF-β:转化生长因子- β;KLF:Kruppel样因子;Runx2:Runt相关转录因子2;Cav-1:微囊蛋白1;DLK1:delta样1同族物;PRDM16:锌指转录因子PR结构域包含蛋白16;UCP-1:解偶联蛋白;Pde1b:磷酸二酯酶1b;Igfbp5:胰岛素样生长因子结合蛋白5;RIP140:受体相互作用蛋白140;ADAM17:解聚素-金属蛋白酶17;Hoxc8:同源框基因8;FGFR1:成纤维细胞生长因子受体1;Pre-ad:前体脂肪细胞;hMADS:人多能脂肪干细胞;MSC:间充质干细胞;ADSC:脂肪干细胞。
3.1 miRNA与白色脂肪细胞分化
PPARγ、C/EBPs是贯穿脂肪细胞分化进程最重要的转录因子,miRNA可以与这些转录因子直接或间接作用来调节细胞分化[3]。Kim等[26]和Lee等[27]发现miR-27a和miR-130a可以与的3′UTR区结合下调的表达量,同时在3T3-L1细胞分化过程中miR-27a和miR-130a表达逐渐下降,与表达呈负相关,而过表达miR-27a和miR-130a时,表达量下降,脂肪细胞分化程度减弱,这些研究表明miR-27a和miR-130a可以靶向作用于从而抑制脂肪细胞分化。Yang等[28]发现在人脂肪干细胞成脂诱导分化过程中miR-138表达量显著下降,而过表达miR-138时,、和等成脂基因表达受到抑制,胞内脂滴聚集减少,进一步研究表明miR-138可以与核共调节因子(EP300 interacting inhibitor of differentiation-1)3′UTR区结合下调表达。EID-1可以抑制TGF-β(Transforming growth factor-β)信号通路以促进脂肪形成,因此,miR-138可以下调靶基因,间接激活TGF-β信号通路从而抑制脂肪细胞分化。Sun等[29]和Tang等[30]发现在人间充质干细胞(MSC)向脂肪细胞诱导分化过程中,miR-31可以靶向作用于脂肪细胞分化重要的转录因子,抑制脂肪细胞分化。Tang等[30]还发现miR-326也可以与作用抑制脂肪形成。Noriko等[31]通过芯片分析发现肥胖小鼠肝脏和脂肪中miR-335上调,且在3T3-L1细胞分化过程中,miR-335与成脂标志基因、和表达量呈正相关,但miR-335的靶基因和作用机制还有待研究。
miRNA还可以与信号转导通路中关键分子的mRNA结合,通过影响细胞信号通路传递间接调控脂肪细胞的分化。Wnt/β-catenin信号通路对脂肪细胞分化具有非常重要的调节作用,激活Wnt通路可以抑制脂肪细胞分化[32]。Chen等[33]发现在3T3-L1分化过程中miR-344可以与Wnt信号通路分子(Glycogen synthase kinase 3-β)的3′UTR区结合,下调其表达量,使GSK3-β下游效应分子β-catenin蛋白表达量上升,从而激活Wnt通路,抑制脂肪细胞分化。Chen等[34]发现miR-183靶向作用于Wnt信号通路分子(Low-density lipoprotein receptor-related protein 6),抑制经典Wnt途径,从而促进3T3-L1前体脂肪细胞分化。ERK(Extracellular regulated protein kinases)、p38、JNK(c-Jun N-terminal kinase)共同构成了MAPK(Mitogen-activated protein kinase)信号通路,MAPK信号通路被激活后,可以引发细胞一系列转录事件和细胞应答[35]。Ling等[36]发现在3T3-L1分化过程中,miR-375能抑制ERK1⁄2 (Extracellular signal regulated kinases 1/2)磷酸化,通过ERK途径上调PPARγ、C/EBPα,增加ap2表达和甘油三脂聚集,从而促进成脂。Chen等[37]在研究miR-143对脂肪干细胞(Adipose derived stem cells, ADSC)分化作用时发现,miR-143可以与MAPKK家族成员的3′UTR区结合影响脂肪形成,但是在不同分化阶段中作用并非完全一致。在克隆扩增阶段,过表达miR-143抑制分化,而在接触抑制和终末分化阶段,过表达miR-143促进分化。另外,Liu等[38]发现在3T3-L1分化中加入TNF-α引起miR-155的上调,miR-155可以与(cAMP- response element binding protein)的3′UTR区作用,通过cAMP/PKA/CREB信号通路抑制脂肪细胞分化。Mi等[39]发现miR-139-5p可以与和的3′UTR区结合,下调Notch1和IRS1表达,通过Notch信号通路和 IRS1/PI3K/Akt信号通路阻碍3T3-L1从克隆增殖期向终末分化期转换,从而抑制脂肪细胞分化。
3.2 miRNA与棕色脂肪细胞分化
PGC-1α、PRDM16、PPARγ和C/EBPs等是棕色脂肪形成非常重要的转录因子,其中PRDM16是决定祖细胞向棕色脂肪细胞分化的开关[24]。Trajkovski等[40]和Yin等[41]发现小鼠在冷刺激后棕色脂肪增多,线粒体活性增强,而miR-133表达下降,进一步研究发现miR-133可以与的3′UTR区结合下调其表达量,因此冷刺激引起miR-133下调能够促进棕色脂肪细胞分化。Chen 等[42]发现在棕色脂肪细胞分化过程中miR-155可以结合的3′UTR区抑制其表达,同时,一些成脂激素引起C/EBPβ上升可抑制miR-155的转录,miR-155和C/EBPβ构成的双向负反馈回路共同调控棕色脂肪形成。Sun等[43]用芯片鉴定出miR- 193b-365在棕色脂肪细胞分化过程中表达量显著上升,miR-193b可以与(Insulin-like growth factor binding protein 5)和这两个成肌相关的细胞因子结合,促进棕色脂肪细胞分化,抑制肌肉形成。Pan等[44]研究发现miR-378转基因小鼠BAT增多,在BAT中miR-378可以与磷酸二酯酶(Phosphodiesterase, Pde1b)的3′UTR区结合抑制cAMP降解,胞内cAMP含量升高,进而促进棕色脂肪形成。
3.3 miRNA与米色脂肪细胞分化
最新研究发现miRNA可以调控白色脂肪细胞棕色化,从而影响米色脂肪形成。Fu等[45]用慢病毒介导饮食诱导肥胖小鼠体内miR-34a下调,发现其血脂降低,脂肪组织线粒体拷贝数和氧化功能增强,同时米色脂肪标志蛋白CD137和UCP-1表达显著升高。后续研究发现miR-34a可以与成纤维细胞生长因子受体(Fibroblast growth factor-1 receptor,)的3′UTR区结合,引起FGFR1表达降低。当miR-34a下调时,FGFR1表达升高,成纤维细胞生长因子21(FGF21)信号增强,而FGF21可以介导胞外调节激酶(ERK)磷酸化,通过MAPK信号通路促进米色脂肪形成。miR-34a的下调还可以增加PGC-1α脱乙酰作用,增强PGC-1α的转录活性,因此miR-34a可以抑制米色脂肪细胞分化。Hu等[46]用β3-肾上腺素激动剂处理或冷刺激小鼠,发现小鼠体内miR-30b/c表达升高,且miR-30b/c可以靶向作用于(Receptor-interacting protein 140),RIP140是脂肪代谢相关途径酶或转录因子的辅阻遏物,RIP140的表达下降可以增加解偶联蛋白UCP-1和Cidea表达,增加WAT产热和棕色化。
4 展望
肥胖的发病率愈来愈高,肥胖引起的脂肪代谢紊乱并发症正逐年上升,肥胖已经成为影响人类健康的最大杀手[1]。近年来发现的米色脂肪细胞源于WAT,高表达线粒体内膜标志蛋白UCP-1,可以增加脂肪组织产热,为肥胖和脂肪代谢异常的治疗开辟新途径[25]。目前已经发现了一些调控脂肪细胞分化的miRNA,它们作用于成脂相关的转录因子和信号通路共同调控脂肪形成,以维持机体脂肪代谢稳态[3, 5]。但是miRNA对脂肪形成调控网络尚需要进一步完善,尤其是调控米色脂肪形成的miRNA还需深度挖掘。研究3种脂肪细胞的起源及分化的分子机制,构建miRNA对脂肪组织形成的调控网络,利用miRNA介导和启动BAT与WAT前体脂肪细胞产热程序将成为未来治疗肥胖的新方向[47]。
参考文献
[1] Haidar YM, Cosman BC. Obesity epidemiology., 2011, 24(4): 205–210.
[2] Adamczak M, Wiecek A.The adipose tissue as an endocrine organ., 2013, 33(1): 2–13.
[3] Son YH, Ka S, Kim AY, Kim JB. Regulation of adipocyte differentiation via microRNAs., 2014, 29(2): 122–135.
[4] Park A, Kim WK, Bae KH. Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells., 2014, 6(1): 33–42.
[5] Chen L, Song JL, Cui JH, Hou J, Zheng XH, Li C, Liu L. MicroRNAs regulate adipocyte differentiation., 2013, 37(6): 533–546.
[6] Trajkovski M, Lodish H. MicroRNA networks regulate development of brown adipocytes., 2013, 24(9): 442–450.
[7] Shenoy A, Blelloch RH. Regulation of microRNA function in somatic stem cell proliferation and differentiation., 2014, 15(9): 565–576.
[8] Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function., 2004, 116(2): 281–297.
[9] Ha MJ, Kim VN. Regulation of microRNA biogenesis., 2014, 15(8): 509–524.
[10] Westholm JO, Lai EC. Mirtrons: microRNA biogenesis via splicing., 2011, 93(11): 1897–1904.
[11] Wilczynska A, Bushell M. The complexity of miRNA-mediated repression., 2015, 22(1): 22–33.
[12] Wu LG, Fan JH, Belasco JG. MicroRNAs direct rapid deadenylation of mRNA., 2006, 103(11): 4034–4039.
[13] Djuranovic S, Nahvi A, Green R. miRNA-mediated gene silencing by translational repression followed by mRNA deadenylation and decay., 2012, 336(6078): 237–240.
[14] Matzke MA, Mosher RA. RNA-directed DNA methylation: an epigenetic pathway of increasing complexity., 2014, 15(6): 394–408.
[15] Wu L, Zhou HY, Zhang QQ, Zhang JG, Ni FR, Liu C, Qi YJ. DNA methylation mediated by a microRNA pathway., 2010, 38(3): 465–475.
[16] Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation., 2011, 12(11): 722–734.
[17] Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential., 2013, 19(10): 1252–1263.
[18] Bartelt A, Heeren J. Adipose tissue browning and metabolic health., 2014, 10(1): 24–36.
[19] Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis., 2012, 481(7382): 463–468.
[20] 吴家睿. 脂肪细胞分化: 一个故事, 两个章节. 科学通报, 2011, 56(17): 1327–1334.
[21] 陈晨. miR-135a和miR-183对3T3-L1前脂肪细胞分化及脂肪形成的调控作用研究[学位论文]. 武汉: 华中农业大学, 2013.
[22] 庞卫军, 李影, 卢荣华, 白亮, 吴江维, 杨公社. 脂肪细胞分化过程中的分子事件. 细胞生物学杂志, 2005, 27(5): 497–500.
[23] Kajimura S, Saito M. A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis., 2014, 76(1): 225–249.
[24] Seale P, Bjork B, Yang WL, Kajimura S, Chin S, Kuang SH, Scimè A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman. PRDM16 controls a brown fat/skeletal muscle switch., 2008, 454(7207): 961–967.
[25] Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang KX, Tu H, Van Marken Lichtenbelt WD, Hoeks J, Enerbäck S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human., 2012, 150(2): 366–376.
[26] Kim SY, Kim AY, Lee HW, Son YH, Lee GY, Lee JW, Lee YS, Kim JB. miR-27a is a negative regulator of adipocyte differentiation via suppressing PPARγ expression., 2010, 392(3): 323–328.
[27] Lee EK, Lee MJ, Abdelmohsen K, Kim W, Kim MM, Srikantan S, Martindale JL, Hutchison ER, Kim HH, Marasa BS, Selimyan R, Egan JM, Smith SR, Fried SK, Gorospe M. miR-130 suppresses adipogenesis by inhibiting peroxisome proliferator-activated receptor γ expression., 2011, 31(4): 626–638.
[28] Yang Z, Bian CJ, Zhou H, Huang S, Wang SH, Liao LM, Zhao RC. MicroRNA hsa-miR-138 inhibits adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells through adenovirus EID-1., 2011, 20(2): 259–267.
[29] Sun FY, Wang JY, Pan QH, Yu YC, Zhang Y, Wan Y, Wang J, Li XY, Hong A. Characterization of function and regulation of miR-24-1 and miR-31., 2009, 380(3): 660–665.
[30] Tang YF, Zhang Y, Li XY, Li C, Tian WD, Liu L. Expression of miR-31, miR-125b-5p, and miR-326 in the adipogenic differentiation process of adipose-derived stem cells., 2009, 13(4): 331–336.
[31] Nakanishi N, Nakagawa Y, Tokushige N, Aoki N, Matsuzaka T, Ishii K, Yahagi N, Kobayashi K, Yatoh S, Takahashi A, Suzuki H, Urayama O, Yamada N, Shimano H. The up-regulation of microRNA-335 is associated with lipid metabolism in liver and white adipose tissue of genetically obese mice., 2009, 385(4): 492–496.
[32] Prestwich TC, MacDougald OA. Wnt/β-catenin signaling in adipogenesis and metabolism., 2007, 19(6): 612–617.
[33] Chen H, Wang SQ, Chen LX, Chen YS, Wu M, Zhang Y, Yu KF, Huang Z, Qin LJ, Mo DL. MicroRNA-344 inhibits 3T3-L1 cell differentiation via targeting GSK3β of Wnt/β-catenin signaling pathway., 2014, 588(3): 429–435.
[34] Chen C, Xiang H, Peng YL, Peng J, Jiang SW. Mature miR-183, negatively regulated by transcription factor GATA3, promotes 3T3-L1 adipogenesis through inhibition of the canonical Wnt/β-catenin signaling pathway by targeting.,2014, 26(6): 1155–1165.
[35] Bost F, Aouadi M, Caron L, Binétruy B. The role of MAPKs in adipocyte differentiation and obesity., 2005, 87(1): 51–56.
[36] Ling HY, Wen GB, Feng SD, Tuo QH, Ou HS, Yao CH, Zhu BY, Gao ZP, Zhang L, Liao DF. MicroRNA-375 promotes 3T3-L1 adipocyte differentiation through modulation of extracellular signal-regulated kinase signalling., 2011, 38(4): 239–246.
[37] Chen L, Hou J, Ye LF, Chen YW, Cui JH, Tian WD, Li C, Liu L. MicroRNA-143 regulates adipogenesis by modulating the MAP2K5-ERK5 signaling., 2014, 4: 3819.
[38] Liu SH, Yang Y, Wu JR. TNFα-induced up-regulation of miR-155 inhibits adipogenesis by down-regulating early adipogenic transcription factors., 2011, 414(3): 618–624.
[39] Mi L, Chen YS, Zheng XL, Li YL, Zhang QL, Mo DL, Yang GS. MicroRNA-139-5p suppresses 3T3-L1 preadipocyte differentiation through notch and IRS1/PI3K/Akt insulin signaling pathways., 2015, 116(7): 1195–1204.
[40] Trajkovski M, Ahmed K, Esau CC, Stoffel M. MyomiR-133 regulates brown fat differentiation through Prdm16., 2012, 14(12): 1330–1335.
[41] Yin H, Pasut A, Soleimani VD, Bentzinger CF, Antoun G, Thorn S, Seale P, Fernando P, van IJcken W, Grosveld F, Dekemp RA, Boushel R, Harper ME, Rudnicki MA. MicroRNA-133 controls brown adipose determination in skeletal muscle satellite cells by targeting Prdm16.,2013, 17(2): 210–224.
[42] Chen Y, Siegel F, Kipschull S, Haas B, Fröhlich H, Meister G, Pfeifer A. miR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit., 2013, 4(4): 1769.
[43] Sun L, Xie HM, Mori MA, Alexander R, Yuan BB, Hattangadi SM, Liu QQ, Kahn CR, Lodish HF.is essential for brown fat differentiation., 2011, 13(8): 958–965.
[44] Pan DN, Mao CX, Quattrochi B, Friedline RH, Zhu LJ, Jung DY, Kim JK, Lewis B, Wang YX. MicroRNA-378 controls classical brown fat expansion to counteract obesity., 2014, 5: 4725.
[45] Fu T, Seok S, Choi S, Huang Z, Suino-Powell K, Xu HE, Kemper B, Kemper JK. MicroRNA 34a inhibits beige and brown fat formation in obesity in part by suppressing adipocyte Fibroblast Growth Factor 21 signaling and SIRT1 function., 2014, 34(22): 4130–4142.
[46] Hu F, Wang M, Xiao T, Yin BQ, He LY, Meng W, Dong MJ, Liu F. miR-30 promotes thermogenesis and the development of beige fat by targeting RIP140., 2015, 64(6): 2056–2068.
[47] Zhou JY, Li LX. MicroRNAs are key regulators of brown adipogenesis., 2014, 1841(11): 1590–1595.
[48] Karbiener M, Fischer C, Nowitsch S, Opriessnig P, Papak C, Ailhaud G, Dani C, Amri EZ, Scheideler M. microRNA miR-27b impairs human adipocyte differentiation and targets PPARγ., 2009, 390(2): 247–251.
[49] Martinelli R, Nardelli C, Pilone V, Buonomo T, Liguori R, Castanò I, Buono P, Masone S, Persico G, Forestieri P, Pastore L, Sacchetti L. miR-519d overexpression is associated with human obesity., 2010, 18(11): 2170–2176.
[50] Gerin I, Bommer GT, McCoin CS, Sousa KM, Krishnan V, MacDougald OA. Roles for miRNA-378/378* in adipocyte gene expression and lipogenesis., 2010, 299(2): E198–E206.
[51] Kennell JA, Gerin I, MacDougald OA, Cadigan KM. The microRNA miR-8 is a conserved negative regulator of Wnt signaling., 2008, 105(40): 15417–15422.
[52] Qin LM, Chen YS, Niu YN, Chen WQ, Wang QW, Xiao SQ, Li AN, Xie Y, Li J, Zhao X, He ZY, Mo DL. A deep investigation into the adipogenesis mechanism: Profile of microRNAs regulating adipogenesis by modulating the canonical Wnt/β-catenin signaling pathway., 2010, 11(1): 320.
[53] Qadir AS, Woo KM, Ryoo HM, Baek JH. Insulin suppresses distal-less homeobox 5 expression through the up-regulation of microRNA-124 in 3T3-L1 cells., 2013, 319(14): 2125–2134.
[54] He AB, Zhu LL, Gupta N, Chang YS, Fang FD. Overexpression of micro ribonucleic acid 29, highly up-regulated in diabetic rats, leads to insulin resistance in 3T3-L1 adipocytes., 2007, 21(11): 2785–2794.
[55] Ling HY, Ou HS, Feng SD, Zhang XY, Tuo QH, Chen LX, Zhu BY, Gao ZP, Tang CK, Yin WD, Zhang L, Liao DF. Changes in microRNA (miR) profile and effects of miR-320 in insulin-resistant 3T3-L1 adipocytes., 2009, 36(9): e32–e39.
[56] Jordan SD, Krüger M, Willmes DM, Redemann N, Wunderlich FT, Brönneke HS, Merkwirth C, Kashkar H, Olkkonen VM, Böttger T, Braun T, Seibler J, Brüning JC. Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism., 2011, 13(4): 434–446.
[57] Yi C, Xie WD, Li F, Lv Q, He J, Wu JB, Gu DY, Xu NH, Zhang YO. MiR-143 enhances adipogenic differentiation of 3T3-L1 cells through targeting the coding region of mouse pleiotrophin., 2011, 585(20): 3303–3309.
[58] El Ouaamari A, Baroukh N, Martens GA, Lebrun P, Pipeleers D, Van Obberghen E. miR-375 targets 3′-phosphoinositide–dependent protein kinase-1 and regulates glucose-induced biological responses in pancreatic β-cells., 2008, 57(10): 2708–2717.
[59] Kim YJ, Hwang SJ, Bae YC, Jung JS. MiR-21 regulates adipogenic differentiation through the modulation of TGF-β signaling in mesenchymal stem cells derived from human adipose tissue., 2009, 27(12): 3093–3102.
[60] Shi XE, Li YF, Jia L, Ji HL, Song ZY, Cheng J, Wu GF, Song CC, Zhang QL, Zhu JY, Yang GS. MicroRNA-199a-5p affects porcine preadipocyte proliferation and differentiation., 2014, 15(5): 8526–8538.
[61] Kinoshita M, Ono K, Horie T, Nagao K, Nishi H, Kuwabara Y, Takanabe-Mori R, Hasegawa K, Kita T, Kimura T. Regulation of adipocyte differentiation by activation of serotonin (5-HT) receptors 5-HT2AR and 5-HT2CR and involvement of microRNA-448-mediated repression of KLF5., 2010, 24(10): 1978–1987.
[62] Huang J, Zhao L, Xing LP, Chen D. MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation., 2010, 28(2): 357–364.
[63] Wilfred BR, Wang WX, Nelson PT. Energizing miRNA research: a review of the role of miRNAs in lipid metabolism, with a prediction that miR-103/107 regulates human metabolic pathways., 2007, 91(3): 209–217.
[64] Xie HM, Lim B, Lodish HF. MicroRNAs induced during adipogenesis that accelerate fat cell development are downregulated in obesity., 2009, 58(5): 1050–1057.
[65] Andersen DC, Jensen CH, Schneider M, Nossent AY, Eskildsen T, Hansen JL, Teisner B, Sheikh SP. MicroRNA-15a fine-tunes the level of Delta-like 1 homolog (DLK1) in proliferating 3T3-L1 preadipocytes., 2010, 316(10): 1681–1691.
[66] Wu Y, Zuo JR, Zhang YC, Xie Y, Hu F, Chen LH, Liu BL, Liu F. Identification of miR-106b-93 as a negative regulator of brown adipocyte differentiation., 2013, 438(4): 575–580.
[67] Walden TB, Timmons JA, Keller P, Nedergaard J, Cannon B. Distinct expression of muscle-specific MicroRNAs (myomirs) in brown adipocytes.2009, 218(2): 444–449.
[68] Sun L, Trajkovski M. MiR-27 orchestrates the transcriptional regulation of brown adipogenesis., 2014, 63(2): 272–282.
[69] Karbiener M, Pisani DF, Frontini A, Oberreiter LM, Lang E, Vegiopoulos A, Mössenböck K, Bernhardt GA, Mayr T, Hildner F, Grillari J, Ailhaud G, Herzig S, Cinti S, Amri EZ, Scheideler M. MicroRNA-26 family is required for human adipogenesis and drives characteristics of brown adipocytes., 2014, 32(6): 1578–1590.
[70] Mori M, Nakagami H, Rodriguez-Araujo G, Nimura K, Kaneda Y. Essential role for miR-196a in brown adipogenesis of white fat progenitor cells., 2012, 10(4): e1001314.
MicroRNA regulates animal adipocyte differentiation
Jinwei Zhang, Yi Luo, Yuhao Wang, Liujun He, Mingzhou Li, Xun Wang
Adipose tissues play a critical role in the regulation of energy metabolism and homeostasis, and is also an important endocrine organ. Adipocyte differentiation is a complicated physiological process during which mesenchymal stem cells differentiate into adipocytes. This process is synergistically regulated by a large number of transcription factors, hormones and signaling pathway molecules. As a class of endogenous non-coding RNA (ncRNA), microRNAs (miRNAs) regulate gene expression mainly through post-transcriptional translational repression. In recent years, numerous studies have demonstrated that miRNA could have an impact on adipocyte differentiation and adipogenesis by modulating the expression levels of several adipogenic transcription factors and key signaling molecules. In this review, we summarize the mechanism of miRNA in regulating the differentiation of white/brown/beige adipocytes and the relevant signaling pathways and key factors, in the hope of providing theoretical guidance and new thoughts for treating obesity and other metabolic diseases.
miRNA; white adipocyte; brown adipocyte; beige adipocyte; differentiation
2015-05-20;
2015-08-17
国家高技术研究发展计划(863计划)项目(编号:2013AA102502),四川省教育厅重点项目(编号:15ZA0008/15ZA0003),四川省青年科技创新研究团队(编号:2015TD0012),长江学者和创新团队发展计划(编号:IRT13083)和重庆市农发资金项目(编号:13410)资助
张进威,硕士研究生,专业方向:猪遗传育种与繁殖。E-mail: JinweiZhang50@163.com
王讯,副教授,硕士生导师,研究方向:猪遗传育种与繁殖。E-mail:wangxun99@163.com
10.16288/j.yczz.15-233
网络出版时间: 2015-9-23 17:06:41
URL: http://www.cnki.net/kcms/detail/11.1913.R.20150923.1706.004.html
(责任编委: 李辉)
