Prevalence of filarial parasites in domestic and stray cats in Selangor State, Malaysia
2015-10-28NazehAlAbdZuraineeMohamedNorMustafaKassimMarzidaMansorAbdulelahAlAdhroeyRomanoNguiSinnaduraiSivanandam
Nazeh M Al-Abd, Zurainee Mohamed Nor, Mustafa Kassim, Marzida Mansor, Abdulelah H. Al-Adhroey, Romano Ngui, Sinnadurai Sivanandam
1Department of Parasitology, Faculty of Medicine, University of Malaya, Kuala Lumpur 50603, Malaysia
2Department of Anesthesiology, Faculty of Medicine, University of Malaya, Kuala Lumpur 50603, Malaysia
Prevalence of filarial parasites in domestic and stray cats in Selangor State, Malaysia
Nazeh M Al-Abd1*, Zurainee Mohamed Nor1, Mustafa Kassim2, Marzida Mansor2, Abdulelah H. Al-Adhroey1, Romano Ngui1, Sinnadurai Sivanandam1
1Department of Parasitology, Faculty of Medicine, University of Malaya, Kuala Lumpur 50603, Malaysia
2Department of Anesthesiology, Faculty of Medicine, University of Malaya, Kuala Lumpur 50603, Malaysia
ARTICLE INFO
Article history:
in revised form
Accepted
Available online
Filarial parasites
Brugia
Wuchereria
Dirofilaria
Cat
Filariasis
Objective: To determine the prevalence of the filarial parasites, ie., Brugia malayi, Brugia pahangi (B. pahangi), Dirofilaria immitis and Dirofilaria repens (D. repens) in domestic and stray cats. Methods: A total of 170 blood sample were collected from domestic and stray cats and examined for filarial worm parasites in two localities, Pulau Carey and Bukit Gasing, Selangor State, Malaysia. Results: The overall prevalence of infection was 23.5% (40/170; 95% CI=17.4-30.6). Of this, 35% (14/40; 95% CI=22.1-50.5) and 50% (20/40; 95% CI=35.2-64.8)were positive for single B. pahangi and D. repens, respectively. The remaining of 15% (6/40;95% CI=7.1-29.1) were positive for mixed B. pahangi and D. repens. In addition, 75% of the infected cats were domestic, and 25% were strays. No Brugia malayi and Dirofilaria immitis was detected. Eighty-four cats were captured at Pulau Carey, of which 35.7% (30/84) were infected. Among the cats determined to be infected, 93% (28/30; 95% CI=78.7-98.2) were domestic, and only 6.7% (2/30; 95% CI=19.0-21.3) were strays. Conversely, the number of infected cats was three times lower in Bukit Gasing than in Pulau Carey, and most of the cats were stray. Conclusions: B. pahangi and D. repens could be the major parasites underlying filariasis in the study area. Adequate prophylactic plans should be administrated in the cat population in study area.
1. Introduction
Cats, dogs, and leaf monkeys are among the known animal hosts that serve as reservoirs for Brugian filarial parasites[1]. Numerous published reports on zoonotic filariae involving cats have originated from several countries including Thailand[2], Indonesia[3], the Philippines[4], and other Southeast Asian countries[5,6]. In the endemic regions, both domestic and stray cats have been reported to be infected with several filarial parasites, such as Brugia malayi (B. malayi), Brugia pahangi (B. pahangi), Dirofilaria immitis (D. immitis)and Dirofilaria repens (D. repens)[2,7,8].
In Malaysia, domestic cats and leaf monkeys have been established as the primary reservoir hosts for these parasites[2]. The currentinvestigation was prompted by the close association and proximity of cats and humans in Malaysia, as well as the evidence of possible natural infections of B. pahangi in man[3,9,0] and the possibility that B. pahangi infection in humans may be underestimated.
In humans, infection with B. malayi causes lymphatic filariasis[11]. Reports of experimental transmission of B. pahangi in humans have indicated that volunteers inoculated with B. pahangi not only developed microfilaria but also suffered from episodes of lymphangitis, lymphadenitis, and oedema in the inoculated limb,each of which began approximately 1 month after the inoculation[12]. Human infection with D. immitis, on the other hand, is very rare,but when infection occurs, it is usually associated with pulmonary lesions or radiological coin lesions in the lung[13,14]. Moreover,humans may become infected with D. repens and present with subcutaneous nodules, pruriginous urticarioid patches, eosinophilia,photophobia, conjunctival irritation, and nodules or cysts in theeye or in the periocular tissues. Infection with D. repens is always amicrofilaremic; thus, it was assumed that larvae introduced into humans died and did not reach maturity[15].
There are limited data available on the prevalence of the filarial parasite in cats in Malaysia, but we believe that a study of the prevalence of these filarial parasites, particularly B. malayi, B. pahangi, and D. immitis, in both domestic and stray cats could have an impact on public health, as such information may help veterinarians to assess the risk of these parasites and establish a control program for zoonotic infections, thereby eliminating infection in cats and reducing the exposure to humans. In addition,estimation of the regional prevalence of filarial infection could increase awareness of this serious problem.
2. Materials and methods
2.1. Study area
Two geographical sites in Selangor state, Malaysia, were included in the current study. Bukit Gasing (3o6´0´´ N, 101o41´1´´ E) is a residential area located in Kuala Lumpur and is a hilly and forested area with individual bungalow houses. Pulau Carey (2o8´53´´ N,101o39´4´´ E), in contrast, is an Orang Asli (Aborigines) village situated east of Kuala Lumpur (Figure 1) that is also forested, and most of the houses are constructed of wood. Both sites included on the study have a tropical climate with high humidity and frequent rainfall throughout the year. The temperature for the sites ranges between 30 ℃ and 36 ℃.
2.2. Collection of domestic and stray cats
In this study, domestic cat refers to a cat living in close proximity to a human household, from which the animal obtains all of its basic requirements for survival (eg., food, water, and shelter). In contrast, stray cat refers to cat living separately from a household,and depending only in part on humans for provision of shelter and sustenance. Trapping of the cats was performed from January to May 2013. Briefly, each cat was approached slowly and restrained by scruffing the loose skin on the back of its neck. Once caught, the cat was allowed a brief period of acclimation and was allowed to regain composure while under humane restraint. The Ethics Committee of the Faculty of Medicine at University Malaya, Kuala Lumpur,Malaysia, approved all procedures involving the cats performed on the current study [Ethical number: PAR/21/11/2011/ZMN (R)].
2.3. Collection of blood
When each cat was calm and comfortable, blood collection was performed by a well-trained veterinarian. With the animal restrained humanely, blood was collected from the ear vein of each cat. All blood samples were collected in EDTA tubes by using a graduated capillary pipette fitted with a simplified non-breakable Sinton pipette. Samples were stored at 4 ℃ until direct transport within 1 d to the laboratory for analysis.
2.3.1. Staining of blood films
Staining of all blood films was performed using Giemsa stain(Innenkorper technique) as previously described[16]. Briefly, thick blood films were prepared on glass slides and allowed to dry at room temperature for 24-48 h. Before staining, the slides were dehemoglobinized in water for 1 min and air-dried. The slides were then fixed with absolute methanol for approximately 30-60 s. The dried blood films were then stained with 2% Giemsa (Merck, NJ) in pH 7.2 phosphate buffer for 35 min, and then rinsed with tap water.
2.3.2. Microscopic examination of blood films
Two independent individuals, using an Olympus CX40 lightmicroscope (Olympus, Japan), examined the stained blood films for microfilaria. The microfilariae were observed at 100× and 400× magnification, and images of the microfilaria detected were captured using an on-board Olympus DP12 digital microscope camera(Olympus, Japan). Data entry and analysis were performed using Microsoft Excel 2010.
3. Results
3.1. Cat population and infection status
The sites selected for this study are detailed in the map in Figure 1. In total, 170 domestic and stray cats (average age, 2 years) were collected. Eighty-four cats were caught from Pulau Carey, including 54 domestic cats and 30 stray cats, whereas 86 cats, including 52 domestic cats and 34 stray cats, were caught from Bukit Gasing. Of the 170 cats included on the study, 49.4% were male and 50.6% were female. Eighty-four cats were captured at Pulau Carey, of which 35.7% (30/84) were infected. Among the cats determined to be infected, 93% (95% CI=78.7-98.2) were domestic, and only 6.7%(95% CI=19.0-21.3) were stray. Conversely, the number of cats infected from Bukit Gasing was three times lower than the number of infected cats from Pulau Carey, and most of the cats were stray(Table 1).
3.2. Identification of parasite species detected in blood films
The morphology of the B. pahangi and D. repens microfilaria detected in the study is shown in Figure 2. Identification of B. pahangi microfilaria (Figure 2a) was based primarily on the size of the innenkorper relative to the whole length of the microfilaria,which was approximately 21% (44-63 μm). Further, the proportional size of the B. pahangi innenkorper was slightly larger than that of B. malayi (subperiodic form), which was approximately 13.7%. Other characteristics of this microfilaria, which were similar to those of other Brugia species, included the presence of two terminal nuclei at the posterior end and a 2:1 cephalic space ratio. Identification of D. repens microfilaria (Figure 2b) was mainly based on the presence of two distinct nuclei in the cephalic space region and the observation that the microfilariae of this parasite lacked a sheath.
3.3. Distribution of the filarial parasites within the cat population
In total, 35% (14/40; 95% CI=22.1-50.5) of the cats determined to have a filarial parasite infection were infected with B. pahangi. Furthermore, all of the cats infected with B. pahangi were from Pulau Carey, and all were domestic. Approximately 50% (20/40;95% CI=35.2-64.8) of the cats tested positive for D. repens, and an equal number of cats was infected by this parasite at each of the study sites. In Pulau Carey, infection with D. repens was detected in eight domestic and two stray cats, whereas in Bukit Gasing, the numbers of infected cats were reversed (two domestic and eight stray cats). Mixed infection with both B. pahangi and D. repens was observed in 15% (6/40; 95% CI=7.1-29.1) of the cats, all of which were from Pulau Carey and included domestic cats only. Similarly,infection occurred in both sexes, with 40% of the infected cats being male (16/40; 95% CI=26.4-55.4) and 60% female (24/40; 95%CI=44.6-73.7). None of the cats in this study were infected with B. malayi or D. immitis (Table 2). Infection rates were higher in the domestic cat population (75%) than in the stray cat population(25%).

Table 1 Number and the type of cats found infected in the study.
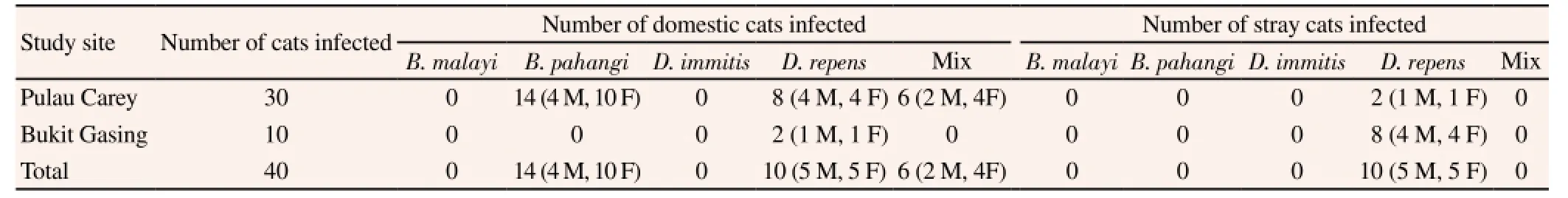
Table 2 Species of parasites detected in the infected cats.
4. Discussion
Cats are known to serve as the host for zoonotic filarial infections,including B. pahangi and D. repens[7,8,10]. However, it is a common practice among humans to keep a cat as a domestic household pet. Because many stray cats roam areas near human populations in search of food, the possibility exists that these cats could serve as hosts and contribute to zoonotic infections[17]. In the current study, 170 cats were captured and evaluated for the presence of microfilaremia. The blood film analyses indicated that 40 of the cats were infected with filarial worms, of which 30 were domestic cats and 10 were strays. The predominant animal filarial parasite species identified included B. pahangi and D. repens. Both B. pahangi and D. repens were detected in domestic cats, but only D. repens was detected in the stray cats. Furthermore, some domestic cats were infected simultaneously with both parasite species, which was not apparent in the stray cat population. Additionally, infection with other filarial parasites, including B. malayi and D. immitis, was not observed in the cat population included in the current study. These results were not unexpected, considering that D. immitis is a parasite found primarily in canine species, whereas B. malayi is a human filarial parasite. Nonetheless, both parasites have been reported to infect cats.
The overall infection rate was determined to be 23.5% for the total cat population examined, where 35% of the affected animals were infected with B. pahangi, 50% were infected with D. repens, and 15% were infected with both B. pahangi and D. repens. Additionally,the results of the current study were similar to those of a previously published report[2]. A prior study that employed polymerase chain reaction-restriction fragment length polymorphism of internal transcribed spacer regions, ITS1, to determine the filarial distribution in 52 domestic cats of south Thailand demonstrated the existence of parasites in 9.5% of the cats, of which 7.6% were infected with B. pahangi. The study also determined that 1.9% of the cats were infected with D. immitis.
A study conducted in Petaling Jaya, another district in Selangor state, reported the occurrence of B. pahangi microfilaria in blood samples from 5 out of 12 domestic cats[10]. In that study, absence of B. malayi in the blood samples of domestic cats was also reported. Based on this report and our findings, we surmised that cats are not the main host for B. malayi infection and that these cats were not from areas endemic for human filariasis. However, our findings contradicted an observation of the occurrence of B. malayi in a cat obtained from an area with endemic lymphatic filariasis in southern Thailand[8].
Reports of several cases of natural human infection with B. pahangi in a suburb of Kuala Lumpur, Malaysia[10], clearly indicated the presence of zoonotic infections, which led the authors to conclude that domestic cats may be the source of B. pahangi. The results of our study support this conclusion, based on the detection of B. pahangi in 35% of the infected cats and the finding that 15% of the cats had mixed infections (B. pahangi and D. repens). In addition, all of the cats infected with B. pahangi were domestic cats.
All of the cats infected with B. pahangi (singly, or mixed with D. repens) were caught at Pulau Carey. None of the cats caught at Bukit Gasing were infected with B. pahangi. The life cycle of B. pahangi involves an intermediate mosquito vector and a primary mammalian host. The Mansonia annulata and Mansonia dives mosquito species are known to be natural vectors of B. pahangi[18], and to thrive in a forest environment similar to that of Pulau Carey, but not in a suburban area such as Bukit Gasing[9].
A previous study revealed the occurrence of D. repens microfilaria in cats and reported that the infected cats exhibited lesional pruritus,concurrent hemobartonellosis, and cutaneous lesions[19]. In our study,D. repens was detected in cats from both areas and affected both domestic and stray cats. Furthermore, although 50% of the affected cats were infected with D. repens and an additional 15% had mixed infections (B. pahangi and D. repens), none of the cats presented conditions such as those described in the previous study[19]. Human infection by this parasite is of great concern because it has become increasingly recognized worldwide as an inadvertent human pathogen[20,21].
In the current study, although the number of cats infected with B. pahangi was lower than the number of cats infected with D. repens, several cats infected with B. pahangi presented a higher number of circulating microfilaria, which increased the likelihood for human infection to occur in the presence of a suitable vector. One of the limitations of the current study was the use of microscopy as the method of diagnosis, as microscopy has low sensitivity compared to other techniques such as the polymerase chain reaction[2,22]. Therefore,it is strongly recommended to use polymerase chain reaction in a future study to accurately determine the true prevalence of filariasis in the area.
The type of filarial parasites reported in the current study, and the associated microfilaria count of each species detected in both domestic and stray cats, could provide insight on the status of zoonotic transmission of parasites of concern in Malaysia. Although B. malayi is the most prominent parasite among all of the filarial parasites in peninsular Malaysia, B. pahangi and D. repens were found to be the major parasites of involved in filariasis in the included study areas. Hence, some attention should be given to B. pahangi because this parasite could potentially pose a threat to the human population.
In conclusion, the data from this study suggest that B. pahangi and D. repens could be the major parasites underlying filariasis in the study area. The identification of these parasites in cats could providean insight into public health, particularly with regard to the extent of the danger of zoonotic infection in the research, as previously reported for several cases of natural human infection with B. pahangi in a suburb of Kuala Lumpur, the capital city of Malaysia. Further studies that will elucidate the role of cats in the zoonotic transmission of microfilarial parasites in the research area are important and are expected to suggest that appropriate prophylaxis should be administered to cats throughout the study area.
Conflict of interest statement
We declare that we have no competing interests.
Acknowledgments
The authors wish to acknowledge the cooperation of Ministry of Health officials, the Head of the Department of Parasitology at the University of Malaya, and guides who provided assistance towards the success of this research. This research was funded by a Ministry of Higher Education Research Grant (FRGS FP011/2011A) and by the University of Malaya (PG085-2012B).
[1] Laing A, Edeson J, Wharton R. Studies on fllariasis in Malaya: the vertebrate hosts of Brugia malayi and B. pahangi. Ann Trop Med Parasit 1960; 53(4): 92-99.
[2] Nuchprayoon S, Junpee A, Nithiuthai S, Chungpivat S, Suvannadabba S,Poovorawan Y. Detection of filarial parasites in domestic cats by PCRRFLP of ITS1. Vet Parasitol 2006; 140(3): 366-372.
[3] Palmieri JR, Ratiwayanto S, Masbar S, Tirtokusumo S, Rusch J,Marwoto HA. Evidence of possible natural infections of man with Brugia pahangi in South Kalimantan (Borneo), Indonesia. Trop Geogr Med 1985;37(3): 239-244.
[4] Rozeboom LE, Cabrera BD. Filariasis caused by Brugia malayi in the Republic of the Philippines. Am J Epidemiol 1965; 81: 200-215.
[5] Irwin PJ, Jefferies R. Arthropod-transmitted diseases of companion animals in Southeast Asia. Trends Parasitol 2004; 20(1): 27-34.
[6] Lim B, Mak J. Human behaviour and zoonotic disease in Malaysia. Human ecology and infectious disease. 2nd ed. London: Academic Press; 1983, p. 49-72.
[7] Chansiri K, Tejangkura T, Kwaosak P, Sarataphan N, Phantana S,Sukhumsirichart W. PCR based method for identification of zoonostic Brugia malayi microfilariae in domestic cats. Mol Cell Probes 2002;16(2): 129-135.
[8] Kanjanopas K, Choochote W, Jitpakdi A, Suvannadabba S, Loymak S, Chungpivat S, et al. Brugia malayi in a naturally infected cat from Narathiwat Province, southern Thailand. Southeast Asian J Trop Med Public Health 2001; 32(3): 585-587.
[9] Muslim A, Fong MY, Mahmud R, Lau YL, Sivanandam S. Armigeres subalbatus incriminated as a vector of zoonotic Brugia pahangi filariasis in suburban Kuala Lumpur, Peninsular Malaysia. Parasit Vectors 2014;6(1): 219.
[10] Tan LH, Fong MY, Mahmud R, Muslim A, Lau YL, Kamarulzaman A. Zoonotic Brugia pahangi filariasis in a suburbia of Kuala Lumpur City,Malaysia. Parasitol Inte 2003; 60(1): 111-113.
[11] Mutafchiev Y, Bain O, Williams Z, McCall JW, Michalski ML. Intraperitoneal development of the filarial nematode Brugia malayi in the Mongolian jird (Meriones unguiculatus). Parasitol Res 2014; 113(5):1827-1835.
[12] Edeson JF, Wilson T, Wharton RH, Laing AB. Experimental transmission of Brugia malayi and B. pahangi to man. Trans R Soc Trop Med Hyg 1960; 54: 229-234.
[13] Genchi C, Bandi C, Kramer L, Epis S. Dirofilaria infections in humans and other zoonotic filarioses helminth infections and their impact on global public health. Springer; 2003, p. 411-424.
[14] Megat Abd Rani PA, Irwin PJ, Gatne M, Coleman GT, Traub RJ. Canine vector-borne diseases in India: a review of the literature and identification of existing knowledge gaps. Parasit Vectors 2010; 3(1): 28.
[15] Kini RG, Leena J, Shetty P, Lyngdoh RH, Sumanth D, George L. Human Dirofilariasis: an emerging zoonosis in India. J Parasit Dis 2013; 37(1):1-6.
[16] Sivanandam S, Fredericks H. The 'Innenkorper' in differentiation between the microfilanriae of Brugia pahangi and B. malayi (sub-periodic form). Med J Malaya 1966; 20(4): 337-338.
[17] Dantas-Torres F, Otranto D. Dogs, cats, parasites, and humans in Brazil:opening the black box. Parasit Vectors 2014; 7: 22.
[18] Edeson JF, Wharton RH, Laing AB. A preliminary account of the transmission, maintenance and laboratory vectors of Brugia pahangi. Trans R Soc Trop Med Hyg 1960; 54: 439-449.
[19] Tarello W. Infestation with the zoonotic nematode Dirofilaria repens in cats from central Italy. Vet On-Line 2002. [Onine]. Avaible from: www. priory.com/vet/filiarisis.htm
[20] Abdel-Rahman S, Mahmoud A, Galal L, Gustinelli A, Pampiglione S. Three new cases of human infection with Dirofilaria repens, one pulmonary and two subcutaneous, in the Egyptian governorate of Assiut. Ann Trop Med Parasitol 2008; 102(6): 499-507.
[21] Pampiglione S, Rivasi F, Gustinelli A. Dirofilarial human cases in the Old World, attributed to Dirofilaria immitis: a critical analysis. Histopathology 2009; 54(2): 192-204.
[22] Silbermayr K, Eigner B, Duscher GG, Joachim A, Fuehrer HP. The detection of different Dirofilaria species using direct PCR technique. Parasitol Res 2014; 113(2): 513-516.
Nazeh M. Al-Abd, Department of Parasitology, Faculty of Medicine, University of Malaya, Kuala Lumpur 50603, Malaysia.
E-mail: Nazehali78@yahoo.com
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Diuretic and antioxidant activities of the aqueous extract of leaves of Cassia occidentalis (Linn.) in rats
- Chemical composition, mechanism of antibacterial action and antioxidant activity of leaf essential oil of Forsythia koreana deciduous shrub
- A pilot study of antioxidant potential of endophytic fungi from some Sudanese medicinal plants
- Genetic variation of Leptospira isolated from rats catched in Yogyakarta Indonesia
- Molecular survey of tick-borne pathogens in Ixodid ticks collected from hunted wild animals in Tuscany, Italy
- Epidemiology of influenza viruses from 2009-2013-A sentinel surveillance report from Union territory of Puducherry, India
