A pilot study of antioxidant potential of endophytic fungi from some Sudanese medicinal plants
2015-10-28AfraKhirallaIetidalMohamedJustinneThomasBenoMignardRosellaSpinaSakinaYagiDominiqueLaurainMattar
Afra Khiralla, Ietidal Mohamed, Justinne Thomas, Benoît Mignard, Rosella Spina, Sakina Yagi, Dominique Laurain-Mattar*
1Université de Lorraine, SRSMC, UMR 7565, BP 70239, F-54506 Vandoeuvre-lès-Nancy, France
2CNRS, SRSMC, UMR 7565, BP 70239, F-54506 Vandoeuvre-lès-Nancy, France
3Botany Department, Faculty of Science, University of Khartoum, P.O. Box 321, Khartoum, Sudan
4Plant Advanced Technologies PAT, c/o ENSAIA - INPL. France
A pilot study of antioxidant potential of endophytic fungi from some Sudanese medicinal plants
Afra Khiralla1,2,3, Ietidal Mohamed3, Justinne Thomas4, Benoît Mignard4, Rosella Spina1,2, Sakina Yagi3, Dominique Laurain-Mattar1,2*
1Université de Lorraine, SRSMC, UMR 7565, BP 70239, F-54506 Vandoeuvre-lès-Nancy, France
2CNRS, SRSMC, UMR 7565, BP 70239, F-54506 Vandoeuvre-lès-Nancy, France
3Botany Department, Faculty of Science, University of Khartoum, P.O. Box 321, Khartoum, Sudan
4Plant Advanced Technologies PAT, c/o ENSAIA - INPL. France
ARTICLE INFO
Article history:
in revised form 20 April 2015
Accepted 22 June 2015
Available online 20 July 2015
Sudanese medicinal plants
Endophytic fungi
Antioxidant activity
Total phenolic content
Objective: To evaluate the total phenolic content and total antioxidant capacity of ethyl acetate extracts of 21 endophytic fungi isolated from five Sudanese medicinal plants: Calotropis procera, Catharanthus roseus, Euphorbia prostrate, Vernonia amygdalina and Trigonella foenumgraecum. Methods: Crude extracts of endophytic fungi and their host plants were tested by classical Folin-Ciocalteu colorimetric method to determine the total phenolic content, also total antioxidant capacity was estimated using 1,1-diphenyl-2-picrylhydrazyl free radical scavenging in vitro method. Results: Among the endophytes, endophytic fungus Aspergillus sp. from Trigonella foenum-graecum seeds demonstrated the highest both total phenolic content in term of gallic acid equivalent (GAE) [(89.9±7.1 mg GAE/g)] and antioxidant activity for 1,1-diphenyl-2-picrylhydrazyl radical scavenging assay [IC50: (18.0±0.1 μg/ mL)]. A high positive linear correlation (R2=0.999 1) was found between total antioxidant capacity and total phenolic content of endophytic fungi isolated from Vernonia amygdalina. Conclusions: The present study revealed that some endophytic fungi from the five Sudanese medicinal plants could be a potential source of novel natural antioxidant compounds.
1. Introduction
Endophytes are organisms that colonize internal plant tissues without causing apparent harm to their host[1]. Endophytic fungi from medicinal plants are a potential antioxidant resource[2]. Vernonia amygdalina (V. amygdalina) Del. (Asteraceae), Calotropis procera (C. procera) Ait. (Asclepiadaceae), Catharanthus roseus (C. roseus) L. (Apocynaceae), Euphorbia prostrata (E. prostrata) Ait.(Euphorbiaceae), and Trigonella foenum-graecum (T. foenumgraecum) L. (Fabaceae) are medicinal plants that have several uses in Sudanese folk medicine. Their extracts have shown some biological activities including antiproliferative activity andantioxidant potential[3]. However, the endophytes mycoflora of these five plants have not been investigated. As part of our ongoing efforts towards finding novel antioxidant agents from natural resources we investigated, for the first time, total phenolic content and total antioxidant capacities of some endophytic fungi from these medicinal plants.
2. Materials and methods
Fresh leaves and stems of C. procera, C. roseus, E. prostrata,V. amygdalina were collected from Khartoum (15°38' N 32 °32' E) and T. foenum-graecum seeds were obtained from Khartoum local market. The plants were identified by Dr. Haider Abdalgadir, taxonomist in the Medicinal and Aromatic Plants Research Institute in Khartoum (Sudan).
Endophytic fungi were isolated from different parts of thecollected medicinal plants after surface sterilization as described by Zhang et al[4]. The sterilized pieces were cultivated on potato dextrose agar medium which was amended with chloramphenicol(500 mg/L) to suppress bacterial growth. The efficiency of the surface sterilization procedure was confirmed by plating the final rinse water. Furthermore, the endophytic fungi were subcultured in order to obtain pure cultures, numbered and reserved at 4 ℃. Identification of the fungal strains was based on the morphology of cultures or hyphae, the characteristics of the spores, and reproductive structures if the feature were discernible[5]. The cultures which failed to sporulate were grouped as mycelia sterilia[6].
Each fungal strain was cultivated on 20 petri dishes potato dextrose agar, and was incubated at 30 ℃ for 7-15 d. The solid fungal culture was crushed and extracted with ethyl acetate overnight, filtered,evaporated and preserved at 4 ℃.
Dry leaves and stems of V. amygdalina, C. procera, C. roseus,E. prostrata and seeds of T. foenum-graecum were ground into fine powder. Each sample (20 g) was extracted with ethyl acetate overnight, filtered, evaporated and stored at 4 ℃.
Total phenolic contents were determined using the Folin-Ciocalteu method as described by Wolfe et al[7]. The absorbance of the resulting was measured with spectrophotometer at 760 nm using a microtiter plate reader (Synergy HT Biotek, logiciel GEN5). Analysis was done in triplicate for each extract. Quantification was based on the standard curve of gallic acid. The results were expressed as gallic acid equivalent (GAE), ie., mg gallic acid/g.
Total antioxidant capacity (TAC) of the extracts was estimated using DPPH in vitro method as described by Yagi et al[8]. The absorbance was measured spectrophotometrically at 517 nm using a microtiter plate reader (Synergy HT Biotek, logiciel GEN5). Ascorbic acid was used as reference antioxidant compound. Each analysis was done in triplicate. The IC50value was calculated from the linear regression of plots of concentration of the test sample against the mean percentage of the antioxidant activity. Results were expressed as mean±SEM and the IC50values obtained from the regression plots (Sigma PlotsR 2001, SPSS Science) had a good coefficient of correlation,(R2=0.998).
3. Results
A total of 21 endophytic fungal strains were isolated from 5 Sudanese medicinal plants: three endophytic fungi from V. amygdalina, five from T. foenum-graecum, four from C. procera,five from C. roseus, and four from E. prostrata. The isolated fungal strains were classified into 12 different taxa (Table 1). Ten strains belong to Ascomycetes, whereas seven strains belong to fungal class Deuteromycetes, four strains were failed to sporulate and were grouped as mycelia sterilia. This group of fungi is a common problem concerning the identification of endophytic fungi[9].
TPC of ethyl acetate crude extracts of 21 endophytes and different parts of their host plants were estimated using the classical Folin-Ciocalteu colorimetric method as shown in (Figure 1). It was found that the five medicinal plants contained TPC values ranged from(0.5±0.1) (T. foenum-graecum seeds extract) to (32.7±2.9) mg GAE/g (V. amygdalina stem extract). TPC values of 21 endophytes revealed variations ranged from (13.6±1.0) to (89.9±7.1) mg GAE/ g. Two Aspergillus spp. of both C. procera and T. foenum-graecum showed the highest TPC values [(77.2±7.5) and (89.9±7.1) mg GAE/ g respectively].
The antioxidant potential using DPPH radical scavenging assay was investigated for the 21 ethyl acetate extracts of endophytic fungi and their medicinal host plants. Table 2 showed that TAC IC50values of the medicinal host plants ranged from (50.0±1.7) μg/mL(V. amygdalina stem) to no activity (T. foenum-graecum seeds). The endophyte extracts revealed extremely wide range of IC50values,from (18.0±0.1) μg/mL for Aspergillus sp. isolated from T. foenumgraecum to (2 686.0±51.7) μg/mL for Phoma sp. in C. roseus.
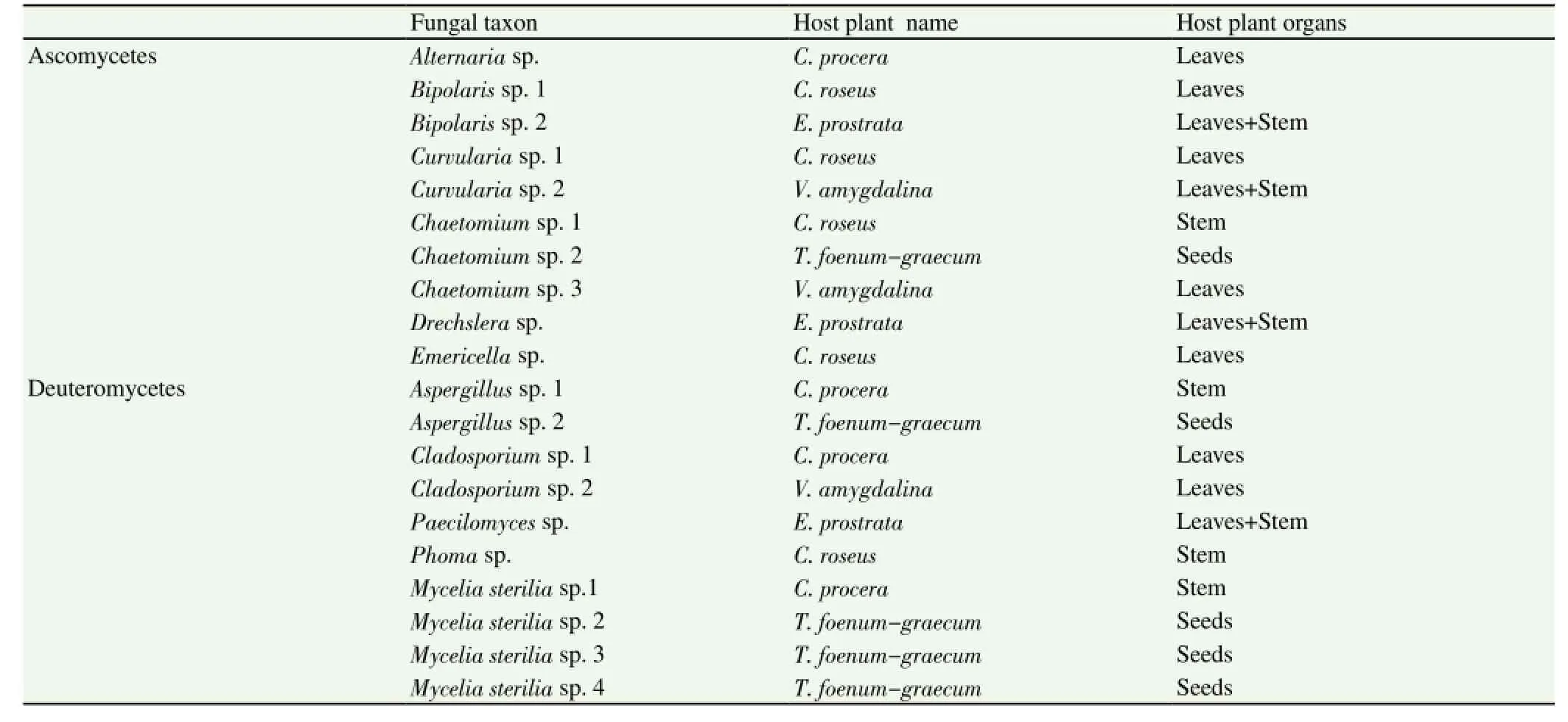
Table 1 Taxonomic identification of the endophytic fungi isolated from various organs of V. amygdalina, C. procera, C. roseus, E. prostrata (stems, leaves) and T. foenum-graecum (seeds).

Table 2 IC50values of 21 endophytic fungi and their host plants by DPPH radical scavenging assay (mean±SD).
A high positive linear correlation (R2=0.999 1) was found between TAC and TPC of endophytic fungi isolated from V. amygdalina. Endophytic fungi isolated from T. foenum-graecum and C. procera showed moderate correlation (R2=0.680 8 and 0.515 6, respectively).
4. Discussion
The majority of the fungal genera isolated from the Sudanese plants were common endophytes (Alternaria, Cladosporium, Phoma,Chaetomium, Drechslera, Curvularia, Bipolaris, Paecilomyces,Emericella and Aspergillus). However Ulocladium were reported only few times as endophytes[10]. The low diversity of the endophytes of the Sudanese medicinal plants may be due to the climate where it is extremely arid for most of the year with about nine months with average rainfall lower than five mm. Some authors[11] reported that a significant variation was detected in the colonization frequency of endophytic species in relation with the environmental factors such as rainfall and atmospheric humidity.
Ethyl acetate is selective solvent which extract low and high molecular weight polyphenols. Despite the high TAC of stems and leaves of V. amygdalina IC50: (50.0±1.7) μg/mL and (63.0±1.8 respectively), their endophyte extracts showed low TAC [IC50:(252.0±5.1) to (480.0±3.9) μg/mL]. In contrario the seed extract of T. foenum-graecum had no antioxidant activity while Aspergillus sp. 2, isolated from the seeds, showed significant TAC [(18.0±0.1)μg/mL]. These results indicated that no correlation between the TACs of the endophytes and the host plants can be established. The main factor is the fungal genus, indeed Aspergillus spp. were recorded the highest TAC. The highest TAC and TPC were obtained with Aspergillus spp. extracts isolated from both C. procera [IC50:(58.0±4.0) μg/mL, TPC: (77.2±7.5) mg GAE/g] and T. foenumgraecum [IC50: (18.0±0.1) μg/mL, TPC: (89.9±7.1) mg GAE/g]. It is noted that crude extract of T. foenum-graecum seeds from Sudanrevealed no antioxidant activity that could be explained by the low concentration of TPC (0.5±0.1) mg GAE/g. In contrario previous works reported that seed ethyl acetate crude extract of T. foenumgraecum demonstrated strong antioxidant activity in relation with high phenolic content (106.316 mg GAE/g)[12].
In conclusion, in this study we investigated the diversity of endophytic fungi of 5 Sudanese medicinal plants. The 21 endophytes were identified and classified. Mycelia sterilia, and Chaetomium, were the dominant fungal taxa isolated. The endophyte diversity was poor in comparison with the results obtained with plants growing in other countries. Our findings revealed the first report on endophytic fungi of 5 Sudanese medicinal plants. Some of them were worthy with phenolic compounds and may serve as potential source of natural antioxidants. The Aspergillus sp. endophyte of T. foenum-graecum was revealed significant antioxidant activity alongside this strain was rich with phenolic compounds, this fungus strain is recommended for further investigations.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The authors thankfully acknowledge the CNRS and the Ministère de l'Enseignement Supérieur, for the financial support.
[1] Arnold AE. Understanding the diversity of foliar endophytic fungi: progress, challenges, and frontiers. Fungal Biol Rev 2007; 21(2-3):51-66.
[2] Huang W, Cai Y, Xing J, Corke H. A Potential antioxidant resource:endophytic from medicinal plant. Econ Bot 2007; 61(1): 14-30.
[3] El Ghazali GE. The promising medicinal plants of the Sudan. National Council for Research: Khartoum University Press; 1997.
[4] Zhang P, Zhou P, Yu L. An endophytic taxol-producing fungus from Taxus media, Cladosporium cladosporioides MD2. Curr Microbiol 2009;59: 227-232.
[5] Watanabe T. Pictorial atlas of soil and seed fungi: Morphologies of cultured fungi and key to species. 2nd ed. Washington, D.C: CRC Press LLC; 2002
[6] Promputtha I, Jeewon R, Lumyong S, Mckenzie EHC, Hyde KD. Ribosomal DNA fingerprinting in the identification of non sporulating edophytes from Magnolia liliifera (Magnoliaceae). Fungal Divers 2005;20: 167-186.
[7] Wolfe K, Wu X, Liu, RH. Antioxidant activity of apple peels. J Agr Food Chem 2003; 51: 609-614.
[8] Yagi S, Drouart N, Bourgaud F, Henry M, Chapleur Y , Laurain-Mattar D. Antioxidant and antiglycation properties of Hydnora johannis roots. S Afr J Bot 2012; 84: 124-127.
[9] Huang WY, Cai YZ, Hyde KD, Corke H, Sun M. Biodiversity of endophytic fungi associated with 29 traditional Chinese medicinal plants. Fungal Divers 2008; 33: 61-75.
[10] Marquez SS, Bills GF, Acuna, LD, Zabalgogeazcoa I. Endophytic mycobiota of leaves and roots of the grass Holcus lanatus. Fungal Divers 2010; 41: 115-123.
[11] Selvanathan S, Indrakumar I, Johnpaul M. Biodiversity of the endophytic fungi isolated from Calotropis gigantean (L) R. Br. Rec Res Sci Tech 2011;3(4): 94-100.
[12] Kenny O, Smyth TJ, Hewage CM, Brunton NP. Antioxidant properties and quantitative UPLC-MS analysis of phenolic compounds from extracts of fenugreek (Trigonella foenum-graecum) seeds and bitter melon (Momordica charantia) fruit. Food Chem 2013; 141: 4295-4302.
in 13 February 2015
Pr. Dominique Laurain-Mattar, Université de Lorraine,SRSMC, UMR 7565, BP 70239, F-54506 Vandoeuvre-lès-Nancy, France; CNRS,SRSMC, UMR 7565, BP 70239, F-54506 Vandoeuvre-lès-Nancy, France.
Tel: +33 3 83 68 21 80
E-mail: dominique.mattar@univ-lorraine.fr
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Diuretic and antioxidant activities of the aqueous extract of leaves of Cassia occidentalis (Linn.) in rats
- Chemical composition, mechanism of antibacterial action and antioxidant activity of leaf essential oil of Forsythia koreana deciduous shrub
- Prevalence of filarial parasites in domestic and stray cats in Selangor State, Malaysia
- Genetic variation of Leptospira isolated from rats catched in Yogyakarta Indonesia
- Molecular survey of tick-borne pathogens in Ixodid ticks collected from hunted wild animals in Tuscany, Italy
- Epidemiology of influenza viruses from 2009-2013-A sentinel surveillance report from Union territory of Puducherry, India
