Molecular survey of tick-borne pathogens in Ixodid ticks collected from hunted wild animals in Tuscany, Italy
2015-10-28ValentinaVirginiaEbaniFabrizioBertelloniBarbaraTurchiDarioFilogariDomenicoCerri
Valentina Virginia Ebani, Fabrizio Bertelloni, Barbara Turchi, Dario Filogari, Domenico Cerri
Department of Veterinary Science, University of Pisa, Pisa, Italy
Molecular survey of tick-borne pathogens in Ixodid ticks collected from hunted wild animals in Tuscany, Italy
Valentina Virginia Ebani*, Fabrizio Bertelloni, Barbara Turchi, Dario Filogari, Domenico Cerri
Department of Veterinary Science, University of Pisa, Pisa, Italy
ARTICLE INFO
Article history:
in revised form 21 April 2015
Accepted 22 June 2015
Available online 20 July 2015
Ixodid ticks
Tick-borne bacteria
Wild animals
PCR
Hunters
Objective: To determine the prevalence of zoonotic tick-borne bacteria in feeding ticks removed from hunted wild animals. Methods: PCR was executed on DNA extracted from 77 tick pools to detect Anaplasma phagocytophilum, Bartonella spp., Borrelia burgdorferi sensu lato, Coxiella burnetii and Rickettsia spp. Results: A total of 432 ticks were collected: 30 (6.94%)Haemaphysalis punctata, 72 (16.7%) Dermacentor marginatus and 330 (76.38%) Ixodes ricinus. For each animal one or two pools of 3 ticks of the same species was constituted. Seventy-seven tick pools were examined by PCR: 58 (75.32%) resulted infected and among them 14 (18.18%)showed co-infections. In particular, 29 (37.66%) pools were positive for Bartonella spp., 23(29.87%) for Anaplasma phagocytophilum, 16 (20.78%) for Rickettsia spp., and 5 (6.49%) for Borrelia burgdorferi s.l. All samples were negative for Coxiella burnetii. Conclusions: The results demonstrate the presence of several zoonotic tick-borne pathogens in the studied area,and underline the risk of exposure to infections for hunters not only during the outdoor activity,but also when they manipulate hunted animals infested by infected ticks.
1. Introduction
Tuscany is a region in central Italy characterized by the presence of wide mountain, hill and plain areas, rich in vegetation, that are frequently used for recreational activity, mainly hunting, mushrooms and chestnuts collecting, and walking.
These areas are home to several species of wild animals that are frequently infested by ticks belonging to different species. The sheep tick Ixodes ricinus (I. ricinus) is the most common hard tick species found in these areas, as well as in the rest of Italy, but Dermacentor sp., [Dermacentor marginatus (D. marginatus)], Hyalomma sp.,Haemaphysalis sp., Rhipicephalus sp. are present too.
These hematophagous arthropods are often vectors of several tickborne agents. Among them, some bacteria are able to infect wild and domestic animals, and humans in which may determine severeclinical forms. Lyme disease by Borrelia burgdorferi (B. burgdorferi)sensu lato spirochetes, granulocytic anaplasmosis by Anaplasma phagocytophilum (A. phagocytophilum), and rickettsiosis due to rickettsiae belonging to the Spotted Fever Group are well known in human medicine[1].
Bartonellosis is a zoonotic vector-borne disease, that is traditionally associated to Bartonella henselae, etiologic agent of the Cat Scratch Disease. However, the genus Bartonella includes several species able to infect humans[2].
Coxiella burnetii (C. burnetii), responsible for Q Fever, is transmitted through ingestion of contaminated food, mainly dairy products, or inhalation of infected aerosol, but infection due to infected ticks is possible, mainly in areas where the arthropod population is abundant[3].
In Italy, investigations on the prevalence of tick-transmitted pathogens have been previously conducted testing ticks collected in wild and urban/periurban habitats[4]. Hunters are at high risk of exposure to tick bites, because they frequent habitat infested by arthropods, but they can be attached by ticks also during manipulation of the carcasses of hunted animals.
The aim of the present study was to determine the prevalence of the main zoonotic bacterial agents transmitted by hematophagous arthropods, in particular A. phagocytophilum, Bartonella spp., B. burgdorferi s.l., C. burnetii and Rickettsia spp., in ticks removed from hunted wild animals living in mountain and hilly areas of Tuscany,central Italy, frequented by visitors for hunting and other recreational activities.
2. Materials and methods
2.1. Study sites and sampling
Feeding ticks were collected from 72 wild animals,in particular 30 fallow deer (Dama dama), 29 red deer (Cervus elaphus), 7 wild boars(Sus scrofa), and 6 roe deer (Capreolus capreolus).
The animals lived in mountain and hilly areas of Tuscany, rich of mixed forests mainly composed by beech (Fagus sylvatica), chestnut(Castanea sativa), fir (Abies alba), oak (Quercus ilex and Quercus robur). Several different bird species live usually in these areas, as well as various large and small mammals such as roe deer, fallow deer, red deer, wild boar, fox (Vulpes vulpes), wolf (Canis lupus), hare(Lepus lepus), marmot (Marmota marmota), European badgers (Meles meles), hedgehog (Erinaceus europaeus), also different rodent species can be found there. Some zones of these areas host farm animals, in particular horses employed for trekking, cattle and sheep. Dogs with their owners are frequently present for hunting activity.
Only adult (male and female) ticks were collected. Six ticks were removed from each animals and placed into sterile tubes. Samples were transferred to the laboratory and kept frozen at -20 ℃. All ectoparasites were stored on ice during the identification procedure,which was done on the basis of their morphologic features by using standard taxonomic keys[5].
2.2. Molecular analysis
2.2.1. DNA extraction
DNA was extracted from each pool constituted by 3 adult ticks belonging to the same species and collected from the same animal. All tick pools were disinfected by immersion into a 70% ethanol solution for 5 min, and then rinsed with sterile phosphate buffered saline. Successively each tick was cut with sterile scalpel and 100 μL of sterile phosphate buffered saline and 20 μL of proteinase K were added.
DNA was extracted using the DNeasy Tissue kit (Qiagen GmbH,Hilden, Germany) according to the manufacturer's instructions and stored at 4 ℃ until used as template for PCR assays.
2.2.2. Polymerase chain reaction
PCR assays to detect DNA of A. phagocytophilum, Bartonella spp., B. burgdorferi s.l., C. burnetii, Rickettsia spp. were performed in thermal cycler (Gene-Amp PCR System 2700, Perkin-Elmer,Norwalk, Connecticut, USA) using the EconoTaq PLUS 2 ×Master Mix (Lucigen Corporation, Middleton, Wiskonsin, USA).
Sterile distilled water instead of DNA was included as negative control to ensure the absence of contamination in each reaction mixture. Genomic DNA, obtained from immunofluorescent slides(Fuller Laboratories Fullerton, California, USA) for each pathogen,was used as positive control.
A. phagocytophilum: A nested PCR was carried out using the primers GE3a and GE10r for the primary reaction which amplifies a 932 bp fragment of the 16S rRNA gene of A. phagocytophilum, and the primers GE9f and GE2 for the secondary assay, which amplified a 546 bp fragment of the same gene[6].
Bartonella spp.: DNA samples were employed in a PCR assay to identify the Bartonella genus. The primers p24E and p12B,previously described by Relman et al (1990)[7], were used in this protocol to amplify a 296 bp fragment of the Bartonella 16S rRNA gene.
B. burgdorferi s.l.: Primers JS1 and JS2 were used to amplify a 261 bp fragment of the 23S rRNA gene of B. burgdorferi s.l. [8].
C. burnetii: C. burnetii was identified by amplifying a 687 bp fragment of the IS1111a gene using primers Trans-1 and Trans-2 as described by Berri et al (2009)[3].
Rickettsia spp.: PCR with the primers Rr190.70p and Rr190.701 were carried out to amplify a 632 bp fragment of the gene encoding the outer surface protein ompA of Rickettsia spp. as described by Roux et al (1996)[9]. Since this protocol does not allow detecting Rickettsia helvetica, Rickettsia akari, Rickettsia australis, and Rickettsia bellii, a second PCR assay was performed using the primers RpCS.877p and RpCS.1258n which amplify a 381 bp fragment of the gltA gene[10].
All the amplification products were analyzed by electrophoresis on 1.5% agarose gel at 100 V for 45 min; gel was stained with ethidium bromide and observed. GelPilot 100 bp Plus Ladder (Qiagen) was used as DNA marker.
2.2.3. Sequencing
Bartonella spp. and Rickettsia spp. amplicons were sent to PRIMM(Milano, Italy) for DNA sequencing; the nucleotide sequences were compared with those present in GenBank database using the basic local alignment search tool (BLAST).
3. Results
A total of 432 ticks were collected and resulted belonging to the species Haemaphysalis punctata (H. punctata) (n ticks=30, 6.94%),D. marginatus (n=72, 16.7%) and I. ricinus (n=330, 76.38%). For each animal one pool was constituted with 3 ticks of the same species. For five animals two pools were obtained. A total of 77 tick pools (10 for H. punctata, 12 for D. marginatus, 55 for I. ricinus) was examined by PCR. Fifty-eight (75.32%) pools resulted infected, and among them 14 (18.18%) showed co-infections (Table 1).
A. phagocytophilum was detected in 23 (29.87%) tick pools. More in detail, the DNA was found in 22 I. ricinus pools collected from 11fallow deer, 8 red deer, 2 roe deer, and 1 H. punctata pool collected from a fallow deer.
Twenty-nine (37.66%) tick pools were positive to amplification of 16S rRNA gene of Bartonella genus. Sequencing analysis identified 2 (2.6%) Bartonella acomydis, 4 (5.2%) Bartonella bacilliformis, 10(12.98%) Bartonella bovis (B. bovis), 9 (11.68%) Bartonella chomelii(B. chomelii), 2 (2.6%) Bartonella tribocorum, 2 (2.6%) Bartonella vinsonii subsp. berkoffii (B. vinsonii berkoffii). Table 2 shows the tick species in which Bartonella DNA was found and the animals from which ticks were collected.
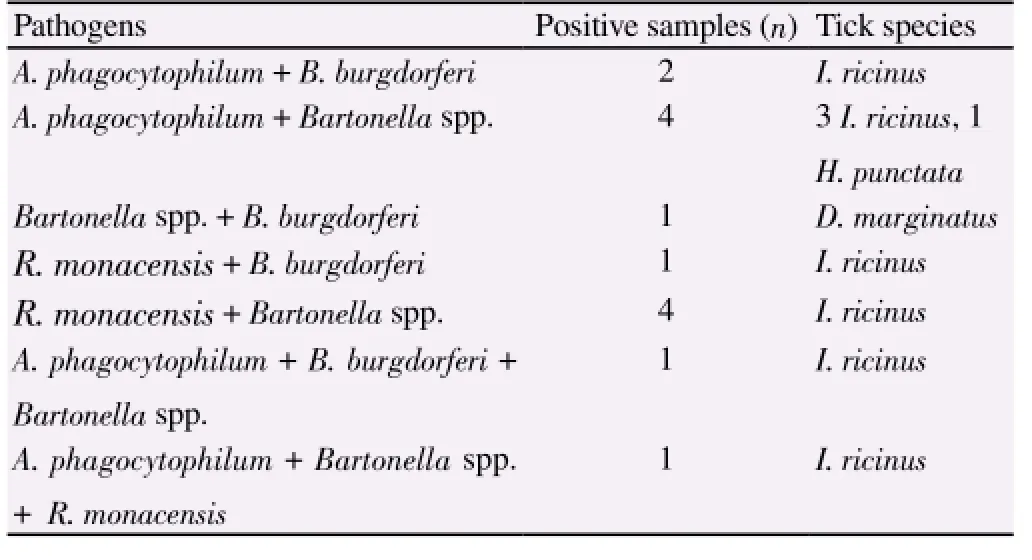
Table 1 Co-infections detected in the examined tick pools.
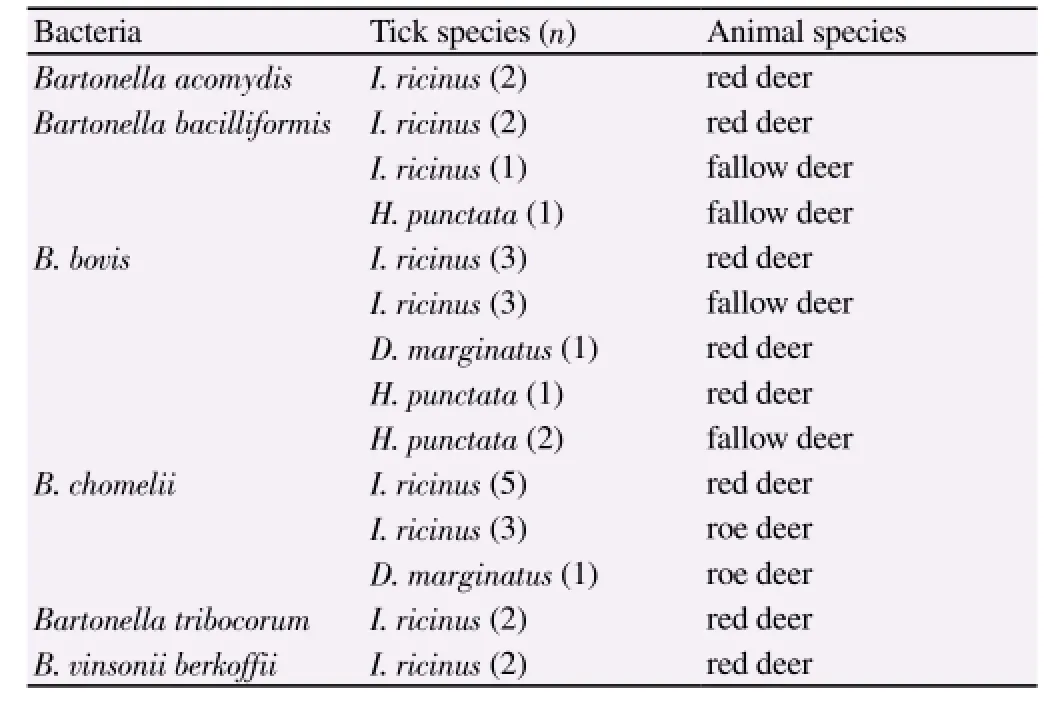
Table 2 Bartonella species detected in tick pools in relation to the animal source.

Table 3 Rickettsia species detected in tick pools in relation to the animal source.
B. burgdorferi s.l. DNA was found in 5 (6.49%) tick pools: 4 I. ricinus pools from fallow deer, and 1 D. marginatus pool from a red deer. All tick pools resulted negative for C. burnetii.
Rickettsia infection was detected in 16 (20.78%) samples; in particular Rickettsia monacensis (R. monacensis) was found in 14(18.18%) pools, and Rickettsia slovaca (R. slovaca) in 2 (2.6%). Positive tick species and animal source are reported in Table 3.
4. Discussion
The present study has been conducted on ticks collected from wild animals captured by hunters in areas largely frequented for hunting and other recreational activities. Among the tick species found, I. ricinus was the most frequently detected (76.38%), confirming its wide distribution in wild environment. A total of 77 tick pools were examined by PCR and a high percentage (75.32%) of positive results was obtained. Dual and triple infections were recognized in 18.18% of cases, suggesting the potential risk of multiple infections from a single tick bite.
A relevant prevalence (37.66%) for Bartonellosis was observed. A recent study conducted in questing ticks collected in an urban park in Rome, Italy, has not found Bartonella spp.[4], but other investigations carried out in Italy and in the rest of Europe have detected Bartonella spp. DNA in hematophagous arthropods[11,12].
B. bovis and B. chomelii were present in the highest number of the examined tick pools. Cattle constitute the reservoir of these species that usually do not cause clinical signs. However, B. bovis was identified as a cause of bovine endocarditis and long lasting bacteremia. In Europe, B. bovis infections in cattle have been reported in France[13,14], Italy[15] and Poland[16], whereas B. chomelii is considered the most frequent species infecting cattle in Spain[17]. The pathogenic role of the other detected Bartonella species has not been fully determined, however all bartonellae are considered potential pathogens for animals and humans[2].
A. phagocytophilum was found in 29.87% of samples. This is a predictable result, because this pathogen has been frequently detected in central Italy that is considered an endemic area. Moreover, the obtained results confirm that I. ricinus is one of the most important vectors of this pathogen in Europe, and wild ruminants represent its reservoirs[18].
The 20.78% of examined tick pools were positive for Rickettsia spp. In particular, two species belonging to the Spotted Fever Group were found: R. monacensis and R. slovaca.
R. monacensis is distributed all over Europe and vector mainly by I. ricinus, as demonstrated by our survey too. The prevalence of tick infection reaches 34.6% in some zones of Europe. Human cases with a clinical picture similar to that caused by the Mediterranean Spotted Fever by Rickettsia conorii, have been associated to R. monacensis[19]. R. slovaca, which causes a human clinical syndrome named tickborne lymphadenopathy or Dermacentor-borne necrosis-erythemalymphadenopathy characterized by inoculation eschar on the scalp and enlarged cervical lymph nodes[20], has been detected in D. marginatus and H. punctata ticks collected from herbivores in Sicily,south Italy[21], and in ticks D. marginatus removed from humans in Tuscany[22]. In this study, R. slovaca DNA was found in 2 pools of D. marginatus collected from red deer, confirming this tick as the main vector.
C. burnetii has not been detected during the present investigation. This result could mean that the Q fever agent was not present in the geographic areas in which ticks had been sampled, or that, despite the presence, it was not spread in arthropod population. However,other recent studies carried out in ticks and animals from centralItaly have found C. burnetii DNA, suggesting that the pathogen is circulating in this geographic area[4,23].
In Italy, the reported B. burgdorferi s.l. prevalence in questing ticks varies considerably among the study areas: from 1.3% to 40.0% in northern Italy, from 8.7% to 30.0% in central Italy[4]. Different results are related to differences in environmental features, climatic conditions and collected tick samples (number, species, and stage). In our study, in which only adult ticks directly collected from hunted animals were tested, 6.49% prevalence was found. This value is not high, but still confirms the circulation of B. burgdorferi s.l. in the studied geographic area.
In conclusion, findings of the present survey underline the potential risk of transmission of zoonotic tick-borne pathogens to humans,which for recreational or work activities frequent rural and forestry environments. Hunters are generally exposed to risk of tick bites during their outdoor activity, but in particular when they manipulate the carcasses of hunted animals. In fact, ticks quite rapidly leave the killed animals going to the surrounding environment and on present people.
The circulation of arthropod-borne pathogens is not only of public health concern, but also of veterinary interest. In fact, several tickborne bacteria are able to infect domestic animals (horse, cattle,dogs), which share the same environment with wild animals, causing diseases varying from asymptomatic to severe forms.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The present research was carried out with funds from the University of Pisa.
[1] Parola P, Raoult D. Ticks and tick-borne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis 2001; 32: 897-928.
[2] Breitschwerdt EB. Bartonellosis: one health perspectives for an emerging infectious diseases. ILAR J 2014; 55: 46-58.
[3] Berri M, Rekiki A, Boumedine A, Rodolakis A. Simultaneous differential detection of Chlamydophila abortus, Chlamydophila pecorum, and Coxiella burnetii from aborted ruminant's clinical samples using multiplex PCR. BMC Microbiol 2009; 9: 130.
[4] Mancini F, Di Luca M, Toma L, Vescio F, Bianchi R, Khoury C, et al. Prevalence of tick-borne pathogens in an urban park in Rome, Italy. Ann Agric Environ Med 2014; 21 (4): 723-727.
[5] Kolonin GV. Fauna of Ixodid ticks of the world (Acari, Ixodidae).[Online]. Available from: http://www.kolonin.org/ [Accessed on 2009].
[6] Massung RF, Slater K, Owens JH, Nicholson WL, Mather TN, Solberg VB, et al. Nested PCR assay for detection of granulocytic Ehrlichiae. J Clin Microbiol 1998; 36: 1090-1095.
[7] Relman DA, Loutit SJ, Schmidt TM, Falkow S, Tompkins S. The agent of bacillary angiomatosis. An approach to the identification of uncultured pathogens. N Engl J Med 1990; 323: 1573-1580.
[8] Chang YF, Novosol V, McDonough SP, Chang CF, Jacobson RH, Divers T, et al. Experimental infection of ponies with Borrelia burgdorferi by exposure to Ixodid ticks. Vet Pathol 2000; 37: 68-76.
[9] Roux V, Fournier PE, Raoult D. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J Clin Microbiol 1996; 34: 2058-2065.
[10] Regnery RL, Spruill CL, Plikaytis BD. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol 1991; 173: 1576-1589.
[11] Angelakis E, Billeter SA, Breitschwerdt EB, Chomel BB, Raoult D. Potential for tick-borne bartonellosis. Emerg Infect Dis 2010; 16: 385-391.
[12] Corrain R, Drigo M, Fenati M, Menandro ML, Mondin A, Pasotto D,et al. Study on ticks and tick-borne zoonoses in public parks in Italy. Zoonoses Public Health 2012; 6: 468-476.
[13] Bermond D, Boulouis HJ, Heller R, Van Laere G, Monteil H, Chomel BB, et al. Bartonella bovis and Bartonella capreoli sp. nov., isolated from European ruminats. Int J Syst Evol Microbiol 2002; 52: 383-390.
[14] Maillard R, Petit E, Chomel B, Lacroux C, Schelcher F, Vayssier-Taussat M, et al. Endocarditis in cattle caused by Bartonella bovis. Emerg Infect Dis 2007; 13: 1383-1385.
[15] Martini M, Menandro ML, Mondin A, Pasotto D, Mazzariol S, Lauszi S,et al. Detection of Bartonella bovis in a cattle herd in Italy. Vet Rec 2008;162: 58-59.
[16] Welc-Fal ciak R, Grono K. The first cases of Bartonella bovis infection in cattle from Central Europe. Vet Microbiol 2013; 162: 954-956.
[17] Antequera-Gomez ML, Lozano-Almendral L, Barandika JF, Gonzales-Martin-Nino RM, Rodriguez-Moreno I, Garcia-Perez AL, Gil H. Bartonella chomelii is the most frequent species infecting cattle grazing in communal mountain pastures in Spain. Appl Environ Microbiol 2015; 81:623-629.
[18] Stuen S, Granquist EG, Silaghi C. Anaplasma phagocytophilum - a wide spread multi-host pathogen with highly adaptive strategies. Front Cell Infect Microbiol 2013; 3: 1-33.
[19] Madeddu G, Mancini F, Caddeo A, Ciervo A, Babudieri S, Maida I, et al. Rickettsia monacensis as cause of Meditterranean Spotted Fever-like illness, Italy. Emer Infect Dis 2012; 18: 702-703.
[20] Oteo JA, Portillo A. Tick-borne rickettsioses in Europe. Ticks Tick Borne Dis 2012; 3: 270-277.
[21] Beninati T, Genchi C, Torina A, Caracappa S, Bandi C, Lo N. Rickettsiae in ixodid ticks, Sicily. Emerg Infect Dis 2005; 11: 509-510.
[22] Selmi M, Bertolotti L, Tomassone L, Mannelli A. Rickettsia slovaca in Dermacentor marginatus and tick-borne lympadenopathy, Tuscany, Italy. Emerg Infect Dis 2008; 14: 817-820.
[23] Ebani VV, Nardoni S, Fognani G, Mugnaini L, Bertelloni F, Rocchigiani G, et al. Molecular detection of vector-borne bacteria and protozoa in healthy hunting dogs from Central Italy. Asian Pac J Trop Biomed 2015;5(2): 108-112.
in 23 February 2015
Valentina Virginia Ebani, DVM, PhD, Department of Veterinary Science, Viale delle Piagge, Pisa, Italy.
Tel: 00390502216968
Fax: 00390502216941
E-mail: valentina.virginia.ebani@unipi.it
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Effect of CXCR4 pretreated with ultrasound-exposed microbubbles on accelerating homing of bone marrow mesenchymal stem cells to ischemic myocardium in AMI rats
- Correlation between microRNA-21 and expression of Th17 and Treg cells in microenvironment of rats with hepatocellular carcinoma
- Anti-proliferation and radiosensitization effects of chitooligosaccharides on human lung cancer line HepG2
- Clinical significance of microRNA-130b in osteosarcoma and its role in cell growth and invasion
- Effect of microRNA-208a on mitochondrial apoptosis of cardiomyocytes of neonatal rats
- Comparative analysis of biological characteristics of adult mesenchymal stem cells with different tissue origins
