Chemical composition, mechanism of antibacterial action and antioxidant activity of leaf essential oil of Forsythia koreana deciduous shrub
2015-10-28XiaoNanYangImranKhanSunChulKang
Xiao-Nan Yang, Imran Khan, Sun Chul Kang
Department of Biotechnology, College of Engineering, Daegu University Kyungsan, Kyungbuk 712-714, Republic of Korea
Chemical composition, mechanism of antibacterial action and antioxidant activity of leaf essential oil of Forsythia koreana deciduous shrub
Xiao-Nan Yang, Imran Khan, Sun Chul Kang*
Department of Biotechnology, College of Engineering, Daegu University Kyungsan, Kyungbuk 712-714, Republic of Korea
ARTICLE INFO
Article history:
in revised form 20 July 2015
Accepted 15 August 2015
Available online 20 September 2015
Antibacterial activity
Antioxidant activity
Cell material released
Essential oil
GC-MS
Potassium ion flux
Objective: To identify the chemical constituents of leaf essential oil of Forsythia koreana(F. koreana) and evaluate its effects on bacterial strains. Methods: The essential oil of leaf of F. koreana was extracted by using hydrodistillation process and the volatile components investigated with the help of gas chromatography coupled with mass spectrometry. The antibacterial study was carried out with the help of agar disc diffusion method, MIC, MBC and viable count. The mode of action was determined with help of potassium ion flux, cellular material release and scanning electron microscopy. The antioxidant activity was determined with the help of 2, 3-diphenyl-2-picrylhydrazyl method, nitric oxide scavenging activity and superoxide anion radical scavenging assay. Results: Total ten compounds were identified as trans-phytol (42.73%), cis-3-hexenol (12.95%), β-linalool (10.68%), trans-2-hexenal(8.86%), trans-2-hexenol (8.86%), myrcenol (3.86%), 4-vinylphenyl acetate (3.86%), (4Z)-4,6-heptadien-1-ol (3.18%), lemonol (2.73%) and benzeneacetaldehyde (2.27%) by gas chromatography coupled with mass spectrometry. The antibacterial study was demonstrated that leaf essential oil of F. koreana act against foodborne and other pathogenic bacteria. The mode of action revealed that this essential oil acted on the cytoplasmic membrane, resulting in loss of integrity and increased permeability. In addition, leaf essential oil of F. koreana was shown to be rich in linalool, which contributes to improved antioxidant activity. Conclusions:These results show that leaf essential oil of F. koreana has great potential as a natural food preservative, antibacterial and antioxidant agent.
1. Introduction
Foodborne diseases include a series of illnesses and are a growing public health problem worldwide. For example, the Grampositive bacterium Staphylococcus aureus (S. aureus) is mainly responsible for post-operative wound infections, toxic shock syndrome, endocarditis, osteomyelitis, and food poisoning[1,2]. Listeria monocytogenes (L. monocytogenes) is responsible for severefoodborne illness listeriosis, which is recognized as one of the emerging zoonotic diseases over the last two decades[3]. The Gram-negative bacteria Escherichia coli (E. coli) is present in the human intestine and causes urinary tract infection, coleocystitis,or septicemia[4]. Often, chemical preservatives such as synthetic antimicrobial agents are used in the food industry to prevent growth of foodborne and food-spoiling microbes. However, concerns about the safety of these chemicals have increased along with consumer demands for naturally processed foods. Currently, there is growing interest in natural antibacterial materials, such as extracts of herbs and spices, for the preservation of foods. Plant-derived essential oils of various species of edible and medicinal plants, herbs, and spices have long been used as natural agents for food preservation in thefood industry due to the presence of antimicrobial compounds[5]. In general, plant-derived essential oils are considered as non-phytotoxic compounds and are potentially effective against microorganisms. In this context, identification and evaluation of natural products to control food pathogens and assure a safe, wholesome, and nutritious food supply has become an important international challenge.
Plant-derived essential oils are composed of various chemical components, each of which has its own significant activity. Plantderived essential oils can be obtained from various plants parts,including the flower, seeds, buds, leaves, twigs, woods, fruits,and roots. Activities of plant-derived essential oils can vary from one strain to another[6]. These essential oils have antimicrobial[7],antioxidant[8,9], antimycotic[10], antiviral[11], antiparasitic[12,13],antitoxicogenic[14,15] and insecticidal[16,17] properties. Each component present in essential oils is directly related to the properties mentioned above.
Forsythia is a genus of flowering plants in the family Oleaceae as a deciduous shrub. The species Forsythia koreana (F. koreana) is widely cultivated in South Korea and blooms in the months of March to April with vast yields of flowers. Fruits of F. koreana have been investigated and are known to have anti-inflammatory, diuretic and dampheat-clearing effects[18]. However, there are no phytochemical and biological studies on leaves of F. koreana so far. Regardless of the considerable data on fruit oils of the Forsythia genus from different regions, there is a lack of information on the chemical compositions and bioactivities of other parts of F. koreana planted in South Korea. Therefore, the aim of the present investigation was to analyze the chemical composition and antibacterial activity of leaf essential oil of F. koreana.
2. Materials and methods
2.1. Chemicals and reagents
1,2-diphenyl-2-picrylhedrazyl (DPPH), sulfanilamide, naphthalenediaminedihydrochloride, nitroferrycyanide, (Ⅲ) dehydrate, xanthine oxidase, and Folin-Ciocalteau's phenol reagent were obtained from Sigma-Aldrich (St. Louis, MO USA). Nitro-blue tetrazolium was purchased from fluka (Buchs, Switzerland). All other chemicals and solvents were of the highest commercial grade.
2.2. Plant materials
Leaves of F. koreana were collected from the campus of Daegu University, Kyungsan, Kyungbuk, South Korea. The plant was identified by its morphological features and database present in the library at the University. Euchre specimen (DU-FK968-L) was preserved in Daegu University for further reference.
2.3. Isolation of leaf essential oil
Fresh leaves of F. koreana were subjected to hydrodistillation for 4 h using a modified clevenger type apparatus. The resulting oil-water mixture was then extracted using dichloromethane. The organic layer was then separated, dried over anhydrous Na2SO4, filtered, and the solvent volatilized. The resulting yellowish oil was preserved in a sealed vial at 4 ℃ in the dark until further analysis.
2.4. Microbial strains (Food spoiling and foodborne pathogens)
A panel of foodborne pathogenic bacteria, including Salmonella enteritidis (S. enteritidis) KCTC 12243, E. coli ATCC 8739, S. aureus ATCC 6538, L. monocytogenes ATCC 19118, L. monocytogenes ATCC 19111, L. monocytogenes ATCC 19166, L. monocytogenes ATCC 19116, and L. monocytogenes ATCC 10943 were used in this study. Bacterial cultures were revived on Luria-Bertani (LB) Agar plates and then transferred into LB broth medium maintained at 37 ℃ for overnight.
2.5. Gas chromatography-mass spectrometry (GC-MS)analysis
Quantitative and qualitative analyses of essential oil was performed using a GC-MS (Model QP 2010, Shimadzu, Japan) equipped with a ZB-1 MS fused silica capillary column (30 m×0.25 mmi. d., film thickness 0.25 μm). For GC-MS detection, an electron ionization system with ionization energy of 70 eV was used. Helium gas was used as a carrier gas at a constant flow rate of 1 mL/min. Injector and mass transfer line temperatures were set at 220 and 290 ℃, respectively. The oven temperature was programmed from 50 ℃to 150 ℃ at a rate of 3 ℃/min, held for 10 min, and finally raised to 250 ℃ at a rate of 10 ℃/min. Diluted sample (1/100, v/ v, in dichloromethane) of 1 μL was manually injected in split less mode. The relative percentages of oil constituents were expressed as percentage by peak area normalization.
Identification of components of essential oil was based on their retention indices, relative to a homologous series of n-alkane (C8-C20) on ZB-1 capillary column under the same operating conditions and computer matching with NIST MS libraries.
2.6. Assay for antibacterial potential
Standard agar disc diffusion method was used for antibacterial assay. Firstly, active cultures were diluted with LB broth to achieve an optical density of 107CFU/mL for the test organisms at 600 nm by an UV/Vis Spectrophotometer (Optizen 2120 UV). Petri plates were prepared by pouring 20 mL of LB agar medium, which was allowed to solidify. Standardized inoculum (0.1 mL) containing107CFU/mL of bacterial suspension was poured onto an LB plate,uniformly spread, and then allowed to dry for 5 min. Whatman No.1 sterile filter paper discs (6 mm diameter) were impregnated with 5 μL/disc of essential oil or 5 μg/disc of positive compounds(streptomycin and tetracyclin) placed on inoculated LB agar. The plates were left at room temperature for 30 min to allow the oil to diffuse into the agar, after which the plates were incubated at 37 ℃for 24 h. Antibacterial activity was evaluated by measuring the diameter of the inhibition zone against the tested bacteria.
2.7. Determination of minimum inhibitory concentration(MIC) and minimum bactericidal concentration (MBC)
The MIC of essential oil was tested by the method described by Chandrasekaran and Venkatesalu (2004)[19]. The oil was dissolved in DMSO and incorporated into tubes containing LB broth medium to obtain a final concentration from 0 to 1% (v/v). Standardized suspension (10 μL) of each fresh tested organism (107CFU/mL)was transferred to separate tubes, which were incubated at 37 ℃ in a shaking incubator for 24 h. The lowest concentration of the test samples, which did not show any visual growth of test organisms after macroscopic evaluation, was determined as MIC. Further,concentrations showing complete inhibition of bacteria were identified. For this, 50 μL of each culture broth was transferred onto an agar plate, which incubated for a specified time and temperature as mentioned above. The complete absence of growth on the agar surface at the lowest concentration of the sample was defined as MBC. Streptomycin was used as a positive control.
2.8. Effect of essential oil on viable counts of tested bacteria
For viable counts, each tube containing a bacterial suspension(approximately 107CFU/mL) in 2 mL of LB broth was inoculated with the MIC level of essential oil and incubated at 37 ℃ in a shaking incubator. Bacterial suspensions (10 μL) for viable counting were removed from tubes at 0, 20, 40, 60, 90, 120,150, 210, and 300 min time intervals and then diluted appropriately with sterile water. Each dilution (50 μL) was spread on the LB agar surface. The colonies were counted after 24 h of incubation at 37 ℃. The controls without essential oil inoculation were treated under same experimental conditions as mentioned above.
2.9. Scanning electron microscopy (SEM)
Sample preparation for SEM was carried out according to Kockro method[20] with some modifications. Bacterial cells of E. coli ATCC 8739 and L. monocytogenes ATCC 10943 were treated with and without essential oil at the MIC level for 60 min, washed three times using 50 mM phosphate buffer solution (PBS, pH 7.3),and then centrifuged at 4 000 rpm. After removing supernatant, centrifuged cells were suspended in new PBS. A thin smear of the suspension was then spread on a glass slide and fixed in 2.5% (v/v)glutaraldehyde (Electron Microscopy Science, Washington, USA)for 2 h. The specimen was dehydrated using sequential exposure per ethanol concentrations ranging from 30%-100%. The ethanol was replaced by tertiary butyl alcohol. After dehydration, the specimen was dried with CO2. The dried cells were coated with gold in a sputter coater (Hitachi, Japan). Samples were observed under a scanning electron microscope (Hitachi-S4300, Japan).
2.10. Assay of potassium ion flux
The concentration of free potassium ions in the bacterial suspension was measured after exposure to essential oils (at MIC value) in sterile peptone water (0.1%) for 0, 60, 120, 180, 240 and 300 min. At each pre-established interval, the extracellular potassium concentration was measured by following a photometric procedure using a Kalium/Potassium kit (Quantofix, GmbH, Wiesbaden,Germany). Control tubes without essential oils were tested. Results were expressed as the amount of extracellular free potassium (μM)ion at each interval of incubation.
2.11. Release of cellular material
Release of cellular materials at 260 nm was carried out in 2 mL aliquots of sterile peptone water (0.1% w/v) containing bacterial inoculum, after which essential oils (at MIC value) were added to the tubes. After 0, 60, 120, 180, 240, and 300 min of treatment, cells were centrifuged at 3 500 rpm, and the absorbance of the obtained supernatant was determined at 260 nm by an Optizen UV/Vis Spectrophotometer. Control tubes without essential oils were tested. Results were expressed as the percentage of absorbing material of 260 nm min each interval with respect to time.
2.12. Antioxidant activity
Free radical scavenging activity was measured by stable 2,DPPH according to Orhan and colleagues (2003)[21] with minor modifications. To test antioxidant activity, three concentrations of sample (25, 50, and 100 μg/mL) were dissolved in methanol. Each concentration (100 μL) was mixed with DPPH solution (900 μL of 0.004% w/v in methanol) and vortexed. Remaining amount of DPPH was determined based on decrease in absorbance at 517 nm. Inhibition of DPPH as a percentage was calculated by the formula:
Where Acontrolis the absorbance of the control reaction, which contains all reagents except the test sample, and Asampleis theabsorbance of the test compounds.
2.13. Measurement of nitric oxide scavenging activity
Nitric oxide scavenging activity was determined by the Griess reaction (Griess et al, 1879)[22]. Nitrite is one of the products of oxidative metabolism of NO. Sample (100 μL) was mixed with the same volume of Griess reagent (2% sulfanilamide in 4% phosphoric acid and 0.2% naphthylethylenediaminedihydrochloride in water)and incubated at room temperature for 10 min. The absorbance of the resulting chromophore was read at 540 nm. The activity was compared with sodium nitrite, which was used as a standard antioxidant.
2.14. Superoxide anion scavenging activity
Superoxide anion radical scavenging assay was carried out according to the method of Suh (Suh et al. 2010)[23]. The reaction mixture consisted 0.25 mL of 0.8 mM xanthine in 0.1 mM potassium phosphate (pH 7.8), 0.15 mL of 0.5 mM nitro-blue tetrazolium in 0.1 mM potassium phosphate (pH 7.8), and 0.05 mL of sample solution. After incubation at 25 ℃ for 10 min, the reaction was started by adding 0.5 mU xanthine oxidase. The samples were kept at 25 ℃ for 20 min and then stopped. The results were calculated as the percentage of scavenging activity according to the following formula:
%scavenging= [1-(S-Sb) /(C-Cb)]×100
Where S, Sb, C, and Cbare the absorbance's of the sample treated with enzyme, sample without enzyme, control treated with enzyme,and control without enzyme, respectively.
2.15. Statistical analysis
The data were expressed as mean ± SD. Statistical significance was calculated between control and essential oil treated group by using the Student's t-test. It was regarded as significant difference as P<0.05.
3. Results
3.1. Chemical composition of essential oil
The yield of leaf essential oil was about 1.2%, (v/w). Upon GC-MS analysis, 10 compounds were identified as trans-phytol (42.73%),cis-3-hexenol (12.95%), β-linalool (10.68%), trans-2-hexenal(8.86%), trans-2-hexenol (8.86%), myrcenol (3.86%), 4-vinylphenyl acetate (3.86%), (4Z)-4,6-heptadien-1-ol (3.18%), lemonol (2.73%),and benzeneacetaldehyde (2.27%) (Table 1).

Table 1 Composition of essential oil of F. koreana leaf.
3.2. Antibacterial activity
The in vitro antibacterial activity of leaf essential oil of F. koreana against foodborne pathogenic bacteria was assessed based on the presence or absence of inhibition zones (Table 2). Essential oil(5 μL/disc) exhibited potent antibacterial activity against all test pathogens, including S. enteritidis KCTC 12243, E. coli ATCC 8739, and S. aureus ATCC 6538 with inhibition zone diameters of 12.3, 8.0, 9.3 mm, respectively. Strains of L. monocytogenes (ATCC 19118, ATCC 19111, ATCC 19166, ATCC 19116, and ATCC 10943)showed inhibition zone diameters of 10.0, 6.9, 6.8, 6.2 and 9.7 mm,respectively.
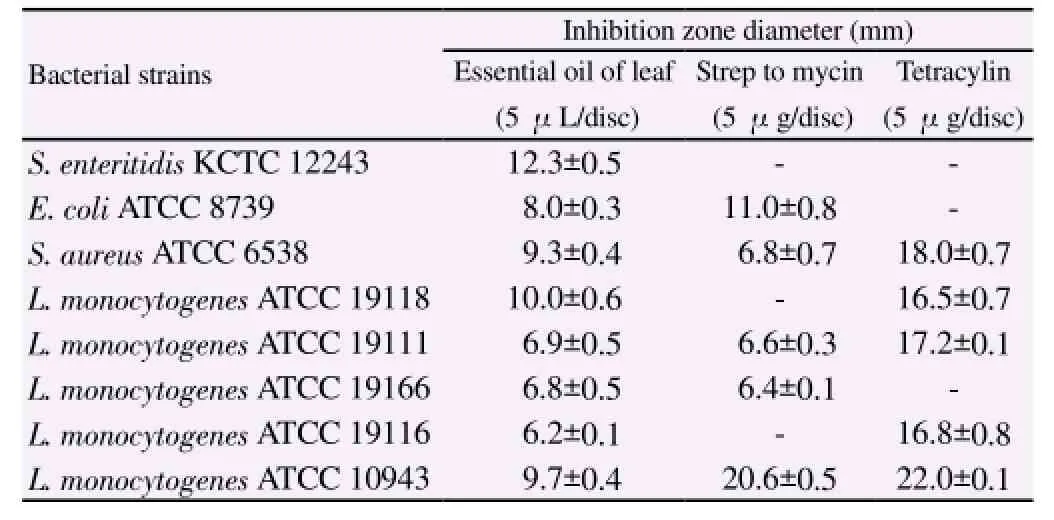
Table 2 Inhibition zone diameter produced by the essential oil of F. koreana leaf on the test pathogens.
3.3. MIC and MBC
As shown in Table 3, leaf essential oil showed greater susceptibilityagainst Gram-positive S. aureus ATCC 6538 and one strain of L. monocytogenes (ATCC 10943) with MIC values of 0.2% and 0.1% (v/v), respectively. Leaf essential oil also exhibited moderate antibacterial activity with MIC values from 0.3% to 0.5% (v/v)against two Gram-negative bacteria, S. enteritidis KCTC 12243 and E. coli ATCC 8739, and the other four strains of Gram-positive L. monocytogenes.

Table 3 MIC and MBC of essential oil of F. koreana leaf against the test pathogens.
3.4. Effect of essential oil on cell viability
Reducing effects of leaf essential oil of F. koreana on cell viabilities of S. enteritidis KCTC 12243, E. coli ATCC 8739, S. aureus ATCC 6538, and L. monocytogenes ATCC 10943 were observed during treatment for 300 min (Figure 1A). Leaf essential oil reduced the growth of test bacteria at minimum inhibitory concentrations, and 50% inhibition of cell viabilities was observed within 90 min of exposure in all test strains. After 150 min, 80% inhibition of Grampositive bacteria (S. aureus ATCC 6538 and L. monocytogenes ATCC 10943) was observed, and exposure for 210 min to leaf essential oil resulted in complete inhibition of CFU numbers of these two strains. After 300 min, all test bacteria were completely inhibited by leaf essential oil.
3.5. SEM
SEM was carried out to visualize the effects of leaf essential oil of F. koreana on cellular morphologies of E. coli ATCC 8739 and L. monocytogenes ATCC 10943 (Figure 2 and 3). Essential oil exhibited strong detrimental effects on morphologies of E. coli ATCC 8739(Figure 2B) and L. monocytogenes ATCC 10943 (Figure 3B and 3C). Control cells (without essential oil treatment) showed a regular,smooth surface as shown in Figure 2A and 3A. In contrast, cells inoculated with essential oil at MIC levels for 60 min showed changes in cell wall morphology, cell wall lysis (Figure 2B, 3B and 3C), and pore formation (Figure 3B). Release of cell materials also occurred in L. monocytogenes ATCC 10943 upon essential oil treatment (Figure 3B).
3.6. Release of potassium ions and 260-nm-absorbing material
Release of potassium ions and 260-nm-absorbing cell material from E. coli ATCC 8739 treated with leaf essential oil of F. koreana at the MIC level (3 000 μg/mL) is depicted in Figure 1B and 1C. Efflux of potassium ions from test bacteria occurred immediately after addition of essential oil, followed by a steady reduction along the evaluated intervals. The OD260nmof filtrates of cells exposed to leaf essential oil revealed elevated release of cell materials according to time of exposure.
As shown in Figure 1D and 1E, release of potassium ions and 260-nm-absorbing cell material from L. monocytogenes ATCC 1093 treated with leaf essential oil of F. koreana at the MIC level (1 000 μg/mL) occurred immediately after addition of essential oil, followed by a steady reduction along the evaluated intervals. The OD260nmof filtrates of cells exposed to leaf essential oil revealed elevated release of cell materials according to time of exposure.
3.7. Antioxidant activity
The IC50(50% free radical inhibitory concentration) values of leaf essential oil were (660±20), (1 280±70), and (1 080±40) μg/mL,respectively. Leaf essential oil showed improved antioxidant activity as possessed lower IC50value on the three free radicals.
4. Discussion
Much attention has been given to plant-derived essential oils, as they possess various pharmaceutical, antibacterial, antioxidant,antiviral, antiinsecticidal, antimycotic, and antitoxinogenic activities[8,10,11,13]. The volatile constituents of F. koreana leaves are a mix of oxygenated terpenes (β-linalool, myrcenol, lemonol, and trans-phytol) and oxygenated derivatives, including aldehydes (trans-2-hexenal and benzeneacetaldehyde), alcohols [cis-3-hexenol, trans-2-hexenol, and (4Z)-4, 6-heptadien-1-ol], and esters (4-vinylphenyl acetate).
Historically, many plant oils have been used as topical antiseptics. The use of essential oils may also improve food safety and quality. In the present study, the results of MIC determination showed that leaf essential oil of F. koreana exhibits potent activities against food spoilage and foodborne pathogenic bacteria, such as S. enteritidis KCTC 12243, E. coli ATCC 8739, S. aureus ATCC 6538, L. monocytogenes ATCC 19118, ATCC 19111, ATCC 19166,ATCC 19116, and ATCC 10943 with IC50values ranging from 0.1 to 0.5% (v/v). The antibacterial activity of leaf essential oil could be attributed to components such as β-linalool, myrcenol, and lemonol, which have been found to be the major constituents of several essential oils or exhibit potential antimicrobial activity[24,25]. Other components are also critical and may have a synergistic effect or potentiating influence. SEM study of E. coli ATCC 8739 and L. monocytogenes ATCC 10943 determined that essential oil acted on cell membranes, resulting in loss of integrity and increased permeability. Increased leakage of potassium ions indicates that the cell membrane structure was damaged by essential oil as compared to the control group.
Release of potassium ions and 260-nm-absorbing material from E. coli ATCC 8739 and L. monocytogenes ATCC 10943 was stimulated by exposure of cells to leaf essential oil of F. koreana. The results indicate that essential oil acted on the cytoplasmic membrane,resulting in increased permeability. The cytoplasmic membrane acts as a permeability barrier to the passage of ions such as H+, K+, Na+,and Ca2+[8]. Increased leakage of K+indicates that the membrane structure was damaged by the essential oil. Further, SEM analysis of these two bacterial cells also demonstrated the destructive effect of essential oil on cell membranes as compared to the control group. Choi and associates (2000)[9] examined the DPPH scavenging activities of 21 authentic compounds, including linalool (DPPH scavenging activity of linalool was 50.3 mg trolox equiv/mL). In short, leaf essential oil of F. koreana caused physical destruction via loss of cytoplasmic integrity. Further, the high content of linalool could be responsible for the increased antioxidant activity of leaf essential oil against the tested pathogens, which supports the notion that leaf essential oil of F. koreana is a promising food preservative. In summary, leaf essential oil of F. koreana is rich in oxygenated diterpenes, oxygenated monoterpenes, and alcohols and displays significant antibacterial activity against foodborne and pathogenic bacteria such as S. enteritidis KCTC 12243, E. coli ATCC 8739, S. aureus ATCC6538, and several strains of L. monocytogenes ATCC 19118, L. monocytogenes ATCC 19111, L. monocytogenes ATCC 19166, L. monocytogenes ATCC 19116, and L. monocytogenes ATCC 10943. Therefore, leaf essential oil of F. koreana might be used as an anticorrosive additive to improve food quality and control growth of food-spoiling microbial pathogens. Future research is necessary to understand the involved mechanisms, especially against otherfoodborne pathogens and as a food preservative. Furthermore,essential oils will be studied using human tumor cell lines to explore their role as a therapeutic agent.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Hennekinne JA, De Buyser ML, Dragacci S. Staphylococcus aureus and its food poisoning toxins: characterization and outbreak investigation. FEMS Microbiol Rev 2012; 36: 815-836.
[2] Krakauer T, Stiles BG. The staphylococcal enterotoxin (SE) family SEB and siblings. Virulence 2013; 4: 759-773.
[3] Cossart P, Lebreton A. A trip in the ‘New Microbiology'' with the bacterial pathogen Listeria monocytogenes. FEBS Lett 2014; 588: 2437-2445.
[4] Ulett GC, Totsika M, Schaale K, Carey AJ, Sweet MJ, Schembri MA. Uropathogenic Escherichia coli virulence and innate immune responses during urinary tract infection. Curr Opin Microbiol 2013; 16: 100-107.
[5] Dahiya P, Purkayastha S. Phytochemical screening and antimicrobial activity of some medicinal plants against multi-drug resistant bacteria from clinical isolates. Indian J Pharm Sci 2012; 74: 443-450.
[6] Kalemba D, Kunicka A. Antibacterial and antifungal properties of essential oils. Curr Med Chem 2003; 10: 813-829.
[7] Parimala M, Shoba FG. In vitro antimicrobial activity and HPTLC analysis of hydroalcoholic seed extract of Nymphaea nouchali Burm. F. BMC Complem Altern Med 2014; 14: 314-361.
[8] Doughari JH, Ndakidemi PA, Human IS, Benade S. Antioxidant,antimicrobial and antiverotoxic potentials of extracts of Curtisia dentata. J Ethnopharmacol 2012; 141: 1041-1050.
[9] Choi HS, Song HS, Ukeda H, Sawamura M. Radical-scavenging activities of citrus essential oils and their components: detection using 1,1-diphenyl-2-picrylhydrazyl. J Agric Food Chem 2000; 48: 4156-4164.
[10] Cannas S, Molicotti P, Ruggeri M, Cubeddu M, Sanguinetti M, Marongiu B, et al. Antimycotic activity of Myrtus communis L. towards Candida spp. from isolates. J Infect Dev Countr 2013; 7: 295-298.
[11] Rothan HA, Zulqarnain M, Ammar YA, Tan EC, Rahman NA, Yusof R. Screening of antiviral activities in medicinal plants extracts against dengue virus using dengue NS2B-NS3 protease assay. Trop Biomed 2014;31; 286-296.
[12] Pandey R, Kalra A, Tandon S, Mehrotra N, Singh HN, Kumar S. Essential oil compounds as potent source of nematicidal compounds. J Phytopathol 2000; 148: 501-502.
[13] Sukphan P, Sritularak B, Mekboonsonglarp W, Lipipun V,Likhitwitayawuid K. Chemical constituents of Dendrobium venustum and their antimalarial and anti-herpetic properties. Nat Prod Commun 2014;9: 825-827.
[14] Ultee A, Smid EJ. Influence of carvacrol on growth and toxin production by Bacillus cereus. Int J Food Microbiol 2001; 64: 373-378.
[15] Doughari JH, Ndakidemi PA, Human IS, Benade S. Antioxidant,antimicrobial and antiverotoxic potentials of extracts of Curtisia dentata. J Ethnopharmacol 2012; 141: 1041-1050.
[16] Ali MS, Ravikumar S, Beula JM, Anuradha V, Yogananth N. Insecticidal compounds from Rhizophoraceae mangrove plants for the management of dengue vector Aedesa egypti. J Vector Borne Dis 2014; 51: 106-114.
[17] Konstantopoulou I, Vassilopoulou L, Mavragani-Tsipidou P, Scouras ZG. Insecticidal effects of essential oils. A study of the effects of essential oils extracted from eleven Greek aromatic plants on Drosophila auraria. Experientia 1992; 48: 616-619.
[18] Kim NY, Kang TH, Song EK, Pae HO, Chung HT, Kim YC. Inhibitory effects of butanol fraction of the aqueous extract of Forsythia koreana on the nitric oxide production by murine macrophage-like RAW 264.7 cells. J Ethnopharmacol 2000; 73: 323-327.
[19] Chandrasekaran M, Venkatesalu V. Antibacterial and antifungal activity of Syzygium jambolanum seeds. J Ethnopharmacol 2004; 91: 105-108.
[20] Kockro RA, Hampl JA, Jansen B, Peters G, Scheihing M, Giacomelli R. Use of scanning electron microscopy to investigate the prophylactic efficacy of rifampin- impregnated CSF shunt catheters. J Med Microbiol 2000; 49: 441-450.
[21] Orhan I, Aydin A, Colkesen A, Sener B, Isimer, AI. Free radical scavenging activities of some edible fruit seeds. Pharm Biol 2003; 41:163-165.
[22] Griess P, Bemerkungen zu der abhandlung der HH. Weselsky und Benedikt 'Uber einige azoverbindungen'. Berichte der Deutschen Chemischen Gesellschaft 1879; 12: 426-428.
[23] Suh HJ, Kim SR, Lee KS, Park S, Kang SC. Antioxidant activity of various solvent extracts from Allomyrina dichotoma (Arthropoda: Insecta)larvae. J Photochem Photobiol 2010; 99: 67-73.
[24] Inouyea S, Takizawab T, Yamaguchia H. Antibacterial activity of essential oils and their major constituents against respiratory tract pathogens by gaseous contact. J Antimicrobial Chemother 2001; 47: 565-573.
[25] Chiang HM, Chiu HH, Lai YM, Chen CY, Chiang HL. Carbonyl species characteristics during the evaporation of essential oils. Atmos Environ 2010; 44: 2240-2247.
15 June 2015
Prof. Sun Chul Kang, Department of Biotechnology,College of Engineering, Daegu University, Kyungsan, Kyungbuk712-714, Republic of Korea.
Tel: +82-53-850-6553
Fax: +82-53-850-6559
E-mail: sckang@daegu.ac.kr
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Diuretic and antioxidant activities of the aqueous extract of leaves of Cassia occidentalis (Linn.) in rats
- A pilot study of antioxidant potential of endophytic fungi from some Sudanese medicinal plants
- Prevalence of filarial parasites in domestic and stray cats in Selangor State, Malaysia
- Genetic variation of Leptospira isolated from rats catched in Yogyakarta Indonesia
- Molecular survey of tick-borne pathogens in Ixodid ticks collected from hunted wild animals in Tuscany, Italy
- Epidemiology of influenza viruses from 2009-2013-A sentinel surveillance report from Union territory of Puducherry, India
