Diuretic and antioxidant activities of the aqueous extract of leaves of Cassia occidentalis (Linn.) in rats
2015-10-28ThophileDimoFidleNtchapdaJosephBaramaDavidRomainKemetaAzambouPaulFaustinSekeEtet
Théophile Dimo, Fidèle Ntchapda, Joseph Barama, David Romain Kemeta Azambou, Paul Faustin Seke Etet
1Department of Animal Biology and Physiology, Faculty of Science, University of Yaoundé 1, P.O. Box 812, Yaoundé, Cameroon
2Department of Biological Sciences, Faculty of Science, University of Ngaoundéré, P.O. Box 454, Ngaoundéré, Cameroon
3Department of Basic Health Sciences, College of Applied Medical Sciences, Qassim University, Buraydah 51452, Saudi Arabia
Diuretic and antioxidant activities of the aqueous extract of leaves of Cassia occidentalis (Linn.) in rats
Théophile Dimo1, Fidèle Ntchapda2*, Joseph Barama2, David Romain Kemeta Azambou2, Paul Faustin Seke Etet3
1Department of Animal Biology and Physiology, Faculty of Science, University of Yaoundé 1, P.O. Box 812, Yaoundé, Cameroon
2Department of Biological Sciences, Faculty of Science, University of Ngaoundéré, P.O. Box 454, Ngaoundéré, Cameroon
3Department of Basic Health Sciences, College of Applied Medical Sciences, Qassim University, Buraydah 51452, Saudi Arabia
ARTICLE INFO
Article history:
in revised form 20 July 2015
Accepted 15 August 2015
Available online 20 September 2015
Cassia occidentalis
Diuretic
Antioxidant
Urine
kidney function
Electrolytes
Objective: To assess the putative diuretic and antioxidant properties of Cassia occidentalis(C. occidentalis) leaves' aqueous extract. Methods: Adult rats were administered with C. occidentalis leaves aqueous extract acutely (24-h) and subchronically (7 d), at doses 80, 160,240, 320, and 400 mg/kg (per os). Negative control group received only an equivalent volume of distilled water, while the two positive control groups received the diuretic drugs furosemide(20 mg/kg, i.p.) and hydrochlorothiazide (HCTZ, 20 mg/kg, i.p.). Urinary elimination of electrolytes in response to treatments was evaluated, together with changes in concentrations of creatinine, urea, aldosterone, glucose, and albumin in urine and plasma. Various urinary indicators of kidney function and plasmatic markers of oxidative stress were also assessed. Results: The acute administration of C. occidentalis increased the urinary excretion of 107.58% at the higher dose tested, compared to negative control. The reference drugs furosemide and HCTZ induced increases of 84.27 % and 48.05 %, respectively. Acutely, the extract induced Na+and Cl-elimination, whereas subchronically an increase in K+elimination was also observed. The extract also improved the kidney function indexes and oxidative stress markers. These effects were dose-dependent and comparable with positive control observations. Conclusions: Our findings strongly suggest that C. occidentalis aqueous extract has diuretic and antioxidant activities, and deserves further studies considering the potential for the treatment of hypertension.
1. Introduction
Arterial hypertension is among the most frequent pathologies in elderly worldwide, with an incidence ranging from 40% (about 65 years patients) to 90 % (patients older than 85) in developed countries[1,2]. This pathology raises more concerns as it constitutesa major risk of cardiovascular accident. In developing countries,partly due to the relatively inexpensive costs several hypertensive patients have been relying on medicinal plants for their treatment. Furthermore, various reports suggesting that conventional antihypertensive drugs may increase the risk for cardiovascular accidents have resulted in an increased interest of the research community for medicinal plants[3,4]. Not surprisingly, WHO reports(particularly in the last decade) have been encouraging studies on medicinal plants and alternative medicine for priority diseases like hypertension and its cardiovascular complications.
Cassia occidentalis (C. occidentalis) is a tropical plant used in African and Asian traditional medicines to treat or improve anumber of diseases and conditions, in particular cardiovascular disorders[5,6]. As in various other countries, in Cameroun roasted seeds are used as coffee substitutes, while other parts of the plants are used by traditional healers to treat metabolic and cardiovascular diseases. Interestingly, experimental evidence supports the applications in traditional medicine. Phytochemical studies of C. occidentalis leaves revealed the presence of a number of pharmacologically active families of molecule, including tanins,saponins, cardiac glycosides, terpenoides and anthroquinones,terpenes, and inorganic elements[7,8]. Extracts of this plant were reported antifungal, antiviral[9,10], antibacterial, anthelmintic[11,12],antispasmodic, analgesic, antipyretic, anti-inflammatory[13,14],and hepatoprotective properties in humans and experimental models[15,16]. Verma and colleagues showed the effect of ethanolic extract of C. occidentalis for the management of alloxan-induced diabetic rats[17,18], and Sreejith and colleagues reported anti-allergic,anti-inflammatory and anti-lipid peroxiding effects[19].
The present work aim was to measure the diuretic and antioxidant activities of the aqueous extract of C. occidentalis leaves in rats.
2. Materials and methods
2.1. Animals and procedures
Rattus norvegicus (172.3 ± 4.3 g) of both sexes obtained from Yaoundé's Pasteur Institute (Cameroon) were reared in the Department of Biological Sciences, Faculty of Sciences (University of Ngaoundéré, Cameroon). Animals were housed under controlled temperature (24 ± 2) ℃ and relative humidity (45 ± 10) %, and had ad libitum access to food[pellets from Cameroonian National Veterinary laboratory (LANAVET)] and tap water. Animal health status and housing conditions were monitored by a veterinary physician.
Preliminary tests were performed as previously described[20]. Briefly, rats received distilled water per os (10 mL/kg body weight),and placed individually in metabolic cages. After 6-h, urine was collected and the volume measured. Animals excreting at least 40% of the volume of distilled water received were selected for the study, and conversely, those excreting less than 40% were excluded. Then, selected animals were placed individually in metabolic cages,and allowed 7 d for acclimation. Eight experimental groups were obtained by treating n=5 rats (per group) with a specific solution,i.e.: vehicle solution (distilled water, per os) for the negative control group, one of the 5 different doses of extract investigated for the 5 test groups (per os), and the diuretic drugs furosemide (20 mg/kg,i.p.) or hydrochlorothiazide (HCTZ) (20 mg/kg, i.p.) for the positive control groups. Animals were sacrificed by decapitation at the end of the experiment. Arteriovenous blood was collected in heparinized tubes and centrifuged (3 000 rev/min for 10 min). The plasma collected was stored at -20 ℃ for biochemical analyses. The liver and kidneys were dissected out, cleaned of fat material, weighed and stored at -20 ℃ for biochemical analyses.
Experimental procedures were approved by the institutional Animal Care and Use Committee and the research was approved by the Ethics Committee of the University of Ngaoundéré.
2.2. Plant extract preparation
2.2.1. Plant material collection
Leaves of C. occidentalis Linn were harvested in Mora (60 km from Maroua, the largest city of the Far North region of Cameroon)during rainy season. They were identified by experts of the National Herbarium of Cameroon and a sample was deposited (specimen N0 21057/SFR/CAM).
2.2.2. Aqueous extract preparation
Fresh leaves of C. occidentalis were soaked in distilled water (1 000 g for 1 L at room temperature) for 12 hours. The macerate was filtered through Whatman filter paper No 3, and the filtrate concentrated in a rotary evaporator at 40 ℃ for 24 hours. This process was repeated until an oily paste extract was obtained (130 g), which represented the concentrated crude extract of C. occidentalis leaves. The extract was stored at -20 ℃ until use.
2.2.3. Aqueous extract doses
The solution of C. occidentalis extract with the highest concentration tested was prepared by dissolving 800 mg of the concentrated crude extract obtained previously in 10 mL of distilled water (80 mg/mL concentration). The other solutions used in the study were 4:5, 3:5,2:5, and 1:5 dilutions of this solution in distilled water. Solutions were given per os in a volume of 5 mL/kg body weight, thus, the increasing doses of aqueous extract of C. occidentalis tested were 80,160, 240, 320, and 400 mg/kg.
2.3. Diuretic effect evaluation
2.3.1. Acute diuretic effect evaluation
Urine was collected and the volume determined each hour from the treatment for 6-h (i.e. 1, 2, 3, 4, 5, and 6-h after treatment) and 24-h after in all experimental groups. Electrolyte concentrations (Na+, K+,and Cl-1) were measured in 24-h urine and in blood plasma obtained from animals sacrificed 24-h after treatment.
2.3.2. Subchronic diuretic effect evaluation
Based on preliminary observations from acute diuretic effectevaluation, the experimental group receiving the highest dose of extract tested (400 mg/kg), and the positive and negative control groups were treated daily for 7 d at the same time each day. Urine was collected daily, its volume measured, and electrolyte concentrations (Na+, K+, Cl-1) determined.
2.4. Determination of urinary and/or plasma concentrations
2.4.1. Osmolarity and electrolytes
Osmolarity of plasma and urine samples were measured by cytometry using an osmometer (Knauer). Urinary and plasma concentrations of Na+, K+and Cl- ions were evaluated using flame photometry (Jenway PFP 7, Bibby Scientific, USA), following standard protocols. Urinary natriuresis was measured during the diuretic response, particularly at the maximum excretion rate. Doses of Na+and K+were calculated as indicators of saluretic activity and the ratio Na+/K+was calculated for the natriuretic activity. And to estimate the carbonic anhydrase inhibition activity, the ratio of Clions to Na+and K+ions was calculated[21].
Osmolar clearance was calculated using plasma osmolality urinary osmolarity and urine flow (V) according to the following formula:
Osmolar clearance = Urinary osmolarity × V /Plasma osmolality
2.4.2. Concentrations of other blood molecules
A two-way digital spectrophotometer (Secomam RS232C, Secomam SAS, France) was used to determine the concentrations of urea,glucose, albumin, and creatinine in plasma and urine samples. Similarly malondialdehyde concentration was determined in plasma, and catalase, hydroperoxide and protein concentrations were determined in hemolysates of blood pellets and in liver homogenates. Aldosterone concentration in the plasma was measured using radioimmunoassay(assay kit Aldo RIACT, ALPCO Diagnostics, USA).
2.5. Phytochemical studies
In order to identify the chemical structure of the compounds responsible for the diuretic activity, preliminary tests of the phytochemical study were conducted following the procedures described by Trease and Evans [22]. Briefly, Essential oils from the aqueous extract of C. occidentalis and urine were extracted with hexane. These extracts were then stitched onto plates of thin layer chromatography on silica, the first disclosure was obtained by ultraviolet radiation (254 nm and 365 nm) and then with vanillin. Analytical tests for the identification of different families of metabolites in crude extracts of the leaves were performed at the national Institute of Medicinal Plants for Medicinal research (IMPM, Cameroon).
2.6. Statistical analyses
Data from test groups and positive control groups were compared to negative control group using one-way ANOVA followed by LSD test for post hoc analysis, using Origin software(OriginLab, Northampton, MA, USA). Changes with P values lower than 0.05 were considered significant. Data are presented as mean ± SEM.
3. Results
3.1. Effect of the acute administration of C. occidentalis extract on urination and associated urinary and serum parameters
3.1.1. Overload eliminated after 1 h
The overload eliminated after 1-h by rats acutely treated with the three highest doses of C. occidentalis aqueous extract tested(240, 320, and 400 mg/kg), administered with an equivalent volume of distilled water (negative control), or rats treated with one of the two diuretic drugs (positive control) is shown in Figure 1A. All treatments induced at least a 3-fold increase in the overload elimination compared with negative control group. The response of the extract was dose-dependent, and eliminated 78.79% of overload at 240 mg/kg (P<0.001 against negative control group), 90.87% at 320 mg/kg (P<0.01) and 126.87% at 400 mg/kg (P<0.001). Furosemide eliminated 100% (P<0.001),HCTZ 88.43% (P<0.001), whereas only 35.35% overload was eliminated in the negative control group.
3.1.2. Latency to first urination, urine volume and pH
The bar graph of Figure 1B represents the latency to first urination of rats acutely treated with the three highest doses of C. occidentalis aqueous extract tested, and of negative and positive control groups. Negative control group average first urination latency was 42 min, furosemide 18.27 min (P<0.01), and HCTZ 19.82 min (P<0.05). The extract displayed a dose-dependent decrease as the first urination latency with 17 min (P<0.05), 15 min (P<0.01), and 11 min (P<0.01) at doses 240, 320 and 400 mg/kg.
The urinary pH (7.11 in negative control group) was slightly decreased in groups treated with furosemide (6.79) or HCTZ(6.42), and slightly increased in groups receiving the extract(7.7, 7.5, 7.6 at doses 240, 320 and 400 mg/kg). However, these changes were not statistically significant (Figure 1B).
Figure 2 shows the changes in the total volume of urine excreted by rats following acute treatment with various doses of C. occidentalis aqueous extract (80, 160, 240, 320, and 400 mg/kg). On the overall, the extract and the diuretic drugs increased the volume of urine excreted. After 24-h, an average volume of urine of 218.34 mL/kg body weight was excreted in the control group, whereas 402.35 mL/kg of urine were excreted in furosemide-treated (P<0.001), 323.27 mL/kg in HCTZ-treated (P<0.05), and 453.24 mL/kg (P<0.001), in groups receiving the extract at 400 mg/kg, respectively (Figure 2C).
3.1.3. Electrolyte excretion
The effect of acute treatment with C. occidentalis aqueous extract or diuretic drugs (compared with negative control groups) on various electrolyte excretion indexes and parameters in the urine produced in the 24-h following treatment are shown in Figure 3, Table 1 and 2.
As shown in Table 1, saluretic and natriuretic activities were significantly increased by diuretic drugs (P<0.001) and by the extract(P<0.001 at the highest dose tested). Similarly, carbonic anhydrase inhibition was also increased by diuretic drugs (P<0.01) and by the extract (P<0.01 at the highest dose tested). On the same hand,carbonic anhydrase inhibition, saluretic, natriuretic, diuretic, as well as Na+and Cl-indexes were high for in all groups, but the extract effect at 400 mg/kg (higher dose used) was 2-fold higher than the diuretic drugs at the dose used (Table 2). Notably, K+index was only slightly increased by the diuretic drugs and the extract (Table 2).
The total volume of urine excreted after 6-h (A), 12-h (B), and 24-h (C) by rats following acute treatment with various doses of C. occidentalis aqueous extract,administration of an equivalent volume of distilled water (negative controls), or treatment with one of the two diuretic drugs used as positive controls. Note that the highest doses of extract and the diuretic drugs increased the volume of urine excreted. Note also that the response of the extract was dose-dependent.
dH2O, distilled water; Furos, furosemide; HCTZ, hydrochlorothiazide. ANOVA+LSD test against negative control group: *P<0.05, **P<0.01, *** P<0.001.
All treatments increased the urinary excretion of Na+and Clcompared to negative control group; the extract response was dose-dependent (Figure 3). After 24-h, the extract significantly(P<0.05) increased the urinary excretion of Na+(Figure 3C) and Cl-(Figure 3F) from 16.58 and 16.67 mEq/kg (negative controlgroup), respectively, to 167.39 and 168.36 mEq/kg at the dose 400 mg/kg. The urinary excretion of Na+and Cl-induced by furosemide were 93.38 and 94.67 mEq/kg (P<0.05), respectively, and HCTZ 72.43 and 75.41 mEq/kg (P<0.05) (Figure 3C, F). No statistically significant change was observed in the mount of K+excreted in the urine after 6-h (Figure 3G), 12-h (Figure 3H), and 24-h (Figure 3I).
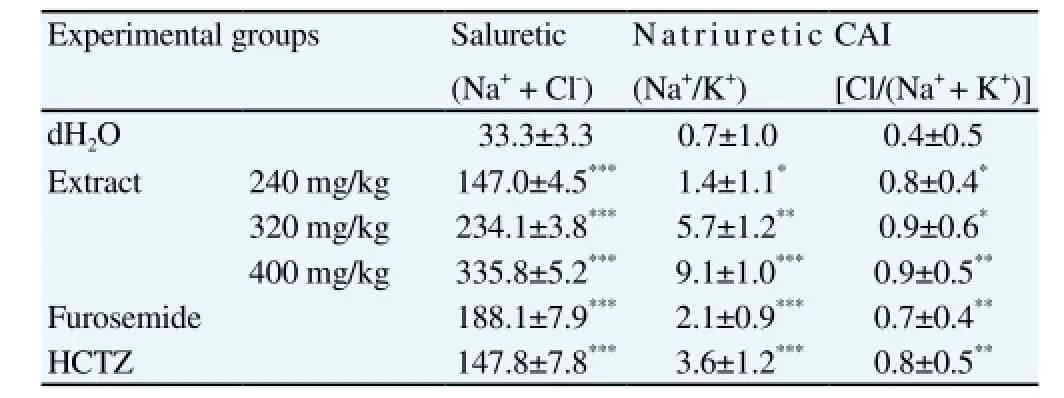
Table 1 Effect of acute treatment with C. occidentalis aqueous extract on carbonic anhydrase inhibition, and on saluretic and natriuretic activities, observed in urine produced in the 24-h following the treatment.
3.1.4. Urinary indexes of kidney function
The effect of C. occidentalis aqueous extract and diuretic drug treatment (compared to negative control group) on indexes of kidney function in urine produced in the 24-h following the treatment are shown in Table 3. Treatments with the various doses of extract and the diuretic drugs decreased the glomerular filtration rate from 1.66 mL/min in the negative control group to 1.37 mL/min (furosemide,P<0.001), 1.38 mL/min (HCTZ, P<0.001), and 1.43 mL/min (extract at 400 mg/kg, P<0.01). Creatinine clearance and urea concentration in the urine were significantly decreased as well, but no significant change was observed in urinary creatinine rate (Table 3). Osmolar clearance and free water clearance were increased, while urinary osmolarity decreased. Glucose and albumin were not detected in the urine.
3.1.5. Serum parameters
Concentrations of Na+and K+ions were significantly increased(596.44% and 311.17% respectively in animals receiving the extract at 400 mg/kg, P<0.05) (Table 4). Furosemide and HCTZ and the extract (at 400 mg/kg) induced significant increases (4.56%, 2.31%,and 4.57% respectively, P<0.05) in albumin level (Table 4). Albumin level increased from 43.17 g/L in the control group to 45.18 g/L in animals treated with the extract at 400 mg/kg.
Increases in plasma osmolality and aldosterone levels were observed (P<0.001) (Table 4). Similarly, the extract and diuretic drugs induced significant increases (P<0.05) in serum creatinine and urea levels. Despite a slight increase in rat receiving the extract and diuretic drugs, glycemia ranged between (91.12±6.34) mg/dL and(97.65±7.15) mg/dL in all experimental groups.
3.2. Effect of the sub-chronic administration of C. occidentalis extract on urination and urine parameters
3.2.1. Urine volume
The total volumes of urine excreted daily by rats following subchronic treatment with the highest dose of C. occidentalis aqueous extract tested (400 mg/kg), administration of an equivalent volume of distilled water (negative controls), or treatment with one of the two diuretic drugs used as positive controls are shown in Figure 4A. C. occidentalis and diuretic treatments significantly increased the volume of urine excreted, compared to the negative control group. Compared to the negative control group (y = 1.91x + 208.4, R = 0.77), furosemide (y = 21.3x + 389.9, R = 0.90), HCTZ (y = 14.7x + 292.3, R = 0.99), and extract-treated groups (y = 23.5x + 425.6, R = 0.99) had a significantly higher and positive slope (P>0.01), i.e. the effects were more marked with time. Notably, from the first day of treatment the volume of urine excreted daily was significantly increased by all these treatments, compared to the negative control group.
3.2.2. Urinary electrolyte excretion
The estimated amounts of Na+(Figure 4B), Cl-(Figure 4C), and K+(Figure 4D) excreted daily in the urine by rats following subchronic treatment with C. occidentalis extract at 400 mg/kg, administration of an equivalent volume of distilled water, or treatment with one of the two diuretic drugs used as positive controls are shown in Figures 4. Na+and Cl-amounts were significantly increased by all these treatments, compared to the negative control group from the first day of treatment. All experimental groups had a significantly higher slope (P>0.01) than the negative control group (y = 0.14x + 15.9, R = 0.56): furosemide (y = 10.6x + 81.9, R = 0.99), HCTZ (y = 9.4x + 60.9, R = 0.98), and extract-treated groups (y = 12.1x + 154.6, R = 0.98). The effects of treatment with extract or diuretic drugs on K+amount excreted was also more marked with time. However, the effect were more marked from two to six days of treatment (Figure 4D): furosemide (y = 6.8x + 33.9, R = 0.98), HCTZ (y = 5.8x + 12.1,R = 0.99), and extract-treated groups (y = 3.6x + 13.2, R = 0.97).
3.2.3. Oxidative stress markers
Table 5 showed the effects of the subchronic administration of C. occidentalis aqueous extract (400 mg/kg) on markers of oxidative stress. Catalase activities in liver homogenates and in hemolysates were significantly decreased (P<0.05). The extract also induced a significant decrease in hydroperoxide amount in liver homogenates(P<0.001), and an increase in blood plasma (P<0.05). Plasma and liver malondialdehyde amounts were significantly decreased(P<0.05). Glutathione concentration in plasma were decreased(P<0.01). Protein amounts were decreased in liver homogenates(P<0.01) and increased in hemolysates (P<0.05).
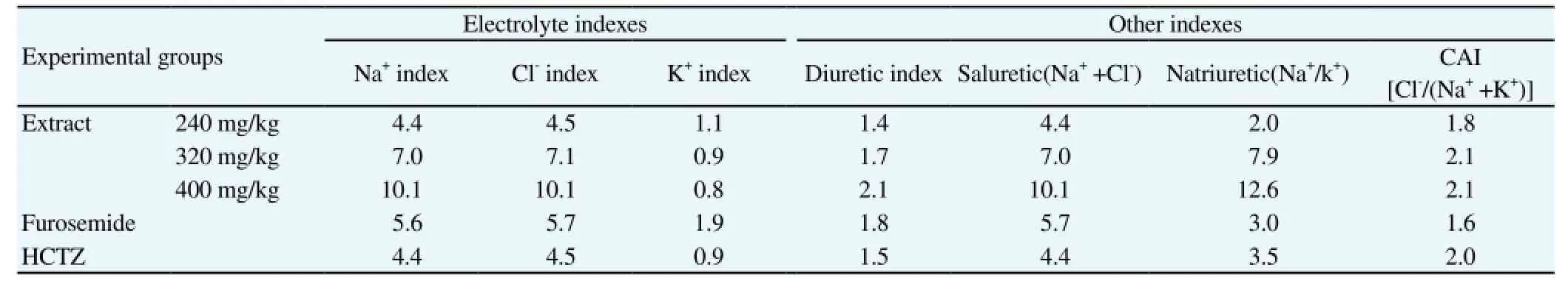
Table 2 Effect of acute treatment with C. occidentalis aqueous extract on electrolyte excretion indexes in urine produced in the 24-h following the treatment.
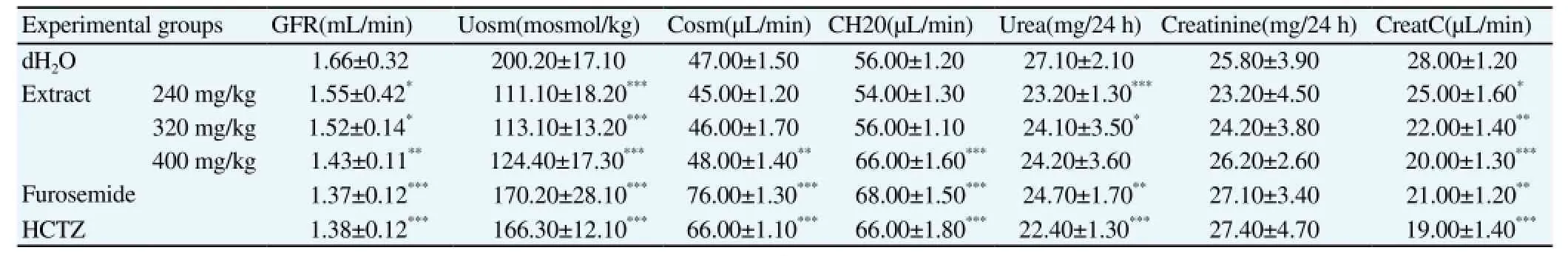
Table 3 Effect of acute treatment with C. occidentalis aqueous extract on indexes of kidney function in urine produced in the 24-h following the treatment.
3.3. Phytochemical study
Phytochemical screening performed on crude extracts revealed the presence of several primary and secondary metabolites such as fatty acids, anthraquinones, glycosides, anthracenes, saponins,tannins, and coumarins. Phenolic compounds, triterpenes, volatile oils and sterols, but also flavonoids and alkaloids were also present in the extract. Thin layer chromatography (TLC) showed that the hexane extract and urine of treated rats contained four chemical fractions. These initial observations and findings suggest that the aqueous extract of leaves of C. occidentalis contains several chemical compounds which biological potential activity deserves further investigation.
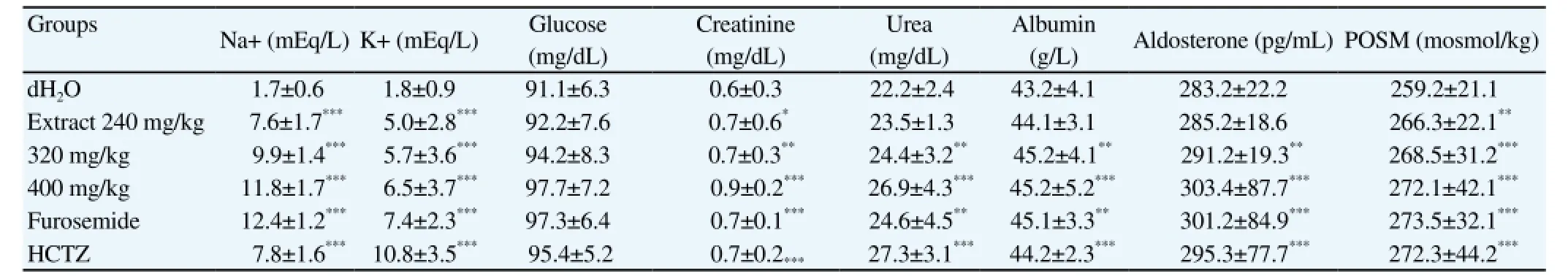
Table 4 Effect of acute treatment with C. occidentalis aqueous extract on serum parameters.

Table 5 Effect of the subchronic administration of C. occidentalis aqueous extract on markers of oxidative stress.
4. Discussion
Results of the present study suggest that the aqueous extract of leaves of C. occidentalis administrated per os had stronger diuretic effects than reference drugs at doses used, both in acute (24-h) and subchronic (7 d) studies. The acute administration of the extract at the dose with the more marked response (400 mg/kg) induced an increase of 107.58 % in urinary excretion (compared to negative control group), against 84.27 % and 48.05 % with furosemide and HCTZ, respectively. Still in the acute study, the extract also accelerated the elimination of fluid overload and decreased the latency of the first urination (152.43%, at 400 mg/kg, against 114.08% and 42.34% furosemide and HCTZ, respectively) and the diuretic index of groups treated with the extract was higher (2.07 at 400 mg/kg) than furosemide-treated (1.84) and HCTZ -treated (1.48). The pharmacological response of the extract was dose-dependent and comparable results were also obtained in the subchronic study. Notably, the volume of urine excreted increased with the dose of extract, particularly in the first hour after administration. Similar observations were reported in studies assessing the other plants with diuretic activity such as Randia echinocarpa (R. echinocarpa)[23], and Ficus glumosa (F. glumosa)[20]. Such rapid diuretic activity may be due to very high concentration of active molecules of the saponin,flavonoïd and anthraquinone families[24], which presence in extracts of C. occidentalis was detected by phytochemical analysis in our study and previously reported[25].
C. occidentalis extract caused a more marked increase in natriuresis than furosemide and HCTZ compared to the negative control group, more specifically 909.59% at 400 mg/kg against 463.20% and 336.85%, respectively. Furosemide increases urinary excretion of sodium by inhibiting Na+/K+/2Cl-1symporter (co-transporter system) in the thick ascending limb of the Henley loop[20], while HCTZ inhibits the Na+/Cl-1symporter (co-transporter system) in the distal convoluted tubule, by competing for the Cl-1binding site,and increasing the excretion of Na+and Cl-1[20]. Whether the extract induces the suppression of renal tubular reabsorption of water and electrolytes by one of these processes or by another mechanism is still to be determined. However, although during acute testing the extract only induced a strong elimination of Na+, as both diuretic reference drugs, K+elimination by extract subchronic treatment became marked only from 6 d of treatment onward, against 2 d for furosemide and 4 d for HCTZ-treated. These observations suggest that C. occidentalis acted as a K+-sparing diuretic[26,27].
C. occidentalis was well tolerated with an encouraging safety profile in subchronic administration. Glucose and albumin were not present in treated rats' urine, and no significant change was observed in the urinary creatinine levels.Instead, a marked reduction was observed in the concentration of urea in the urine compared to negative control group, the K+plasmatic concentration was increased, and Na+and Cl-concentrations in the plasma were significantly decreased. Taken together, these results indicate that C. occidentalis may act as a loop diuretic which inhibit the Na+/K+/Cl-co-transporter system in the thick ascending loop of the nephron, thus increasing natriuresis and kaliuresis[28].
C. occidentalis also caused the acidification of urine. There was a significant reduction in the osmolarity of urine in rats treated with the extract. C. occidentalis may impair the basal secretion of ADH and reduce the responsiveness of uriniferous tubules to the action of ADH. Inhibition of ADH causes polyurea with low osmolarity[29]. Furthermore, in our study radioimmunoassay revealed a decrease in aldosterone in animals treated with the extract, further suggesting that the stimulation of diuresis by the aqueous extract of the leaves of C. occidentalis may be comparable to furosemide mechanism. Notably, glomerular filtration rate measured by creatinine clearance was reduced, together with glomerular filtration rate, probably due to increases in the Na+load available for Na+/K+exchange[30,31],which indicates that the increase in diuresis may also originate from changes in glomerular filtration, besides changes at tubular level.
Findings of the study also indicated that C. occidentalis may have antioxidant effects. Comparable observations were reported in a number of other plants with diuretic properties[32]. In addition,the extract also decreased hydroperoxide levels in homogenates,malondialdehyde levels in plasma, and the activity of catalase in homogenates and hemolysates, which are markers of oxidative stress[33].
The results of the present study strongly suggest that C. occidentalis leaves' aqueous extract have potent and dose-response diuretic and antioxidant properties, both in acute and subchronic use in rats. These findings justify at least partly the use of this extract in folk medicine for the treatment of hypertension. Future studies aimed at identifying the active principles accounting for these effects of C. occidentalis leaves' aqueous extract may lead to the discovery of a potent diuretic, potentially with antioxidant properties.
Conflict of interest statement
[1] Fonarow GC, Smith EE, Reeves MJ, Pan W, Olson D, Hernandez AF, et al. Hospital-level variation in mortality and rehospitalization for medicare beneficiaries with acute ischemic stroke. Stroke 2011; 42:159-166.
[2] Barakat LA, Mahmoud RH. The antiatherogenic, renal protective and immunomodulatory effects of purslane, pumpkin and flax seeds on hypercholesterolemic rats. N Am J Med Sci 2011; 3:411-417.
[3] Brandi L. 1alpha(OH)D3 One-alpha-hydroxy-cholecalciferol--an active vitamin D analog. Clinical studies on prophylaxis and treatment of secondary hyperparathyroidism in uremic patients on chronic dialysis. Dan Med Bull 2008; 55:186-210.
[4] Strand V. Are COX-2 inhibitors preferable to non-selective non-steroidal anti-inflammatory drugs in patients with risk of cardiovascular events taking low-dose aspirin? Lancet 2007; 370:2138-2151.
[5] Seethapathy GS, Ganesh D, Santhosh Kumar JU, Senthilkumar U,Newmaster SG, Ragupathy S, et al. Assessing product adulteration in natural health products for laxative yielding plants, Cassia, Senna, and Chamaecrista, in Southern India using DNA barcoding. Int J Legal Med 2014; Article doi:10.1007/s00414-014-1120-z.
[6] Yadav JP, Arya V, Yadav S, Panghal M, Kumar S, Dhankhar S. Cassia occidentalis L.: a review on its ethnobotany, phytochemical and pharmacological profile. Fitoterapia 2010; 81:223-230.
[7] Bukhari NA, Siddique I, Perveen K, Siddiqui I, Alwahibi MS. Synthetic seed production and physio-biochemical studies in Cassia angustifolia Vahl. - a medicinal plant. Acta Biol Hung 2014; 65:355-367.
[8] Epifano F, Fiorito S, Locatelli M, Taddeo VA, Genovese S. Screening for novel plant sources of prenyloxyanthraquinones: Senna alexandrina Mill. and Aloe vera (L.) Burm. F. Nat Prod Res 2015; 29:180-184
[9] Chen L, Yang Y, Yuan P, Yang Y, Chen K, Jia Q, et al. Immunosuppressive Effects of A-Type Procyanidin Oligomers from Cinnamomum tamala. Evid Based Complement Alternat Med 2014; 2014: 365258.
[10] Cong Q, Shang M, Dong Q, Liao W, Xiao F, Ding K. Structure and activities of a novel heteroxylan from Cassia obtusifolia seeds and its sulfated derivative. Carbohydr Res 2014; 393:43-50
[11] Shao F, Chen HJ, Liu RH, Hou YC, Ren G, Huang HL, et al. Effects of heishunpian total alkaloids on Cassia acutifolia induced mice diarrhea and contraction of isolated intestinal smooth muscle in rats. Zhong Yao Cai 2013; 36:1805-1809.
[12] Somova LO, Nadar A, Rammanan P, Shode FO. Cardiovascular,antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine 2003;10:115-121.
[13] Nakamura S, Xu F, Ninomiya K, Nakashima S, Oda Y, Morikawa T, et al. Chemical structures and hepatoprotective effects of constituents from Cassia auriculata leaves. Chem Pharm Bull (Tokyo) 2014; 62:1026-1031.
[14] Purushotham KN, Annegowda HV, Sathish NK, Ramesh B, Mansor SM. Evaluation of phenolic content and antioxidant potency in various parts of Cassia auriculata L.: A traditionally valued plant. Pak J Biol Sci 2014;17:41-48.
[15] Silva CR, Monteiro MR, Rocha HM, Ribeiro AF, Caldeira-de-Araujo A,Leitao AC, et al. Assessment of antimutagenic and genotoxic potential of senna (Cassia angustifolia Vahl.) aqueous extract using in vitro assays. Toxicol In Vitro 2008; 22:212-218.
[16] Matsuzawa N, Takamura T, Kurita S, Misu H, Ota T, Ando H, et al. Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology 2007; 46:1392-1403.
[17] Wang X, Li Q, Shen L, Yang J, Cheng H, Jiang S, et al. Fumigant,contact, and repellent activities of essential oils against the darkling beetle, Alphitobius diaperinus. J Insect Sci 2014; 14:75.
[18] Verma L, Singour PK, Chaurasiya PK, Rajak H, Pawar RS, Patil UK. Effect of ethanolic extract of Cassia occidentalis Linn. for the management of alloxan-induced diabetic rats. Pharmacognosy Res 2010;2:132-137.
[19] Sreejith G, Latha PG, Shine VJ, Anuja GI, Suja SR, Sini S, et al. Antiallergic, anti-inflammatory and anti-lipidperoxidant effects of Cassia occidentalis Linn. Indian J Exp Biol 2010; 48:494-498.
[20] Ntchapda F, Djedouboum A, Kom B, Nana P, Bonabe C, Maguirgue K,et al. Diuretic activity of the aqueous extract leaves of Ficus glumosa Del. (Moraceae) in rats. Sci World J 2014; 2014:693803. Article doi:10.1155/2014/693803.
[21] Vogel GH. Drug discovery and evaluation: Pharmacological assays. Germany: Springer- Verlag; 2002.
[22] Trease GE, Evans MC. Textbook of pharmacognosy. Tindall, London:Bailliere; 1983.
[23] Vargas Solis R, Perez Gutierrez RM. Diuretic and urolithiatic activities of the aqueous extract of the fruit of Randia echinocarpa on rats. J Ethnopharmacol (2002; 83:145-147.
[24] Maghrani M, Zeggwagh NA, Haloui M, Eddouks M. Acute diuretic effect of aqueous extract of Retama raetam in normal rats. J Ethnopharmacol 2005; 99:31-35.
[25] Takahashi M, Sakurai K, Fujii H, Saito K. Identification of indicator components for the discrimination of Cassia plants in health teas and development of analytical method for the components. J AOAC Int 2014;97:1195-1201.
[26] Kreydiyyeh SI, Usta J. Diuretic effect and mechanism of action of parsley. J Ethnopharmacol 2002; 79:353-357.
[27] Rang HP, Dale MM, Ritter JM. Pharmacology. London: Churchill Livingstone; 1995.
[28] Ratnasooriya WD, Pieris KP, Samaratunga U, Jayakody JR. Diuretic activity of Spilanthes acmella flowers in rats. J Ethnopharmacol 2004;91:317-320.
[29] Osorio FV, Teitelbaum I. Mechanisms of defective hydroosmotic response in chronic renal failure. J Nephrol 1997; 10:232-237.
[30] Jouad H, Lacaille-Dubois MA, Eddouks M. Chronic diuretic effect of the water extract of Spergularia purpurea in normal rats. J Ethnopharmacol 2001; 75:219-223.
[31] Jackson EK. Drugs affecting renal and cardiovascular function. In:Hardman JC, Gilman AG, Limbird LE (eds). Goodman and gilman's the pharmacological basis of therapeutics. New York: Pergamon press;1996,p. 685-713.
[32] Gupta RK, Kesari AN, Diwakar S, Tyagi A, Tandon V, Chandra R, et al. In vivo evaluation of anti-oxidant and anti-lipidimic potential of Annona squamosa aqueous extract in type 2 diabetic models. J Ethnopharmacol 2008; 118:21-25.
[33] Ly J, Maquet P. Stroke and aging. Rev Med Liege 2014; 69:315-317.
15 June 2015
s declare no competing financial interests.
Acknowledgments
Dr. Fidèle Ntchapda, PhD, Department of Biological Sciences, Faculty of Sciences, University of Ngaoundere, PO Box 454 Ngaoundere,Cameroon.
Tel: +237677921869/ +2372421584 84 /+237691313746
E-mail: ntchapda71@yahoo.fr
Foundation project: The authors received financial support for the research, by the financing allocated for function of the Laboratory of the Medicinal Plants, Health and Galenic Formulation of the Department of Biological Sciences.
The authors thank Mr ALLARAMADJI Ndohortongar of N'Djaména hospital Le Bon Samaritain, for their assistance in this project. The authors also thank the Laboratory of the Medicinal Plants, Health and Galenic Formulation of the Department of Biological Sciences. The authors received financial support for the research, by the financing allocated for function of the Laboratory of the Medicinal Plants, Health and Galenic Formulation of the Department of Biological Sciences.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Chemical composition, mechanism of antibacterial action and antioxidant activity of leaf essential oil of Forsythia koreana deciduous shrub
- A pilot study of antioxidant potential of endophytic fungi from some Sudanese medicinal plants
- Prevalence of filarial parasites in domestic and stray cats in Selangor State, Malaysia
- Genetic variation of Leptospira isolated from rats catched in Yogyakarta Indonesia
- Molecular survey of tick-borne pathogens in Ixodid ticks collected from hunted wild animals in Tuscany, Italy
- Epidemiology of influenza viruses from 2009-2013-A sentinel surveillance report from Union territory of Puducherry, India
