Effect of PI3K-mediated autophagy in human osteosarcoma MG63 cells on sensitivity to the chemotherapy drug cisplatin
2015-10-28XuDongMiaoLeCaoQiangZhangXiaoYingHuYiZhang
Xu-Dong Miao, Le Cao, Qiang Zhang, Xiao-Ying Hu, Yi Zhang*
1Department of Orthopedics, the Second Affiliated Hospital of Zhejiang University Schhol of Medicine, Hangzhou 310009, Zhejiang, China
2Department of Gynaecology and Obstetrics, the First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou 310006, Zhejiang, China
Effect of PI3K-mediated autophagy in human osteosarcoma MG63 cells on sensitivity to the chemotherapy drug cisplatin
Xu-Dong Miao1, Le Cao1, Qiang Zhang1, Xiao-Ying Hu2, Yi Zhang2*
1Department of Orthopedics, the Second Affiliated Hospital of Zhejiang University Schhol of Medicine, Hangzhou 310009, Zhejiang, China
2Department of Gynaecology and Obstetrics, the First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou 310006, Zhejiang, China
ARTICLE INFO
Article history:
in revised form 20 July 2045
Accepted 15 August 2015
Available online 20 September 2015
Autophagy
Osteosarcoma
LC3
Cisplatin
Proliferation
Objective: To investigate the influence of autophagy on sensitivity to the chemotherapy drug cisplatin (DDP) and the role of PI3K in autophagy. Methods: MTT methods and flow cytometer, with rapamycin up-regulating the autophagy and 3-MA down-regulating the autophagy, were employed to measure the proliferation inhibition rate on DDP-treated osteosarcoma cells and the change in cell cycle. The expression of intracellular protein was detected by Western blot. The autophagy of MG63 cell was observed using fluorescence microscope and transmission electron microscope. Results: Western blot showed that basic autophagy level of MG63 cell was significantly lower than that of hFOB cell. MTT test revealed that the cell proliferation inhibition rate in the group treated with rapamycin and DDP, group treated with 3-MA and DDP, and group only treated with DDP was significantly different. It was demonstrated by the flow cytometry that in group treated with DDP, inhibition on autophagy can increase the cell numbers in G1phase and reduce the cell numbers in S phase of cell cycle. Increase of autophagosome in MG63 cytoplasm was observed under fluorescence microscope. Conclusions: Up-regulating the autophagy significantly reduced the sensitivity of MG63 cell to chemotherapy with DDP. DDP induced autophagy of MG63 cell and blocked the cell cycle at G1phase.
1. Introduction
Osteosarcoma is one of the most common malignant bone tumor in adolescent[1]. With high malignant degree and poorer prognosis,osteosarcoma seriously influences the prognostic survival of patients. Although the present classical surgery combined with neoadjuvant chemotherapy can significantly improve patient's life quality and raise the survival rate, the relapse, metastasis and multi-drug resistance to chemotherapy heavily threaten the longterm survival rate of patients[2-4]. Therefore, it is very important to enhance the sensitivity of osteosarcoma to chemotherapy and studyits molecular mechanism during the process of clinical treatment.
Autophagy is the main means, which is relatively conservative,to degrade autologous organelles or protein in evolutionary of eukaryocyte[5], regulating and controlling the cell proliferation and function. A study showed that the level of autophagy activity plays an important role in occurrence and development of malignant tumor[6]. Microtubule-associated protein 1 light chain 3 (LC3) is located at the membrane surface of preautophagosome and autophagosome, and it is usually used as specific marker of autophagy level[7]. As the main gene for regulation of autophagy,Beclin 1 is also regarded as maeker of autophagy; it can inhibit the tumor growth through enhancement of autophagy, being an important autophagy-associated tumor suppressor gene[8]. Molecular signaling pathway of phosphatidylinositol 3-kinase(PI3K) is involved in regulation of cell proliferation, apoptosis,relapse and metastasis etc. In addition, PI3K/Akt plays an important role in tumor cell proliferation, apoptosis, autophagy, relapse and metastasis as well as degradation of extracellular matrix etc[9].Interference of abnormal activation of PI3K/Akt can facilitate programmed cell death, thus inhibiting the tumor growth. Hence,drugs targeting the inhibition of PI3K/Akt are more and more valued in tumor research.
There is still controversy regarding the role of autophagy in tumor treatment[10], and the inhibition and protective effects of autophagy during tumor treatment need to be further elaborated. In the present study, human osteosarcoma cell MG63 was treated with autophagy inhibitor 3-methyladenine (3-MA), autophagy promoter rapamycin,PI3K inhibitor LY294002 and clinical commonly used chemotherapy drug cisplatin (DDP), to detect cell proliferation activity, cell cycle and changes in molecular level of autophagy, so as to investigate the changes of autophagy activity and molecular mechanism of PI3K/Akt during autophagy in the chemotherapy of osteosarcoma,providing theoretical basis for combined chemotherapy in treatment of osteosarcoma.
2. Materials and methods
2.1. Cell line
Human osteoblast hFOB and osteosarcoma cell MG63 were purchased from Cell Bank of Chinese Academy of Science and cultured in DMEM medium containing 10% fetal bovine serum(Gibco), 100 IU/mL penicillin, 100 μg/mL streptomycin at 37 ℃and 5% CO2condition. Cisplatin, DDP, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 3-MA, rapamycin and LY294002 were purchased from Sigma company.
Respective treatments: No drug was give to the control group;Group DDP was treated with 10 μg/mL DDP; Group PI3K inhibitor was treated with 5 μmol/L PI3K specific inhibitor LY294002;Group RAPA was treated with 10 μmol/L rapamycin; Group 3-MA was treated with 5 mmol/L 3-MA; Group DDP+PI3K inhibitor was treated with 5 μmol/L LY294002 and 10 μg/mL DDP; Group DDP+RAPA was treated with 10 μmol/L rapamycin, and 1 h later,10 μg/mL DDP was added; Group DDP+3-MA was treated with 5 mmol/L 3-MA, and 1 h later, 10 μg/mL DDP was added.
2.2. Detection of cell proliferation activity by MTT method
1×104cells at logarithmic phase were inoculated in 96-well plate with each well. Different drugs were added and the plate was cultured for 24 h. Then 20 μL MTT solution (5 mg/mL) was added into each well and cultured for 4 h. Culture solution was removed,and each well was added with 150 μL dimethylsulfoxide and shaken for 10 min. ELISA plate reader was used to measure the absorbance(OD) at 490 nm. Cell proliferation inhibition rate (%) = (1-ODtestgroup/ ODcontrolgroup)×100%.
2.3. Analysis of cell cycle by flow cytometry
Human osteosarcoma cells MG63 from different groups were inoculated in 6-well plate. After cell adherence, the cells were treated with different drugs for 36 h, and collected, centrifuged, washed with phosphate buffer solution (PBS) and added with 4 ℃ 70% ethanol for overnight. Then the cells were centrifuged, added with 150 μL propidium iodide and 150 μL RNaseA solution for staining for 30 min at 4 ℃ avoiding the light. BD FACSort CellQuest software of flow cytometry was used to analyze the cell cycle and obtain percentage of cell cycle.
2.4. Observation of cell morphological change through transmission electron microscope
Cells at logarithmic phase were interfered with different drugs for 72 h, fixed by 3% glutaraldehyde for 2 h, washed by PBS for 10 min for 3 times, then fixed by 2% osmic acid for 2 h and washed by PBS for 10 min for 3 times. The cells were dehydrated using gradient ethanol which is then replaced with acetone, embedded and aggregated. The paraffin-embedded cells were trimmed, cut into ultrathin sections, stained with uranium and subsequently with lead. Then the cell morphology and structure were observed under transmission electron microscope.
2.5. Real-time quantitative PCR
Cells from different groups were collected and total RNA was extracted according to instruction of TRIzol. About 2 μg of RNA was used to construct 25 μL reverse transcription system, and the reverse transcription was performed at 42 ℃ for 60 min and then 75 ℃ for 10 min. Then 1 μL reverse transcription product was used as template to amplify using SYBR® Premix Ex Taq™Ⅱ. PCR reaction was performed at 94 ℃ for 2 min, 94 ℃ for 30 s and 60 ℃for 34 s with a total of 35 cycles. The value of 2-△△Ctwas used to indicate the relative expression level of target gene mRNA. The primer sequences are shown in Table 1.

Table 1 Primer sequences used for real-time fluorescent quantitative PCR.
2.6. Western blot analysis
Cells were lysed on ice for 20 min using RIPA lysis buffer, and centrifuged at 12 000 rpm at 4 ℃ for 20 min. The supernatant was collected and concentration of protein sample was quantitatively measured by BCA. About 80 μg total protein was separated by SDSPAGE, transferred to PVDF membrane and blocked by 5% skimmed milk at room temperature for 2 h. Proper proportion of primary antibody (Cell Signaling Technology) was added, and the mixture was placed overnight at 4 ℃. Then membrane was washed for 3 times (6 min each wash). HRP-labeled goat anti-rabbit and goat antimouse secondary antibodies were added respectively (1:5 000). The mixture was incubated for 2 h at room temperature and exposed in darkroom by ECL developer. The relative expression was indicated by the gray value ratio of target protein and internal reference protein.
2.7. Intracellular expression of GFP-LC3 fusion protein
MG63 cell was transfected with GFP-LC3 plasmid according to the instruction of Lipofectamine2000. Stable GFP-LC3 fusion protein was treated under different conditions for 24 h. The culture solution was then removed, and cells were washed by PBS for 2 times, and then were observed and photographed by laser scanning confocal microscope.
2.8. Statistical analysis
Statistical analysis was conducted using SPSS13.0 software. Data was expressed as mean±SD. t-test and One-way ANOVA were used to compare data between two groups and data among multiple groups respectively. Difference was statistically significant when P<0.05.
3. Results
3.1. Expression of autophagy associated protein in human osteosarcoma cells
The expression of autophagy associated protein PI3K, Bcl-2 and Beclin-1 as well as LC3-Ⅰ/LC3-Ⅱ protein in MG63 and hFOB was detected by Western blot. LC3, the homologue of yeast Atg,is a marker on membrane of autophagosome. When autophagy is enhanced, expression levels of LC3-Ⅰ and LC3-Ⅱ are lowered and increased respectively. Beclin-1, the homologue of yeast, can accelerate the formation of autophagosome; and its expression level can reveal the degree of autophagy. Relative amount of autophagic marker protein LC3-Ⅱ/LC3-Ⅰ and Beclin-1 in human osteosarcoma cells was significantly lower than that in normal osteoblast (Figure 1) (P<0.01), indicating that the basic autophagic activity of MG63 cell is lower than that of hFOB cell. The expression of anti-apoptotic protein Bcl-2 and PI3K was significantly increased (P<0.01). Therefore, lowering of autophagic level, inhibition on apoptosis and abnormal expression of PI3K play an important role in occurrence and development of osteosarcoma.
3.2. Effect of autophagy on osteosarcoma cell sensitivity to chemotherapy with DDP
Osteosarcoma cell was treated with DDP (10 μg/mL), different concentrations of autophagy inhibitor 3-MA (1, 5 and 50 mmol/ L) and autophagy promoter rapamycin (1, 10 and 100 μmol/L) to verify the effect of autophagy on osteosarcoma cell sensitivity to chemotherapy with DDP. The results of MTT test are showed in Figure 2. Rapamycin at 1 μmol/L and 10 μmol/L can weaken the sensitivity of MG63 cell to DDP, lowering the inhibition rate. This effect was significantly different from that in cells only treated with DDP; when the concentration of rapamycin was increased to 100 μmol/L, it showed a reverse result. Hence, rapamycin at 10 μmol/L was selected to perform the subsequent experiment. 3-MA at 1-50 mmol/L dose-dependently increased the inhibition rate of DDP on MG63 cells. Significant difference was observed at 5 mmol/L and the inhibition rate achieved about 82%. However, the inhibition rate was too high at 50 mmol/L, and cells can hardly survive. Therefore 3-MA at 5 mmol/L was used to conduct the subsequent experiment. This experiment revealed that interdicting autophagy can promote the inhibitory effect of DDP on MG63 cell activity.
3.3. Role of PI3K in MG63 cell autophagy and sensitivity to chemotherapy
MG63 cells at logarithmic phase were treated with different drugs. Cells in each group were incubated for 24 h and used for the subsequent experiment. MTT test revealed that after interdicting autophagy, 3-MA facilitated the inhibition of DDP on MG63 cell growth. After promoting autophagy, rapamycin reduced inhibition of DDP on MG63 cell growth. After inhibiting PI3K, LY294002 reduced inhibition of DDP on MG63 cell growth. These results were significantly different (Figure 3) (P<0.01). In conclusion, it is proved that autophagy plays an important role in sensitivity of MG63 to chemotherapy with DDP, and inhibition on autophagy can enhance the sensitivity to chemotherapy; however, promotion of autophagy weakened the sensitivity to chemotherapy. PI3K exhibited an important inhibition effect on autophagy.
3.4. Influence of DDP on autophagy associated protein in MG63 cell
The expression of mRNA (Figure 4B, C, D) and protein (Figure 4A) of autophagy associated protein PI3K, Bcl-2 and Beclin-1 in MG63 cells from different groups was measured. It was found that DDP can significantly inhibit the expression of PI3K and Bcl-2 and promote the expression of Beclin-1. When 3-MA was used to inhibit autophagy, expression of PI3K and Beclin-1 was partially raised again, but when rapamycin was used to accelerate autophagy,expression of PI3K was partially lowered; PI3K inhibitor LY294002 can significantly enhance the inhibition of DDP on PI3K and Bcl-2,and its promotion on Beclin-1. It is well-proved that DDP inhibited the expression of PI3K and Bcl-2 and promoted the expression of Beclin-1, indicating that DDP exhibited anti-cancer effect partially through enhancement of cell autophagy. PI3K plays an important role in the cell autophagy process; PI3K inhibitor can significantly increase the sensitivity to chemotherapy with DDP, thus enhancing the lethal effect on osteosarcoma cells and providing new insight into new combined chemotherapy for clinical treatment.
3.5. Effect of DDP on degree of MG63 cell autophagy
Flow cytometry was used to detect the autophagy of GFPLC3 fusion protein in MG63 cell. As showed in Table 2, PI3K inhibitor can facilitate the autophagy of MG63 cell, displaying the inhibition effect of PI3K on cell autophagy. Rapamycin can promote autophagy, thus enhance the inhibition effect of DDP.
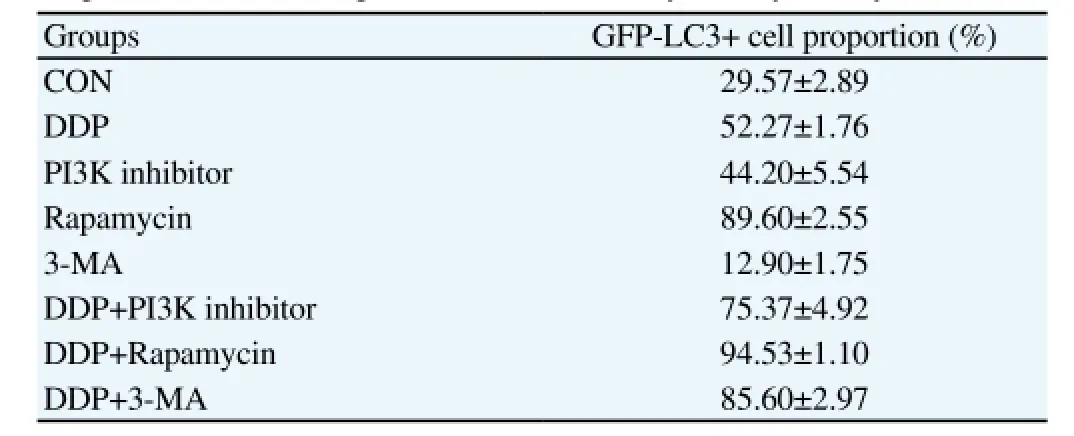
Table 2 Proportion of GFP-LC3 positive cells detected by flow cytometry.
3.6. Impact of DDP on MG63 cell cycle
The impact on GFP-LC3 fusion cell cycle was studied by flow cytometry. As showed in Table 3 and Figure 5, DDP can increase the cell number of MG63 cell at G1 phase, and reduced the cell number at S phase. The results revealed that DDP can block MG63 cells at G1phase of the cell cycle, and PI3K inhibitor can increase the inhibition effect of DDP.

Table 3 Cell cycle of MG63 cells from different groups.
3.7. Changes of DDP-induced cell autophagy detected by GFP-LC3 fluorescence
As showed in Figure 6, autophagy specific marker protein LC3, which is marked by green fluorescent protein, revealed dot-like scattered distribution of autophagy around on the cell membrane. When rapamycin promoted the autophagy, fluorescence agglutination was increase, forming graininess-like fluorescence agglutination spot.
3.8. Influence of DDP on cell morphology and structure observed by transmission electron microscope
Changes of ultramicro morphology and structure of MG63 cells was observed under transmission electron microscope. As showed in Figure 7, organells and chromosome morphology in cytoplasm of control group were normal. After interference of different treatment,Group DDP which promoted the autophagy showed a lot of double membrane structure in cytoplasm and many autophagosome.
4. Discussion
Mechanism of occurrence and development of malignant tumor is complicated, and it has not been clarified yet. With the development of surgery combined neoadjuvant chemotherapy, the prognosis of osteosarcoma is remarkably improved[11]. Resistance to chemotherapy drugs heavily influenced the effectiveness of the chemotherapy drugs. Therefore, discovery of effective drugs that can increase the sensitivity to chemotherapy plays an important role in anti-tumor effect. Chemotherapy drugs kill the tumor cells through autophagy, necrosis, apoptosis and mitotic mutation etc[12]. Chemotherapy drug induces the occurrence of tumor cell autophagy,namely, it together with apoptosis can induce cell necrosis, and it also acts as survival mechanism for the self-protection of tumor cells in adverse environment[13]. Adjuvant chemotherapy is significant in treatment of osteosarcoma, and patient's sensitivity to chemotherapydrugs is the key factor that affects the prognostic[14].
In the present research, the expression of LC3 and Beclin-1, the important regulatory factors for autophagy was studied firstly. LC3 appears as LC3-Ⅰ and LC3-Ⅱ in the cytoplasm, but LC3-Ⅰ is converted to LC3-Ⅱ when autophagy occurs. LC3-Ⅱ is the significant protein of autophagosome which is considered as the molecular marker of autophagosome, so the ratio of LC3-Ⅱ/LC3-Ⅰ was employed to reflect the degree of cell autophagy[15]. Our findings showed that the ratio of LC3-Ⅱ/LC3-Ⅰ in MG63 cell was remarkably lower than that in osteoblast, revealing the lower autophagy activity of MG63 cells compared with normal cells. In addition, we found that the expression of PI3K in osteosarcoma cells are significantly increased, which may be related to the lowering of autophagy activity. Hence in the experiment, MG63 cells were treated with DDP, and the result revealed that DDP can up-regulate the ratio of LC3-Ⅱ/LC3-Ⅰ, down-regulate the expression of Beclin-1, and remarkably decrease the MG63 cell proliferation activity, indicating that DDP can promote the autophagy of MG63 cell. Beclin-1 is a specific gene regulating the autophagy, and it is involved in regulation of autophagy by interaction with multiple protein[16]. The results of our experiment displayed gradual increase of DDP-induced Beclin-1 expression, which illustrated that DDP can induce MG63 cell autophagy. Meanwhile, it can be presumed that DDP facilitates MG63 cell autophagy and inhibites MG63 cell proliferation activity by inhibiting the expression of PI3K according to the decrease in expression of PI3K, Moreover, autophagy was promoted with the increase in concentration of rapamycin, but the proliferation inhibition rate of DDP was obviously lowered. However, autophagy was inhibited with increase in concentration of 3-MA, and the proliferation inhibition rate of DDP was significantly raised. This result indicates that up-regulation of autophagy can remarkably reduce the sensitivity of DDP-treated osteosarcoma cells to chemotherapy. The inhibition effect of autophagy on sensitivity to chemotherapy with DDP fully proves the important role of autophagy in occurrence and development of osteosarcoma. Inhibition on autophagy can significantly enhance the sensitivity of osteosarcoma cells to chemotherapy drugs[17]. Pathway of PI3K/ Akt is vital in cell proliferation, differentiation and apoptosis etc,and it is also an important signaling pathway regulating the cell autophagy[18]. A research stated that under environmental stress,PI3K/Akt can mediate rapamycin protein mTOR, negatively regulating the cell autophagy[19]. When autophagy is inhibited by LY294002, proliferation inhibition rate of DDP was significantly increased. The effect of autophagy on DDP inhibiting MG63 cell activity was observed in this study for the first time. Our findings showed that the cell proliferation inhibition rate in Group DDP + rapamycin was lower than its counterpart in Group DDP, indicating that up-regulation of autophagy can remarkably reduce the sensitivity of osteosarcoma cells to chemotherapy drug DDP, which provide theoretical basis for further anti-cancer treatment.
In conclusion, autophagy participates in the anti-tumor effect of chemotherapy drugs, lowering the sensitivity to chemotherapy. A study found that activation of autophagy can induce cell death, but autophagy can protect the effect of anti-tumor therapy by inhibiting tumor cell apoptosis[20]. The cell cycle analyzed by flow cytopetry in the present study showed that when MG63 cell was treated with DDP, the cell number of MG63 cell increased at G1 phase, and reduced at S phase. PI3K can enhance the inhibition effect of DDP,which displayed that DDP can block the growth cycle of MG63 cells, thus inhibiting the cell growth. PI3K inhibitor can increase the sensitivity to chemotherapy with DDP. The main characteristics of autophagy in cell morphology is the presence of vesicle with double membrane[21]. Observation under transmission electron microscope revealed that after MG63 cell was treated with DDP,there was remarkable increase in formation of autophagosome with double membrane structure around the cytoplasm and organelles. This indicated that DDP can facilitate the activation of MG63 cell autophagy. Through fluorescent protein detection test for LC3, it was found that dot-like green fluorescence was distributed around on the cell membrane, which revealed the activation of autophagy. This fully illustrated that autophagy is involved in osteosarcoma cell proliferation inhibited by DDP as well as the cell cycle. Based on the previous research findings, the present study investigates the influence of DDP on human osteosarcoma cell proliferation and the cell autophagy, but the mechanism of specific molecular signaling pathway need to be further studied. This present study provides research basis for relation of cell cycle, cell proliferation and autophagy, offering theoretical basis for further clinical treatment of osteosarcoma.
Autophagy can scavenge the abnormal organelle, cell nucleus and protein, protecting the cell. During the process of anti-tumor treatment, autophagy can protect the cell, scavenge intracellular toxic substance, reduce toxicity of chemotherapy drugs and lower sensitivity to chemotherapy under environmental stress[22]. Meanwhile, the activation of autophagy is enhance, accelerating the cell death. Therefore, induction and enhancement of tumor cell autophagy activity become the new insight into anti-tumor research[23]. Intensive study on regulatory mechanism of autophagy and effect mechanism of autophagic cell death in development of tumor, how to induce improvement of cell autophagy activity, as well as autophagy in sensitivity to anti-tumor chemotherapy drugs is of very important clinical value for anti-tumor treatment. The present study found that chemotherapy drug DDP can effectively induce autophagic activation of MG63 cell and inhibit the cell proliferation activity, and the significant role of autophagy in drug-resistant tumor cell is also observed. Consequently, subtle regulation of autophagy will be promising in treatment of tumor, and this will provide the important theoretical basis for tumor treatment by combiningchemotherapy drugs with autophagy regulator.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res 2010; 152: 3-13.
[2] He H, Ni J, Huang J. Molecular mechanisms of chemoresistance in osteosarcoma (Review). Oncol Lett 2014; 7(5): 1352-1362.
[3] Mittal N, Kent PM, Ording J. Metastatic and recurrent bone primary bone cancers. Curr Probl Cancer 2013; 37(4): 215-224.
[4] Zhou J, Wu S, Chen Y, Zhao J, Zhang K, Wang J, et al. microRNA-143 is associated with the survival of ALDH1+CD133+ osteosarcoma cells and the chemoresistance of osteosarcoma. Exp Biol Med (Maywood) 2015;doi: 10.1177/1535370214563893.
[5] Ponpuak M, Mandell MA, Kimura T, Chauhan S, Cleyrat C, Deretic V. Secretory autophagy. Curr Opin Cell Biol 2015; 35: 106-116.
[6] Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell 2008; 132(1): 27-42.
[7] Mehta P, Henault J, Kolbeck R, Sanjuan MA. Noncanonical autophagy:one small step for LC3, one giant leap for immunity. Curr Opin Immunol 2014; 26: 69-75.
[8] Wirawan E, Lippens S, Vanden Berghe T, Romagnoli A, Fimia GM,Piacentini M, et al. Beclin1: a role in membrane dynamics and beyond. Autophagy 2012; 8(1): 6-17.
[9] Davis WJ, Lehmann PZ, Li W. Nuclear PI3K signaling in cell growth and tumorigenesis. Front Cell Dev Biol 2015; 3: 24.
[10] Yang ZJ, Chee CE, Huang S, Sinicrope FA. The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther 2011; 10(9): 1533-1541.
[11] Bacci G, Longhi A, Fagioli F, Briccoli A, Versari M, Picci P. Adjuvant and neoadjuvant chemotherapy for osteosarcoma of the extremities: 27 year experience at Rizzoli Institute, Italy. Eur J Cancer 2005; 41(18):2836-2845.
[12] Rajesh E, Sankari LS, Malathi L, Krupaa JR. Naturally occurring products in cancer therapy. J Pharm Bioallied Sci 2015; 7(Suppl 1):S181-S183.
[13] Gump JM, Thorburn A. Autophagy and apoptosis: what is the connection? Trends Cell Biol 2011; 21(7): 387-392.
[14] Robl B, Pauli C, Botter SM, Bode-Lesniewska B, Fuchs B. Prognostic value of tumor suppressors in osteosarcoma before and after neoadjuvant chemotherapy. BMC Cancer 2015; 15: 379.
[15] He R, Peng J, Yuan P, Xu F, Wei W. Divergent roles of BECN1 in LC3 lipidation and autophagosomal function. Autophagy 2015; 11(5): 740-747.
[16] Salminen A, Kaarniranta K, Kauppinen A. Beclin 1 interactome controls the crosstalk between apoptosis, autophagy and inflammasome activation: impact on the aging process. Ageing Res Rev 2013; 12(2): 520-534.
[17] Friedhuber AM, Chandolu V, Manchun S, Donkor O, Sriamornsak P,Dass CR. Nucleotropic doxorubicin nanoparticles decrease cancer cell viability, destroy mitochondria, induce autophagy and enhance tumour necrosis. J Pharm Pharmacol 2015; 67(1): 68-77.
[18] Hirao A. Regulation of cancer behavior mediated by mTOR signal. Nihon Rinsho 2015; 73(5): 773-778.
[19] Singh BN, Kumar D, Shankar S, Srivastava RK. Rottlerin induces autophagy which leads to apoptotic cell death through inhibition of PI3K/ Akt/mTOR pathway in human pancreatic cancer stem cells. Biochem Pharmacol 2012; 84(9): 1154-1163.
[20] Bo Q, Ma S, Han Q, Wang FE, Li X, Zhang Y. Role of autophagy in photoreceptor cell survival and death. Crit Rev Eukaryot Gene Expr 2015;25(1): 23-32.
[21] Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol 2007; 9(10): 1102-1109.
[22] Yang Y, Yang Y, Yang X, Zhu H, Guo Q, Chen X, et al. Autophagy and its function in radiosensitivity. Tumour Biol 2015; doi: 10.1007/s13277-015-3496-x.
[23] Rebecca VW, Amaravadi RK. Emerging strategies to effectively target autophagy in cancer. Oncogene 2015; doi: 10.1038/onc.2015.99.
15 June 2015
Yi Zhang, Department of Gynaecology and Obstetrics, the First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou 310006,Zhejiang, China.
Tel: 13588782069
E-mail: zhangyi00934@163.com
Foundation project: It was supported by Natural Science Funds of Zhejiang Province(Y13H160038).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Diuretic and antioxidant activities of the aqueous extract of leaves of Cassia occidentalis (Linn.) in rats
- Chemical composition, mechanism of antibacterial action and antioxidant activity of leaf essential oil of Forsythia koreana deciduous shrub
- A pilot study of antioxidant potential of endophytic fungi from some Sudanese medicinal plants
- Prevalence of filarial parasites in domestic and stray cats in Selangor State, Malaysia
- Genetic variation of Leptospira isolated from rats catched in Yogyakarta Indonesia
- Molecular survey of tick-borne pathogens in Ixodid ticks collected from hunted wild animals in Tuscany, Italy
