七氟烷后处理对失血性休克猪肠黏膜AQP8和I-FABP表达的影响
2015-06-28陈艳红孙莹杰张毅男王丽晶张铁铮陈克研
陈艳红,孙莹杰,张毅男,王丽晶,张铁铮,陈克研
七氟烷后处理对失血性休克猪肠黏膜AQP8和I-FABP表达的影响
陈艳红,孙莹杰,张毅男,王丽晶,张铁铮,陈克研
目的观察七氟烷后处理对失血性休克巴马小型猪肠黏膜水通道蛋白8(AQP8)和血清肠脂肪酸结合蛋白(I-FABP)表达的影响,探讨七氟烷后处理对失血性休克引起的肠黏膜屏障损伤的保护作用及可能机制。方法18只巴马小型猪随机分为对照组(S组)、失血性休克组(HS组)和七氟烷后处理组(Post/Sev组),每组6只。实验动物术前禁食8h,给予丙泊酚3mg/kg实施麻醉。S组麻醉后经股动脉和颈内静脉置管;HS组麻醉置管后建立失血性休克模型;Post/Sev组于麻醉置管建立失血性休克模型成功后给予2%七氟烷吸入30min。各实验组均于麻醉前(T0)及失血性休克后30min(T1)、1h(T2)、1.5h(T3)、2h(T4)、3h(T5)、4h(T6)时间点颈内静脉取血,ELISA法检测血清I-FABP的含量。失血性休克4h后放血处死实验动物,取肠组织制作病理切片,HE染色观察各组病理组织学变化;用干湿比法计算肠组织的含水量;ELISA法检测肠黏膜AQP8的表达水平。结果与S组相比,HS组和Post/Sev组血清I-FABP含量、肠黏膜AQP8表达水平和肠组织含水量均显著升高,差异有统计学意义(P<0.05);与HS组比较,Post/Sev组上述指标明显降低,差异有统计学意义(P<0.05)。光镜观察显示,S组肠黏膜未见明显变化;HS组肠黏膜损伤严重,可见肠黏膜出血、炎细胞浸润、上皮细胞坏死;Post/Sev组肠黏膜损伤明显减轻,仅见黏膜层腺体轻度扩张,上皮层和固有层中度分离,上皮下间隙轻度水肿,少许炎细胞浸润。结论七氟烷后处理可减轻失血性休克引起的肠黏膜屏障损伤,其机制可能与降低I-FABP和AQP8表达、减轻肠黏膜水肿有关。
肠黏膜;休克,出血性;七氟烷;脂肪酸结合蛋白质类;水通道蛋白质8
失血性休克可以引起“休克肠”的发生,造成肠道损伤,是失血性休克和创伤后的严重并发症。近年来有关失血性休克后肠道损伤及保护的研究逐渐受到关注[1-3]。既往研究表明,七氟烷不仅具有优良的麻醉作用,而且具有一定的器官保护作用[4]。本研究通过建立失血性休克模型,观察七氟烷后处理对巴马小型猪肠黏膜水通道蛋白8(aquaporin 8,AQP8)和血清肠脂肪酸结合蛋白(intestinal fatty acid binding protein,I-FABP)表达的影响,从而探讨七氟烷后处理对失血性休克引起的肠黏膜屏障损伤的保护作用及其可能机制。
1 材料与方法
1.1 动物分组 健康成年巴马小型猪18只,由沈阳军区总医院医学实验动物科提供,雌雄不限,体重22~25kg,随机分3组,每组6只。对照组(S组):麻醉后股动脉置管、颈内静脉置管,观察6h;失血性休克组(HS组):麻醉置管后建立失血性休克模型,观察4h;七氟烷后处理组(Post/Sev组):麻醉置管后建立失血性休克模型,建模成功后吸入2%七氟烷30min,观察4h。
1.2 失血性休克模型的建立 实验动物术前禁食8h,禁水4h,于耳缘静脉给予丙泊酚3mg/kg实施麻醉。麻醉成功后,将巴马小型猪固定于手术台上,进行气管插管(ID 7.5mm)行机械通气,给予2%七氟烷维持麻醉。手术部位备皮,碘伏消毒。右侧股动脉穿刺插管,用于动脉采血和放血建立失血性休克模型;左侧股动脉穿刺,用于监测动脉血压和平均动脉压;右颈内静脉置入三腔中心静脉导管,用于静脉采血;右颈内中心静脉导管下方置入Swan-Ganz导管,用于测量心输出量和温度。置管等操作结束后,将导管缝合固定于皮肤上,停止吸入七氟烷,待猪自主呼吸恢复后,用气体监测仪监测七氟烷浓度,至MAC为0时,在常温(20~22℃)环境下,15min内将40%的血容量(按30ml/kg计算)匀速放出,建立容量控制性失血性休克模型[5]。
1.3 标本采集及检测 分别于T0(实验前)、T1(失血性休克后30min)、T2(失血性休克后1h)、T3(失血性休克1.5h)、T4(失血性休克后2h)、T5(失血性休克后3h)、T6(失血性休克后4h)7个时间点经颈内静脉采血3ml,立即在4℃下3000×g离心10min,分离出血清移入1.5ml离心管中,置于-80℃深低温冰箱保存待测。采用ELISA法检测血清肠I-FABP的含量,所用试剂盒由美国R&D公司提供。
失血性休克后4h放血处死各组动物,立即取空肠、回肠和结肠组织3cm置入组织冻存管中,置于-80℃冰箱保存,采用ELISA法检测肠黏膜AQP8表达的变化,试剂盒由美国R&D公司提供。另取空肠组织3cm,置于4%甲醛溶液中,用于制作病理组织切片,HE染色观察各组病理组织学变化。取空肠、回肠和结肠各5cm,去除肠道内容物和肠系膜,冷盐水冲洗干净,用电子分析天平称量湿重后,置于80℃恒温干燥箱内烤干24h至恒重(两次称重差别≤0.2mg)再称干重,计算肠道组织含水量。肠道组织含水量(%)=(湿重-干重)/湿重×100%。
1.4 统计学处理 采用SPSS 19.0软件进行统计分析,数据结果以表示,组间比较采用单因素方差分析,组内比较采用重复测量设计的方差分析,进一步两两比较采用SNK-q检验,P<0.05为差异有统计学意义。
2 结 果
2.1 血清I-FABP含量的变化 与T0时点比较,各组血清I-FABP含量在T1时点开始升高,HS组和Pre/ Sev组T2-T6时点均显著升高(P<0.05),HS组在休克后1.5h(T3)达到峰值,然后呈下降趋势,Post/Sev组在休克后2h(T4)达到峰值,然后呈下降趋势。在T0时点3组血清I-FABP含量差异无统计学意义(P>0.05),而在T1-T6时点HS组和Pre/Sev组血清中I-FABP含量均明显高于S组,差异有统计学意义(P<0.05),且HS组血清中I-FABP含量明显高于Pre/ Sev组S组,差异有统计学意义(P<0.05,表1)。
2.2 各组肠黏膜AQP8表达水平比较 与S组比较,HS组和Post/Sev组失血性休克4h肠黏膜AQP8表达水平明显升高,差异有统计学意义(P<0.05),且Post/Sev组肠黏膜AQP8表达水平明显低于HS组,差异有统计学意义(P<0.05,表2)。
2.3 各组肠组织含水量比较 与S组比较,HS组和Post/Sev组失血性休克4h空肠、回肠和结肠组织的含水量均明显升高,差异有统计学意义(P<0.05),且Post/Sev组明显低于HS组,差异有统计学意义(P<0.05,表3)。
表1 各组动物血清I-FABP含量比较(ng/L,±s,n=6)Tab. 1 Comparison of the serum I-FABP contention in each group of pigs (ng/L,±s,n=6)
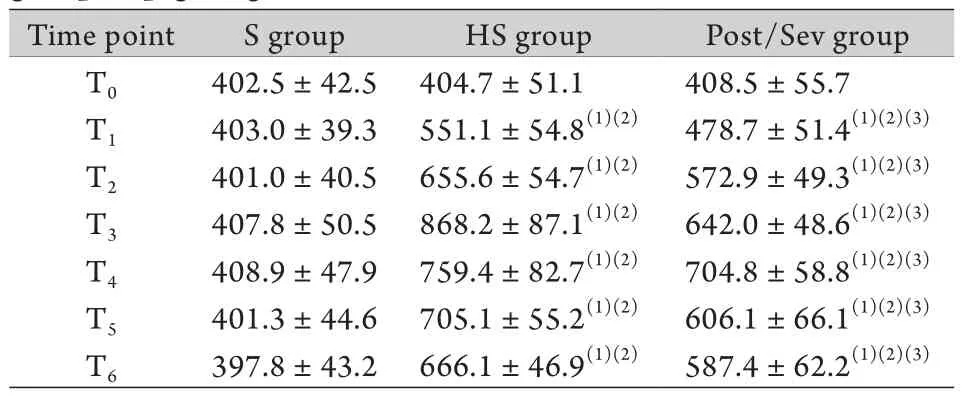
表1 各组动物血清I-FABP含量比较(ng/L,±s,n=6)Tab. 1 Comparison of the serum I-FABP contention in each group of pigs (ng/L,±s,n=6)
(1)P<0.05 compared with T0; (2)P<0.05 compared with S group; (3)P<0.05 compared with HS group
Time point S group HS group Post/Sev group T0 402.5±42.5 404.7±51.1 408.5±55.7 T1 403.0±39.3 551.1±54.8(1)(2) 478.7±51.4(1)(2)(3)T2 401.0±40.5 655.6±54.7(1)(2) 572.9±49.3(1)(2)(3)T3 407.8±50.5 868.2±87.1(1)(2) 642.0±48.6(1)(2)(3)T4 408.9±47.9 759.4±82.7(1)(2) 704.8±58.8(1)(2)(3)T5 401.3±44.6 705.1±55.2(1)(2) 606.1±66.1(1)(2)(3)T6 397.8±43.2 666.1±46.9(1)(2) 587.4±62.2(1)(2)(3)
表2 各组休克后4h时肠黏膜AQP8表达水平比较(ng/ mg,±s,n=6)Tab. 2 Comparison of AQP8 expression levels in intestinal mucosa 4h after hemorrhagic shock in each group of pigs (ng/mg,±s,n=6)
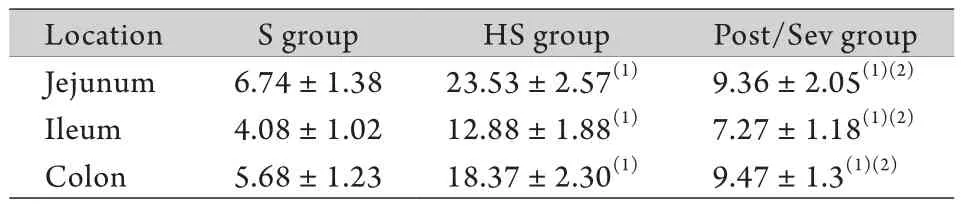
表2 各组休克后4h时肠黏膜AQP8表达水平比较(ng/ mg,±s,n=6)Tab. 2 Comparison of AQP8 expression levels in intestinal mucosa 4h after hemorrhagic shock in each group of pigs (ng/mg,±s,n=6)
(1)P<0.05 compared with S group; (2)P<0.05 compared with HS group
Location S group HS group Post/Sev group Jejunum 6.74±1.38 23.53±2.57(1) 9.36±2.05(1)(2)Ileum 4.08±1.02 12.88±1.88(1) 7.27±1.18(1)(2)Colon 5.68±1.23 18.37±2.30(1) 9.47±1.3(1)(2)
表3 各组休克后4h肠组织含水量比较(%,±s,n=6)Tab. 3 Comparison of the water content after hemorrhagic shock in each group of pigs (%,±s,n=6)
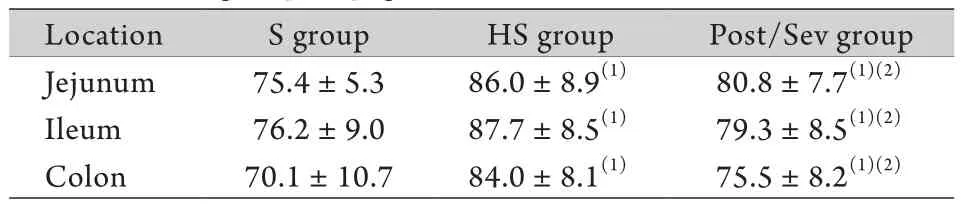
表3 各组休克后4h肠组织含水量比较(%,±s,n=6)Tab. 3 Comparison of the water content after hemorrhagic shock in each group of pigs (%,±s,n=6)
(1)P<0.05 compared with S group; (2)P<0.05 compared with HS group
Location S group HS group Post/Sev group Jejunum 75.4±5.3 86.0±8.9(1) 80.8±7.7(1)(2)Ileum 76.2±9.0 87.7±8.5(1) 79.3±8.5(1)(2)Colon 70.1±10.7 84.0±8.1(1) 75.5±8.2(1)(2)
2.4 巴马小型猪肠黏膜形态学观察 HE染色光镜下观察显示,S组肠黏膜刷状缘正常,无水肿充血,仅见少量炎细胞浸润;HS组肠黏膜损伤严重,可见出血,黏膜层炎细胞浸润,上皮细胞坏死;Post/Sev组肠黏膜损伤较HS组轻,仅见黏膜层腺体轻度扩张,上皮层和固有层中度分离,上皮下间隙轻度水肿,少许的炎细胞浸润(图1)。
3 讨 论
近年研究发现,肠黏膜损伤后的肠道水肿是影响患者转归和住院时长的重要因素[6]。失血性休克后发生的肠道水肿不但与休克时微血管和肠黏膜通透性增加、自由基形成、中性粒细胞激活浸润和细胞因子释放有关,还可能与肠道内的水转运和含水量密切相关。AQP是一类结构和功能类似的非选择性的膜蛋白家族,AQP8在十二指肠、空肠、结肠、肝脏、胰腺中都有表达。研究发现,AQP在肺水肿和脑水肿的形成、肿瘤转归、尿崩症的发展等方面发挥着重要作用[7-9]。Sakai等[10]对5-Fu引起的小鼠腹泻进行研究发现,腹泻小鼠的AQP8表达水平明显下降。Nakano等[11]在研究肠道水代谢的过程中发现,小肠部分切除小鼠的AQP8表达水平增加。Zhao等[12]研究发现,在2,4,6-三硝基苯磺酸(TNBS)诱导的结肠炎大鼠模型中,AQP8和AQP3表达下调。以上研究均提示AQP8对于肠道水液调节具有重要作用。本研究结果显示,休克后4h,HS组巴马小型猪空肠、回肠、结肠AQP8的表达量增加,其肠组织含水量和对照组相比也呈增加趋势,提示AQP8参与了失血性休克后肠组织水肿的形成。
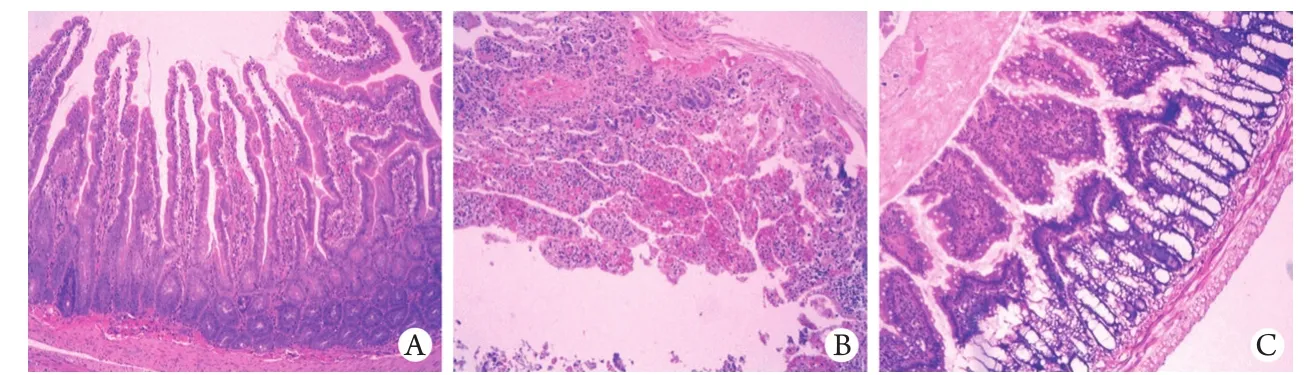
图1 3组肠黏膜病理改变(HE ×10)Fig. 1 Histopathological changes of intestinal mucosa in three groups of pigs (HE ×10) A. S group; B. HS group; C. Post/Sev group
I-FABP是一组存在于小肠的纤毛细胞内、相对分子量为15kD的胞液蛋白。正常情况下外周血中检测不到I-FABP,只有在肠黏膜发生损害时,I-FABP才会进入循环系统中,这一特点使I-FABP成为诊断肠道缺血、肠道屏障损伤的早期敏感指标[13]。近年来针对I-FABP和肠损伤的关系开展了大量研究。Niewold等[14]在大鼠肠道缺血再灌注损伤模型中发现,I-FABP在肠缺血损伤的早期即开始升高。Matsumoto等[15]在208例急性肠缺血患者中发现,对于肠缺血,I-FABP可能是最好的诊断指标。本研究结果显示,HS组巴马小型猪血清I-FABP含量明显高于对照组,结合肠组织形态学观察,提示I-FABP增高与失血性休克后肠道损伤有关。
七氟烷是临床上常用的吸入麻醉药,其对心、脑、肝、肾等器官的保护作用已经得到基础和临床研究的证实[16]。失血性休克是一个不可预期的意外伤害和病理生理学过程,本研究观察了七氟烷后处理对失血性休克巴马小型猪肠屏障功能的影响,结果显示,Post/Sev组巴马小型猪血清I-FABP含量、肠黏膜AQP-8表达水平和肠组织含水量明显低于HS组,可能与七氟烷抑制了肠黏膜细胞AQP8的表达,继而对进入线粒体的水分起到调节作用,最终使线粒体基质达到正常状态,电子传递链激活,线粒体ATP合成增加,从而使肠道水肿和肠道损伤减轻有关。还有实验发现七氟烷后处理可明显增加循环血流量,升高平均动脉压,稳定血流动力学,减少组织灌注不足的发生率,降低乳酸释放,由此推测其可以减轻失血性休克时乳酸堆积引发的渗透梯度改变,从而下调AQP8表达,减轻组织细胞水肿[17]。
综上所述,七氟烷后处理可降低失血性休克巴马小型猪血清I-FABP浓度,减轻休克导致的肠黏膜屏障损伤,其机制可能与七氟烷后处理降低肠黏膜AQP8表达水平、减轻肠组织水肿有关。
[1]Yadav VR, Hussain A, Sahoo K,et al. Remediation of hemorrhagic shock-induced intestinal barrier dysfunction by treatment with diphenyldihaloketones EF24 and CLEFMA[J]. J Pharmacol Exp Ther, 2014, 351(2): 413-422.
[2]Fishman JE, Sheth SU, Levy G,et al. Intraluminal nonbacterial intestinal components control gut and lung injury after traumahemorrhagic shock[J]. Ann Surg, 2014, 260(6): 1112-1120.
[3]Li L, Jiang X, Hou JY,et al. Effects of carbachol matching oral fluid resuscitation on intestinal mucosa blood flow and absorption rate of dogs suffered hemorrhagic shock[J]. Med J Chin PLA, 2011, 36(4): 408-410.[李琳, 蒋纤, 侯经元, 等. 卡巴胆碱对失血性休克犬口服补液时肠黏膜血流量和吸收率的影响[J]. 解放军医学杂志, 2011, 36(4): 408-410.]
[4]Dal Molin SZ, Kruel CR, de Fraga RS,et al. Differential protective effects of anaesthesia with sevoflurane or isoflurane: an animal experimental model simulating liver transplantation[J]. Eur J Anaesthesiol, 2014, 31(12): 695-700.
[5]Chao A, Chen K, Trask S,et al. Time to failure of arterial shunts in a pig hemorrhagic shock model[J]. Am Surg, 2012, 78(10): 1045-1048.
[6]Moore-Olufemi SD, Padalecki J, Olufemi SE,et al. Intestinal edema: effect of enteral feeding on motility and gene expression[J]. J Surg Res, 2009, 155(2): 283-292.
[7]Verkman AS, Yang B, Song Y,et al. Role of water channels in fluid transport studied by phenotype analysis of aquaporin knockout mice[J]. Exp Physiol, 2000, 85: S2335-S2241.
[8]Yang B. The human aquaporin gene family (review)[J]. Current Genomics, 2000, 1(1): 91-102.
[9]Saadoun S, Papadopoulos MC, Hara-Chikuma M,et al. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption[J]. Nature, 2005, 434(7034): 786-792.
[10] Sakai H, Sagara A, Matsumoto K,et al. 5-Fluorouracil induces diarrhea with changes in the expression of inflammatory cytokines and aquaporins in mouse intestines[J]. PloS One, 2013, 8(1): e54788.
[11] Nakano M, Koyama Y, Nogami H,et al. Enhanced aquaporin 8 expression after subtotal colectomy in rat[J]. Open J Gastroenterol, 2013, 3: 253-258.
[12] Zhao G, Li J, Wang J,et al. Aquaporin 3 and 8 are down-regulated in TNBS-induced rat colitis [J]. Biochem Biophys Res Commun, 2014, 443(1): 161-166.
[13] Windsant ICV, Hellenthal FA, Derikx JPM,et al. Circulating intestinal fatty acid-binding protein as an early marker of intestinal necrosis after aortic surgery: a prospective observational cohort study[J]. Ann Surg, 2012, 255(4): 796-803.
[14] Niewold TA, Meinen M, van der Meulen J. Plasma intestinal fatty acid binding protein (I-FABP) concentrations increase following intestinal ischemia in pigs[J]. Res Vet Sci, 2004, 77(1): 89-91.
[15] Matsumoto S, Sekine K, Funaoka H,et al. Diagnostic performance of plasma biomarkers in patients with acute intestinal ischaemia[J]. Br J Surg, 2014, 101(3): 232-238.
[16] Lv BS, Wang ZQ, Wang W,et al. A clinical evaluation of the effect of sevoflurane or propofol in combination with remifentanil in myasthenia gravis patients undergoing thymectomy[J]. Med J Chin PLA, 2013, 38(7): 586-590.[吕宝胜, 王卓强, 王卫, 等.七氟烷或丙泊酚复合瑞芬太尼麻醉在重症肌无力患者胸腺切除术中的应用[J]. 解放军医学杂志, 2013, 38(7): 586-590.]
[17] Jablonski EM, Webb AN, McConnell NA,et al. Plasma membrane aquaporin activity can affect the rate of apoptosis but is inhibited after apoptotic volume decrease[J]. Am J Physiol Cell Physiol, 2004, 286(4): C975-C985.
Effects of post-conditioning with sevoflurane on the expressions of intestinal AQP8 and I-FABP in pigs with hemorrhagic shock
CHEN Yan-hong1,2, SUN Ying-jie1, ZHANG Yi-nan1*, WANG Li-jing1, ZHANG Tie-zheng1, CHEN Ke-yan11Department of Anesthesiology, General Hospital of Shenyang Command, Shenyang 110016, China
2Department of Anesthesiology, People's Hospital of Kangping, Kangping, Liaoning 110500, China
*< class="emphasis_italic">Corresponding author, E-mail: zynan13@126.com
, E-mail: zynan13@126.com
This work was supported by the“Twelfth Five-Year Plan” Medical Science Development Foundation of PLA (BWS12J008)
ObjectiveTo observe the effects of sevoflurane post-conditioning on the expression of Aquaporin 8 (AQP8) and intestinal fatty acid binding protein (I-FABP), in order to investigate the protective role of sevoflurane post-conditioning on intestinal injury and its underlying mechanism.MethodsEighteen bama miniature pigs were randomly divided into three groups (6 each) using a random number table: control group (S group), hemorrhagic shock group (HS group), and sevoflurane post-coditioning group (Post/ Sev group). Experimental animals were fasted for 8 hours before surgery, and propofol 3mg/kg was givenviathe ear vein. Endotracheal intubation was done when the animal fell asleep. Bloodletting from the femoral artery after anesthesia was done to reproduce hemorrhagic shock. In Post/Sev group, 2% sevoflurane was given by inhalation for 30min (post-conditioning) after successful reproduction of the model. Blood samples were collected prior to anesthesia (T0) and 30min (T1), 1h (T2), 1.5h (T3), 2h (T4), 3h (T5), 4h (T6) after hemorrhagic shock. The quantity of blood I-FABP and intestinal AQP8 levels were determined with ELISA. Water content in the intestinal tissue was determined by wet and dry weight method. Histopathological changes in the intestinal tissue were observed with HE staining.ResultsCompared with the control group, the serum I-FABP content, the expressions of intestinal AQP8, and water content inthe intestinal tissue were significantly increased in HS group and Post/Sev (P<0.05) group. Compared with HS group, the above indices in Post/Sev group were significantly lower (P<0.05). These results were confirmed by pathological examination.ConclusionPostconditioning with sevoflurane could improve, to some extent, pig's intestinal barrier function in hemorrhagic shock, and this effect is likely related with lowering of intestinal AQP8 and I-FABP expression and mucosal edema.
intestinal mucosa; shock, hemorrhagic; sevoflurane; fatty acid-binding proteins; aquaporin 8
R605.971
A
0577-7402(2015)11-0911-04
10.11855/j.issn.0577-7402.2015.11.11
2015-04-24;
2015-09-18)
(责任编辑:胡全兵)
全军“十二五”医学科研重点项目(BWS12J008)
陈艳红,副主任医师,硕士研究生。主要从事围术期重要器官保护方面的研究
110016 沈阳 沈阳军区总医院麻醉科 (陈艳红、孙莹杰、张毅男、王丽晶、张铁铮、陈克研);110500 辽宁康平 康平县人民医院麻醉科(陈艳红)
张毅男,E-mail:zynan13@126.com
