Synthesis and Characterization of 12-Acryloyloxystearic Acid and Application in Preparing Environmentally Friendly Magnesium 12-Acryloyloxy Stearate Detergent
2015-06-22
(School of Chemistry and Chemical Engineering, Huangshan University, Huangshan 245041)
Synthesis and Characterization of 12-Acryloyloxystearic Acid and Application in Preparing Environmentally Friendly Magnesium 12-Acryloyloxy Stearate Detergent
Wang Yonglei ; Li Haiyun; Fang Hongxia; Wu Qiong; Lu Lulu
(School of Chemistry and Chemical Engineering, Huangshan University, Huangshan 245041)
In this article, 12-acryloyloxystearic acid was synthesized, which was then used to prepare the magnesium 12-acryloyloxy stearate detergent. Reaction conditions for synthesizing 12-acryloyloxystearic acid, including the molar ratio of 12-hydroxystearic acid to acrylic acid, the catalyst amount, the esterification temperature, and the esterification time, were optimized. Under the optimized conditions, the 12-acryloyloxystearic acid with an acid value of 159 mgKOH/g and a melting range of between 70.4 ℃ and 71.4 ℃ was obtained. The structure of 12-acryloyloxystearic acid was confirmed by FTIR spectroscopy. Results of preparing magnesium 12-acryloyloxy stearate detergent showed the existence of acryloyloxy radical in 12-hydroxystearic acid could improve the quality of lubricant detergent greatly.
12-acryloyloxystearic acid, acrylic acid, environmental-friendly, lubricant detergent, magnesium stearate
1 Introduction
In the internal combustion engine, lubricating oil[1-2]is an indispensable material needed in the course of its operation. However, some acidic degradation products[3-5]would be formed as the consequence of reaction of combustion products on lube oil in the engine, and could corrode engine parts and promote the formation of oil sludges. In order to grapple with this problem, adding alkaline lubricant detergents to lubricating base oil was the most commonly used method[6-8].
In recent years, for the development of environmentally friendly lubricants[9-14], the studies on environmentally friendly lubricant detergents[15-16]using fatty acid as starting materials are also increasing. The detergents using the long-chain saturated fatty acids[17-18](such as stearic acid, palmitic acid and lauric acid, etc.) as starting materials with high viscosity are inconvenient for industrial applications. In our previous studies, a series of environmentally friendly magnesium oleate[19-20]and magnesium linoleate lubricant detergents[21]were synthesized using unsaturated fatty acids and some satisfactory products were obtained in the laboratory. However, in the industrial application process, we found out that the oil-solubility and compatibility of the lubricant detergents made from fatty acids with the environmentally friendly synthetic ester base oil were unsatisfactory because of the linear molecular structure of the long-chain fatty acids.
The synthetic esters are widely used as environmentally friendly lubricant base oil[22-24]thanks to its excellent oil solubility, low temperature fluidity and biodegradability. In order to improve the oil solubility and compatibility with the synthetic ester base oil composed of the environmentally friendly lubricant detergents, the 12-acryloyloxystearic acid was synthesized using 12-hydroxystearic acid and acrylic acid as raw materials, and then it was used to prepare the environmentally friendly magnesium 12-acryloyloxy stearate detergent with excellent comprehensive performance. Since 12-acryloyloxystearic acid is a fatty acid with ester group, the oil solubility and compatibility with the synthetic ester base oil of 12-acryloyloxy stearate detergent could be improved greatly. Meanwhile, in the process of synthesizing the lubricant detergent, the tolerance of the fatty acid with an ester group to alkaline reaction environment was also confirmed. Therefore, this research has the potential to obtain an environmentallyfriendly lubricant detergent with excellent performance using fatty acid with ester group as the starting material.
2 Experimental
2.1 Material
12-Hydroxystearic acid, which was of technically pure grade, was provided by the Tongliao Tonghua Castor Chemical Co., Ltd., Inner Mongolia, China. The diluent oil (trimethylolpropane trioctanoate) was of technically pure grade and provided by the Liyang Ruipu Chemical Technology Research Center, Jiangsu, China. Xylene and methanol were analytical reagents and were provided by the Xilong Chemical Co., Ltd, Guangdong, China. Active-60 magnesium oxide was of technically pure grade and provided by the Shanghai Dunhuang Chemical Plant, China. CO2, which was technically pure, was received from the Huangshan Industrial Air Company, Anhui, China. All other materials were obtained from commercial sources.
2.2 Analytical methods
The acid value (AV) of detergent samples was determined according to the standard test method ASTM D664. The total base number (TBN) of detergent samples was determined according to the standard test method ASTM D2896 and the viscosity (100 ℃) was determined according to the standard test method ASTM D445. The Fourier transform infrared spectrometry using a Nicolet 380 type spectrometer (Nicolet Company, USA) could measure the structures of petroleum products. A GC2014 gas chromatograph (Shimadzu Company, Japan) was used to test the tolerance of 12-acryloyloxystearic acid to alkaline reaction system. A WRS-3 melting point apparatus (Shanghai Precision Instrument Co., Ltd.) was used to measure the melting point of detergent samples. The particle size distribution of colloidal carbonate particles was determined using the Zetasizer Nano-ZS90 instrument (Malvern Instruments Ltd., UK).
2.3 Synthesis of 12-acryloyloxystearic acid
The 12-acryloyloxystearic acid was synthesized in a 250 mL reactor fitted with a stirrer, a heating jacket and a vacuum pump. A calculated amount of acrylic acid and p-toluenesulfonic acid was added into the reactor. When the temperature of the reactants was increased to 70 ℃, 12-hydroxystearic acid was added into the reactor. Under a vacuum of -0.09 MPa, the reaction mixture entered into reaction until the acid value of the product was close to the desired acid value of 155-160 mgKOH/g. Then the reaction products were washed by distilled water to remove the catalyst and unreacted acrylic acid. The residual water and acrylic acid were further distilled off to obtain the 12-acryloyloxystearic acid. The mechanism for synthesis of 12-acryloyloxystearic acid is shown in Scheme 1.
Scheme 1 Mechanism for synthesis of the 12-acryloyloxystearic acid
2.4 Preparation of magnesium 12-acryloyloxystearate detergent
8.8 g of 12-acryloyloxystearic acid, 10 g of diluent oil, 8 mL of methanol, and 100 mL of xylene were added to a three-necked flask. After the stirring was initiated, 12 g of active-60 magnesium oxide was added. The reaction mixture was kept at 50 ℃ for 1 h and then was heated to 65 ℃. 2 mL of ammonia were added to the mixture and gaseous CO2was introduced into the reactor for carbonation reaction. Finally, the magnesium 12-acryloyloxystearate detergent was obtained by removing the waste residue and solvent. The mechanism for synthesis of 12-acryloyloxy stearic acid is shown in Scheme 2.

Scheme 2 Mechanism for synthesis of magnesium 12-acryloyloxy stearate detergent
3 Results and Discussion
3.1 Effect of molar ratio of 12-hydroxystearic acid to acrylic acid
Since the molecule of 12-hydroxystearic acid has a hydroxyl group and a carboxyl group, therefore, to avoid the occurrence of self esterification, the acrylic acid should be introduced in surplus. The effect of molar ratio of 12-hydroxystearic acid to acrylic acid on the acid value, the melting range and the yield of the 12-acryloyloxystearic acid are shown in Table 1.As shown in Table 1, as the molar ratio of 12-hydroxystearic acid to acrylic acid was increased, the acid value of the product decreased gradually, while the yield of the target product also increased gradually. Generally, the error for acid value of the product was less than 0.5 mgKOH/g. Because the theoretic acid value of the 12-acryloyloxystearic acid is about 158 mgKOH/g, the actual acid value of the product was very close to its theoretic acid value when the molar ratio of 12-hydroxystearic acid to acrylic acid was equal to 1:1.5. In addition, the melting range of the product was quite narrow (70.4—71.4 ℃), which demonstrated the high reaction depth and good purity of the product. When the amount of acrylic acid continued to increase, the acid value, melting range and yield of the final product did not change obviously. This suggests that an appropriate molar ratio of 12-hydroxystearic acid to acrylic acid could inhibit the self-esterification of 12-hydroxystearic acid. Thus, the optimal molar ratio of 12-hydroxystearic acid to acrylic acid was specified as 1:1.5.

Table 1 Effect of molar ratio of 12-hydroxystearic acid to acrylic acid on the acid value, melting range and the yield of the product
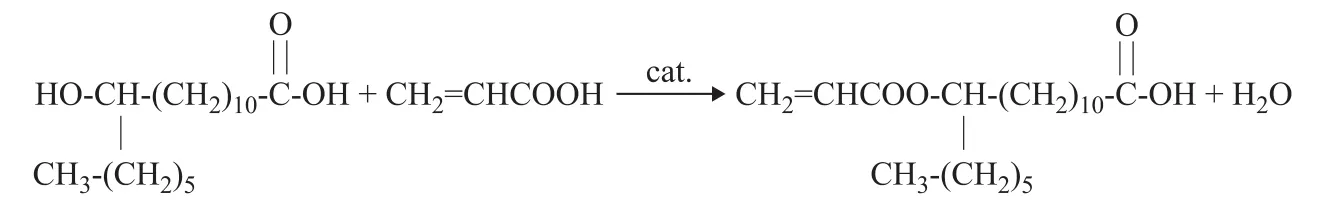
3.2 Catalyst amount
The p-toluenesulfonic acid as a classical catalyst for the esterification reaction has excellent catalytic performance[25-26], and the effects of its weight percentage on the acid value, the melting range and the yield of the target product are shown in Table 2.
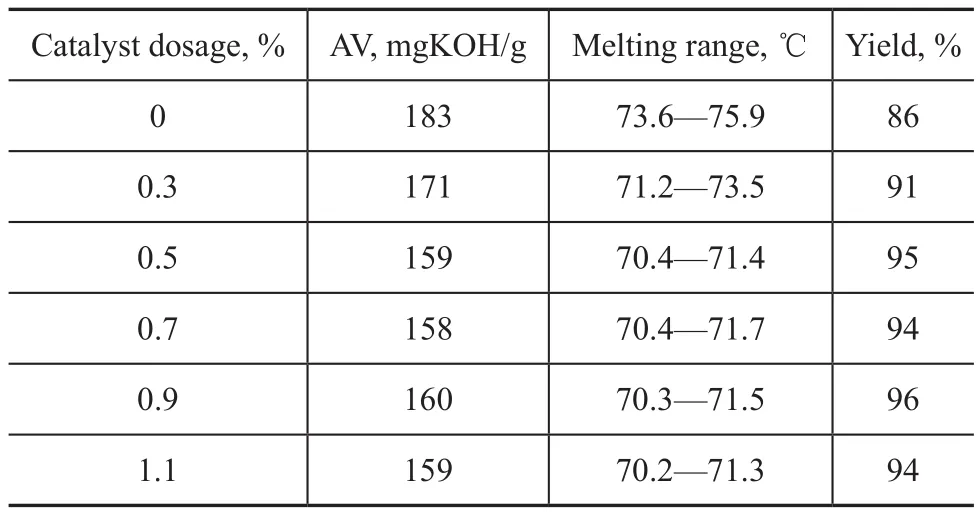
Table 2 Effects of weight percentage of p-toluenesulfonic acid on the acid value, melting range and yield of the target product
As shown in Table 2, when the amount of p-toluenesulfonic acid was increased, the acid value of the product decreased and the yield increased gradually. At an esterif ication time of 4 h, the acid value and yield of the product with 0.5%, 0.7%, 0.9% and 1.1% (w%) of p-toluenesulfonic acid used as the catalyst, respectively, showed minor differences. Upon considering the reduction of catalyst cost, a dosage of 0.5% of p-toluenesulfonic acid was selected as the optimal catalyst amount used.
3.3 Esterification temperature
The effects of esterification temperature on the acid value, the melting range and the yield of 12-acryloyloxystearic acid are shown in Table 3.
As shown in Table 3, with an increasing esterification temperature, the acid value of the product decreased rap-idly and the yield of the target product first increased and then decreased. At the same time, the melting range of the target product also showed a downward trend. These phenomena showed that the esterification efficiency was low when the esterification temperature was below 70 ℃, and the possible reasons were as follows. Firstly, the low temperature could hardly distill off the water timely. Secondly, when the temperature was below 70 ℃, the 12-hydroxystearic acid was still in a solid state, so the esterification efficiency was poor. Then, with an increasing esterification temperature, the acid value decreased along with an increase in the yield of the product. However, a too high temperature would make acrylic acid evaporate from the reaction system, so the self esterification reaction of 12-hydroxystearic acid could occur[27-28]because of insufficient amount of acrylic acid in the reactor, which would cause a further decrease in acid value of the product, coupled with the decreased melting point and the reduced yield of the target product. Therefore, upon considering the appropriate reaction rate and the avoidance of side reactions, an optimal reaction temperature of 70 ℃ was feasible.
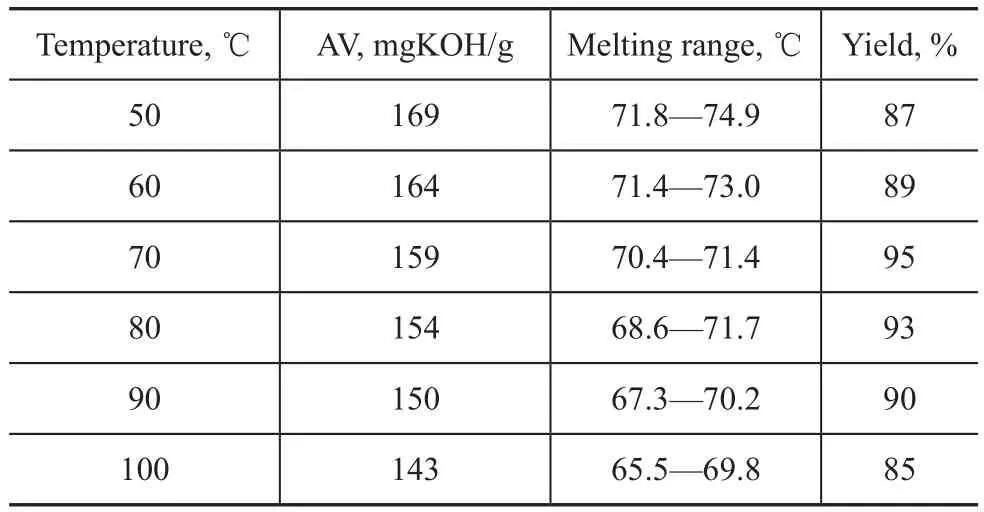
Table 3 Effects of esterification temperature on acid value, melting range and yield of the target product
3.4 Esterification time
In order to obtain a satisfactory product yield, a sufficient esterification time was necessary. The effects of esterif ication time on the acid value, the melting range and the yield of 12-acryloyloxystearic acid are shown in Table 4. As shown in Table 4, when the esterification time was increased, the acid value of the product decreased gradually and the yield of the product at first increased and thendecreased slightly. The melting range of the product was almost the narrowest when the esterification time was 4 h. This showed that the esterification reaction was incomplete when the esterification time was equal to 2 h. Then the yield of the product increased along with the extension of esterification time. However, a too long reaction time may increase the self-esterification reaction so that the acid value and yield of the product decreased slightly. The quality of the product was best at an esterification time of 4 h. Therefore, the optimal esterification time was set at 4 h.3.5 Infrared spectroscopic analysis

Table 4 Effects of esterification time on acid value, melting range and yield of the target product
Figure 1 Typical IR spectra of (a) acrylic acid; (b) 12-hydroxystearic acid; and (c) 12-acryloyloxystearic acid
The chemical structures of 12-acryloyloxystearic acid were confirmed by infrared spectrometry. Figure 1 shows the typical infrared spectra of raw materials and the target product. In Figure 1a (acrylic acid), the broad adsorption peaks at 2 900—3 300 cm-1were the characteristic association adsorption peaks of carboxyl and hydroxyl groups; the adsorption peak at 1 705 cm-1was the characteristic carbonyl absorption peak; the adsorption peaks at 1 635 cm-1and 1 617 cm-1were the characteristic adsorption peaks of the carbon-carbon double bonds. In Figure 1b (12-hydroxystearic acid), the broad adsorption peaks at 3 000—3 300 cm-1were the characteristic association adsorption peaks of carboxyl and hydroxyl groups, and its association extent was weaker than that of acrylic acid; similarly, the adsorption peak at 1 697 cm-1was ascribed to the characteristic carbonyl groups. In Figure 1c (12-acryloyloxy stearic acid), the adsorption peak at 1 585 cm-1was the characteristic adsorption peak of the carbon-carbon double bond of acryloyloxy group, which confirmed the introduction of the acrylic acid. In addition, other characteristic adsorption peaks of the 12-acryloyloxystearic acid was similar to that of the 12-hydroxystearic acid in Figure 1b (carboxyl absorption peaks at 3 193 cm-1and 1 697 cm-1), which showed the carboxyl group of 12-hydroxystearic acid did not participate in the reaction and the final product was 12-acryloyloxystearic acid.
3.6 Tolerance to alkaline system
Generally, organic esters are prone to hydrolysis in an alkaline environment[29]. However, the hydrolysis of the 12-acryloyloxystearic acid should be avoided in the weak alkaline reaction system for synthesizing lubricant detergent, which also determined whether it could be used as the starting material for synthesizing lubricant detergent. Therefore, the tolerance of 12-acryloyloxystearic acid to the alkaline reaction system for synthesizing lubricant detergent was investigated through testing the amount of acrylic acid at different reaction stages, with the results presented in Table 5.
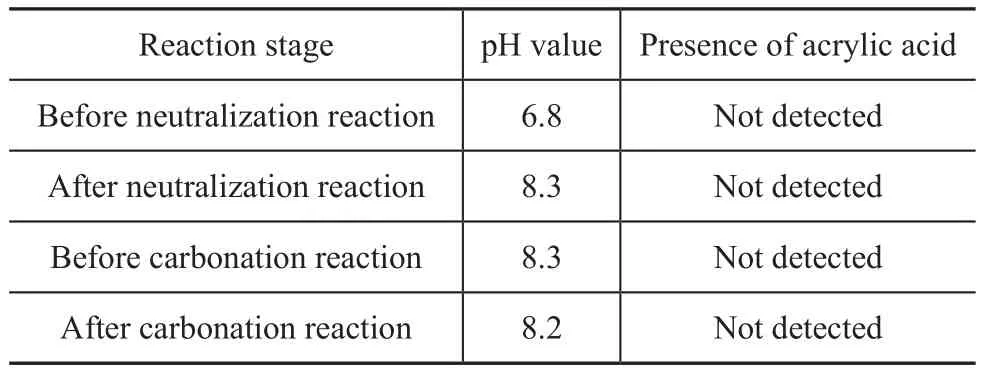
Table 5 Tolerance of 12-acryloyloxystearic acid to the alkaline reaction system
Table 5 shows that, at different stages for synthesizing magnesium 12-acryloyloxy stearate detergent, the acrylic acid was not detected in the reaction system by gas chromatography. Test results also demonstrated that the tolerance of 12-acryloyloxystearic acid to the alkaline reaction system (at pH values in the range of 6.8—8.3) for synthesizing lubricant detergent was excellent, so it could be used as an environmentally friendly fatty acid with ester group to synthesize lubricant detergent.
3.7 Preparation of magnesium 12-acryloyloxy stearate detergent
In order to explore the possibility of synthesizing the lubricant detergent using an organic acid with ester group as the starting material, the magnesium 12-hydroxy stearate detergent and the magnesium 12-acryloyloxy stearate detergent were both studied, with the results presented in Table 6.It can be seen that under the same reaction conditions, there are significant differences in the final properties of the lubricant detergent, which was formed at first by reacting 12-hydroxystearic acid on acrylic acid followed by reaction of the product—12-acryloyloxystearic acid —as the starting material with magnesium oxide. In comparison with the magnesium 12-hydroxystearate detergent, the TBN of the magnesium 12-acryloyloxy stearate detergent increased significantly, while the viscosity of the magnesium 12-acryloyloxy stearate detergent also decreased. Meanwhile, after 30 days of storage, the surface skinning of magnesium 12-hydroxystearate detergent was obvious, but the magnesium 12-acryloyloxy stearate detergent showed almost no surface skinning, which demonstrated that the oil solubility and compatibility with trimethylolpropane trioctanoate (diluent oil) of the magnesium 12-acryloyloxy stearate detergent were better than the magnesium 12-hydroxystearate detergent. The particle size distribution analysis of colloidal carbonate particleshowed that the particle size and polydispersity index of the magnesium 12-acryloyloxy stearate detergent were significantly smaller than that of the magnesium 12-hydroxy stearate detergent, indicating that in comparison with the colloidal particles of the magnesium 12-hydroxystearate detergent, the average size of the colloidal particles of the 12-acryloyloxystearate detergent was smaller and more uniform. This fact again confirmed that the oil solubility and dispersing ability of 12-hydroxystearic acid were improved after the introduction of acrylate group. Meanwhile, the successful preparation of magnesium 12-acryloyloxystearate detergent also indicated the fatty acid with an ester group was able to adapt to the alkaline environment of synthesizing lubricant detergent. Therefore, synthesizing excellent lubricant detergent using organic acid with an ester group as the starting material was feasible.

Table 6 Properties of magnesium 12-hydroxystearate detergent and magnesium 12-acryloyloxy stearate detergent
4 Conclusions
In this study, 12-acryloyloxystearic acid was synthesized via the esterification reaction of 12-hydroxystearic acid upon acrylic acid. Its structure was characterized by FTIR analysis. Under the optimized reaction conditions (viz.: a molar ratio of 12-hydroxystearic acid to acrylic acid of 1:1.5 using p-toluenesulfonic acid as the catalyst at a dosage of 0.5 m% at an esterification temperature of 70 ℃ for an esterification duration of 4 h ), the product—12-acryloyloxystearic acid with an acid value of 159 mgKOH/g and a melting range of 70.4—71.4 ℃ could be obtained. Then the 12-acryloyloxystearic acid was used as the starting material for preparing the magnesium 12-acryloyloxystearate detergent and the results demonstrated that in comparison with the magnesium 12-hydroxystearate detergent, the quality of magnesium 12-acryloyloxy stearate detergent was greatly improved. Experiments indicated that the formation of acryloyloxy radical in the molecule of 12-hydroxystearic acid could contribute greatly to the good performance of lubricant detergent. Hence, synthesizing the lubricant detergent using organic acid with an ester group as the starting material was feasible, so more single organic acids with ester groups will have the possibility to be used for preparing excellent lubricant detergents.
Acknowledgement: This work was supported by the National Undergraduate Innovative Training Program (201410375004). the Scientific Research Foundation for Introduced Scholars, Huangshan University (2015xkjq002) and the Scientific Research Foundation of Huangshan University (2014xkj012).
[1] Dong L, Shu G, Liang X. Effect of lubricating oil on the particle size distribution and total number concentration in a diesel engine[J]. Fuel Process Technol, 2013, 109: 78-83
[2] Al-Zahrani S M, Putra M D. Used Lubricating oil regeneration by various solvent extraction techniques[J]. J Ind Eng Chem, 2013, 19(2): 536-539
[3] Galsworthy J Hammond S, Hone D. Oil-soluble colloidal additives[J]. Curr Opin Colloid Interface Sci, 2000, 5: 274-279
[4] Fu J Z, Lu Y F, Campbell C B, Papadopoulos K D. Acid neutralization by marine cylinder lubricants inside a heating capillary: strong/weak-stick collision mechanisms[J]. Ind Eng Chem Res, 2006, 45(16): 5619-5627
[5] HudsonlK, Eastoe J, Dowding P J. Nanotechnology in action: overbased nanodetergents as lubricant oil additives[J]. Adv Colloid Interface Sci, 2006, 123: 425-431
[6] Besüergil B, Akın A, Celik S. Determination of synthesis conditions of medium, high, and overbased alkali calcium sulfonate[J]. Ind Eng Chem Res, 2007, 46(7): 1867-1873
[7] Chen Z, Xiao S, Chen F, et al. Calcium carbonate phase transformations during the carbonation reaction of overbased calcium heavy alkylbenzene sulfonate nanodetergents preparation[J]. J Colloid Interface Sci, 2011, 359(1): 56-67
[8] Greenall A, Neville A, Morina A, Sutton M. Investigation of the interactions between a novel, organic anti-wear additive, ZDDP and overbased calcium sulphonate[J]. Tribo Int, 2012, 46(1): 52-61
[9] Erhan S Z, Asadauskas S. Lubricant basestocks from vegetable oils[J]. ind Crop Prod, 2000, 11(2): 277-282
[10] Cermak S C, Isbell T A. Physical properties of saturated estolides and their 2-ethylhexyl esters[J]. Ind Crop Prod 2002, 16(2): 119-127
[11] Lathi P S, Mattiasson B. Green approach for the preparation of biodegradable lubricant base stock from epoxidized vegetable oil[J]. Appl Catal, B: Environmental, 2007, 69:207-212
[12] Singh A K. Castor oil-based lubricant reduces smoke emission in two-stroke engines[J]. Ind Crop Prod, 2011, 33(2): 287-295
[13] Sharma R V, Dalai A K. Synthesis of bio-lubricant from epoxy canola oil using sulfated Ti-SBA-15 catalyst[J]. Appl Catal, B: Environmental, 2013, 142:604-614
[14] Pham M Q, Yoon H S, Khare V, et al. Evaluation of ionic liquids as lubricants in micro milling - process capability and sustainability[J]. J Clean Prod, 2014, 76: 167-173
[15] Wang Y, Eli W, Liu Y F, et al. Synthesis of environmentally friendly calcium oleate detergent[J]. Ind Eng Chem Res, 2008, 47(22): 8561-8565
[16] Wang Y, Eli W, Zhang L, et al. Synthesis of environmentally friendly composite-metal (calcium and magnesium) oleate detergent[J]. Ind Eng Chem Res, 2011, 50(3): 1530-1535
[17] Mohammed A H A-K, Ahmad M R, Al-Messri Z A K. Synthesis, characterization and evaluation of overbased magnesium fatty acids detergent for medium lubricating oil[J]. Iraqi Journal of Chemical and Petroleum Engineering, 2013, 14(3): 1-9
[18] Wang Y, Eli W. Synthesis of biodegradable high-alkali magnesium oleate detergent[J]. Ind Eng Chem Res, 2010, 49(6): 2589-2592
[19] Wang Y, Eli W. Synthesis of environmentally friendly overbased magnesium oleate detergent and high alkaline dispersant/magnesium oleate mixed substrate detergent[J]. Ind Eng Chem Res, 2010, 49(19): 8902-8907
[20] Wang Y, Li H, Fang H, Ni Z. Synthesis of environmentally friendly magnesium linoleate detergent[J]. China Petroleum Processing and Petrochemical Technology, 2014, 16(1): 96-100
[21] Nagendramma P, Kaul S. Development of ecofriendly/biodegradable lubricants: an overview [J]. Renew Sust Energ Rev, 2012, 16(1): 764-774
[22] Wu Y, Li W, Zhang M, Wang, X. Improvement of oxidative stability of trimethylolpropane trioleate lubricant[J]. Thermochimica Acta, 2013, 569: 112-118
[23] Zhang L, Cai G X, Eli W. Synthesis and characterization of novel liquid ester-phenolic antioxidant based on dipentaerythritol[J]. Lubri Sci, 2013, 25(3): 209-216
[24] Wu Y, Li W, Zhang M, Wang X. Oxidative degradation of synthetic ester and its influence on tribological behavior[J]. Tribol Int, 2013, 64: 16-23
[25] Liu W, Ma H, Zhang W. Methyl esterification of high acid value oil catalyzed by p-toluene sulphonie acid[J]. China Oils and Fats, 2008, 33(8): 54-56 (in Chinese)
[26] Bita B. Application of p-toluenesulfonic acid (PTSA) in organic synthesis[J]. Curr Org Chem, 2011, 15(17): 3091-3097
[27] Wang Y, Eli W, Zhang L. Synthesis and characterization of poly-12-hydroxystearic acid-polyethylene polyamine hyperdispersant[J]. Textile Auxiliaries, 2011, 28(1): 18-20 (in Chinese)
[28] Wang Y, Eli W, Nueraimaiti A, et al. Synthesis and characterization of polyol poly-12-hydroxystearic acid: applications in preparing environmentally friendly overbased calcium oleate detergent[J]. Ind Eng Chem Res, 2009, 48: 3749-3754
[29] Zeng X, Li Q, Chen M. The effect of mixed micelle of surfactant on the alkaline hydrolysis of esters [J]. Chemical Journal of Chinese Universities, 1995, 16(10): 1605-1609 (in Chinese)
date: 2014-11-13; Accepted date: 2015-01-09.
Dr. Wang Yonglei, Telephone: +86-559-2546612; E-mail: wylei@hsu.edu.cn.
杂志排行
中国炼油与石油化工的其它文章
- Pyrolysis Characteristics and Kinetics of Methyl Oleate Based on TG-FTIR Method
- Synthesis of Waterborne Polyurethane Modified by Nano-SiO2Silicone and Properties of the WPU Coated RDX
- A Highly Efficient and Selective Water-Soluble Bimetallic Catalyst for Hydrogenation of Chloronitrobenzene to Chloroaniline
- Curing Mechanism of Condensed Polynuclear Aromatic Resin and Thermal Stability of Cured Resin
- A Novel Thermally Coupled Reactive Distillation Column for the Hydrolysis of Methyl Acetate
- Effects of Fe2+, Co2+and Ni2+Ions on Biological Methane Production from Residual Heavy Oil
