A Highly Efficient and Selective Water-Soluble Bimetallic Catalyst for Hydrogenation of Chloronitrobenzene to Chloroaniline
2015-06-22ZhouYafenYangWenjuanZhouLimeiWangManmanMaXiaoyan
Zhou Yafen; Yang Wenjuan; Zhou Limei; Wang Manman; Ma Xiaoyan
(1. Chemical Synthesis and Pollution Control Key Laboratory of Sichuan Province, College of Chemistry and Chemical Engineering, China West Normal University, Nanchong 637002; 2. College of Materials and Chemistry & Chemical Engineering, Chengdu University of Technology, Chengdu 610059)
A Highly Efficient and Selective Water-Soluble Bimetallic Catalyst for Hydrogenation of Chloronitrobenzene to Chloroaniline
Zhou Yafen1; Yang Wenjuan1; Zhou Limei1; Wang Manman1; Ma Xiaoyan2
(1. Chemical Synthesis and Pollution Control Key Laboratory of Sichuan Province, College of Chemistry and Chemical Engineering, China West Normal University, Nanchong 637002; 2. College of Materials and Chemistry & Chemical Engineering, Chengdu University of Technology, Chengdu 610059)
Selective hydrogenation of chloronitrobenzene (CNB) to chloroaniline (CAN) catalyzed by water-soluble Ru/Pt bimetallic catalyst in an aqueous-organic biphasic system was studied. It was found that the catalytic activity increased obviously due to the addition of platinum. Ru/Pt bimetallic catalysts exhibited a strong synergistic effect when the molar ratio of Pt was in the range of 5%—80%. Under the mild conditions including a temperature of 25 ℃, a hydrogen pressure of 1.0 MPa and a Pt molar ratio of 20%, the conversion of p-chloronitrobenzene (p-CNB) reached 99.9%, with the selectivity to p-chloroaniline (p-CAN) equating to 99.4%. The Ru/Pt catalyst also showed high activity and selectivity for the hydrogenation of other chloro- and dichloro-nitrobenzenes with different substituted positions. In addition, the catalyst can be recycled five times without significant loss of activity.
water-soluble bimetallic catalyst; hydrogenation; chloronitrobenzene; chloroaniline
1 Introduction
Haloanilines are important intermediates for synthesis of organic fine chemicals, such as pharmaceuticals, agrochemicals, and dyes. The main routes for their production involve the Bechamp reaction in a metal-acid system or selective hydrogenation of the corresponding halonitrobenzenes over heterogeneous metal catalysts[1]. Much attention has been focused on the hydrogenation route, owing to its lower impact on the environment and the high quality of the target product. Catalytic hydrogenation of halonitrobenzenes over various catalysts, such as platinum[2-8], nickel[1,9-11], ruthenium[12-14], palladium[15-16], gold[17-18], and silver[19]has been widely investigated. A key problem in the catalytic reduction is that the process is usually accompanied with extensive dehalogenation type side reactions, which can lead to the decrease of selectivity to haloaniline. To solve this problem, several approaches have been developed, which include preparation of the designated catalysts (alloying, controlling the metal particle dispersion and metal/support interaction, etc.), or introduction of special additives (promoters, inhibitors). Coq, et al. investigated the hydrogenation of p-CNB to p-CAN over several supported Pt and Ru/M (M=Sn, Pb, Ge) catalysts with varying dispersions[2,12]. Liu, et al. studied the effect of metal cations and metal complex on the hydrogenation of p-CNB over poly-vinylpyrrolidone (PVP)-stabilized Pt or Ru colloid catalysts[3,13]. Zheng, et al. discussed the influence of support and transition metal on the hydrogenation of p-CNB over supported Pt catalysts[4]. Recently, there has been growing interest in bimetallic catalysts, as the addition of a second metal can drastically improve the activity and/or selectivity towards the desired product[20-23]. For example, Liu, et al. also studied the hydrogenation of o-CNB to o-CAN over PVP-stabilized Ru/Pd or Pd/Pt bimetallic colloidal clusters[20-21]. However, from the point of view of green chemistry, the homogeneous catalytic systems as mentioned above cannot be easily applied in industry due to the difficulties in separation.
During the past decades, catalytic reactions using water-soluble catalysts in aqueous/organic biphasic system have been intensively studied, which can carve out a route towards the application of clean and cheap water solvent and the facile separation of the catalyst from the reaction system[24].
Herein, we report our preliminary results of a synergistic effect which we experienced in the course of biphasic hydrogenation of chloronitrobenzene to chloroaniline using water-soluble Ru/Pt catalyst formed in-situ, while the recycle of the Ru/Pt catalyst system was also studied.
2 Experimental
2.1 Materials and reagents
DPPE [1,2-bis(diphenylphosphino)ethane] and DPPES [1,2-bis(3-sulfonatophenyl) phosphine ethane] were prepared according to the reported methods[25-26]. RuCl3.xH2O and H2PtCl6·6H2O were purchased from the Kunming Institute of Noble Metals, China. Hydrogen gas (>99.99%) was supplied by the Sichuan Tianyi Science & Technology Co., Ltd. Other chemicals were commercially available and used as received.
2.2 Catalytic tests
Catalytic hydrogenation of chloronitrobenzene was carried out in a 60-mL stainless autoclave equipped with a magnetic stirrer and an electric temperature controller. The catalyst was synthesized in-situ in the reaction system. In a typical experiment, certain amounts of the metal salt, DPPES, the substrate and solvent were added into the reactor. The mixture was purged with H2gas for five times and then H2was introduced to reach a desired pressure. When a prescribed temperature was reached, the reaction was started by switching on the stirrer. Samples were analyzed by a GC-7890 (Agilent) chromatograph equipped with a FID detector and a quartz capillary column (SE-30, 30 m × 0.25 mm × 0.25 um) and the products were identified by comparison with standard samples and results of GC-MS analysis.
3 Results and Discussion
3.1 Effect of metal cations
It was reported that the addition of metal ions to the colloidal PVP-Pt catalyst can markedly increase both the activity and selectivity for the hydrogenation of o-CNB and some metal ions added to the PVP-Ru system can improve the activity, while retaining good selectivity to o-CAN[3,13]. Therefore, the effect of metal cations was also investigated in the water-soluble catalyst system, and p-CNB was employed as the model substrate for comparing the catalytic performance of these catalysts (Table 1). As shown in Table 1, all the metal cations surveyed here had no influence on the selectivity to p-CAN and a high selectivity was maintained with addition of each metal cation. But introduction of metal cations changed the activity to varying degrees. The metal cations of group II B, Zn2+ions and transition metal ions, Cr3+ions could poison the catalyst. The VIII group ions, Ni2+ions and transition metal ions, Mn2+ions had little effect on the activity. Introduction of the VIII group ions, Fe3+, Co2+and IV A group ions, Sn2+and Sn4+ions, Cu2+ions of II B group, especially Ca2+ions of II A group obviously increased the activity. The maximum activity was obtained by adding H2PtCl6to the catalytic system. Platinum is known to have a good activity for the hydrogenation of halonitrobenzenes. However, with respect to selectivity to haloani-lines, the performance of ruthenium is not satisfactory. Hereby, upon combining the high activity of platinum with the high selectivity of ruthenium, the Ru/Pt-DPPES catalyst exhibited the best catalytic performance, showing remarkable synergistic effect.

Table 1 Catalytic behavior of Ru-DPPES and Ru/M-DPPES for the hydrogenation of p-CNB
3.2 Effect of platinum content
As it has been discussed above that the addition of platinum can promote the hydrogenation of p-CNB to p-CAN remarkably. The effect of platinum content in the Ru/Pt-DPPES bimetallic system was further investigated. The results are summarized in Table 2 and Figure 1. As it can be seen that a striking feature of Ru/Pt-DPPES catalyst indicated that its activity was much higher than that of the monometallic Ru-DPPES and Pt-DPPES catalysts when the molar ratio of platinum [x(Pt)] in the bimetallic catalyst varied from 5% to 80%, while the selectivity to p-CAN was maintained at around 99%. The synergistic effect also can be observed even if the molar ratio of platinum was only 5%, with improvements of TOF [TOF = (mol of converted substrate)/((mol of catalyst)·min)] value varied from 0.437 mol/mol·min in Ru-DPPES to 14.8 mol/mol·min in Ru/Pt-DPPES. Upon increasing the platinum content in the bimetallic system, the TOF increased at first and then decreased. When the molar ratio of platinum increased to 20%, the hydrogenation fin-ished within 10 min at 70 ℃, giving a maximum TOF of 44.4 mol/mol·min. Besides, the hydrogenation reaction catalyzed by Ru/Pt-DPPES with a Pt molar ratio of 20% could be completed within 120 min even at ambient temperature (25 ℃), leading to a good p-CAN selectivity of 99.4%.
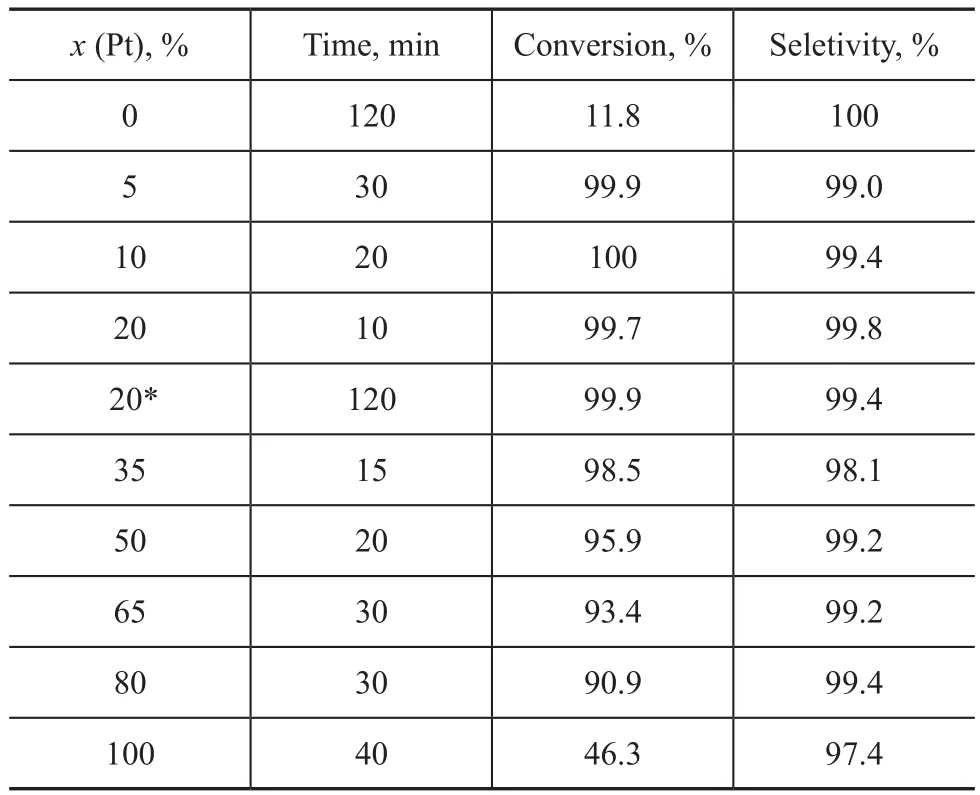
Table 2 Effect of platinum content in the Ru/Pt-DPPES catalyst on the hydrogenation of p-CNB

Figure 1 Effect of platinum content on the TOF
3.3 Effect of molar ratio of DPPES to ruthenium and platinum
The effect of molar ratio of DPPES to ruthenium and platinum on the hydrogenation reaction of p-CNB was also investigated. It can be seen from Table 3 that when the molar ratio of DPPES to ruthenium and platinum was below 3, the formation of active complex became easier with an increasing molar ratio value, so that both the conversion of p-CNB and selectivity to p-CAN were improved. However, when the molar ratio value reached 6, the selectivity to p-CAN obviously decreased. This phenomenon may be ascribed to the coordinative competition between too much DPPES and substrate to the center metal ions, which is disadvantageous to the coordination between the substrate and the catalytic active species.

Table 3 Effect of molar ratio of DPPES to Ru and Pt [n(DPPES)/n(Ru+Pt)] in the Ru/Pt-DPPES catalyst on hydrogenation of p-CNB
3.4 Hydrogenation of different chloronitrobenzenes
The results of hydrogenation of various substituted chloro- and dichloro-nitrobenzenes catalyzed by Ru-DPPES and Ru/Pt-DPPES are summarized in Table 4. The reactions catalyzed by Ru-DPPES and Ru/Pt-DPPES were carried out at 70 ℃ and 25 ℃, respectively. It can be seen that although the selectivity to CANs or DCANs (dichloroanilines) was maintained nearly at 99%, the conversion of CNBs or DCNBs (dichloronitrobenzenes) was below 10% when the Ru-DPPES catalyst was used. However, in the case of Ru/Pt-DPPES catalyst, all of the CNBs and DCNBs investigated could be converted to their corresponding CANs or DCANs completely, with the selectivity reaching over 99%. In any case, the synergistic effect of the bimetallic catalyst was remarkable.
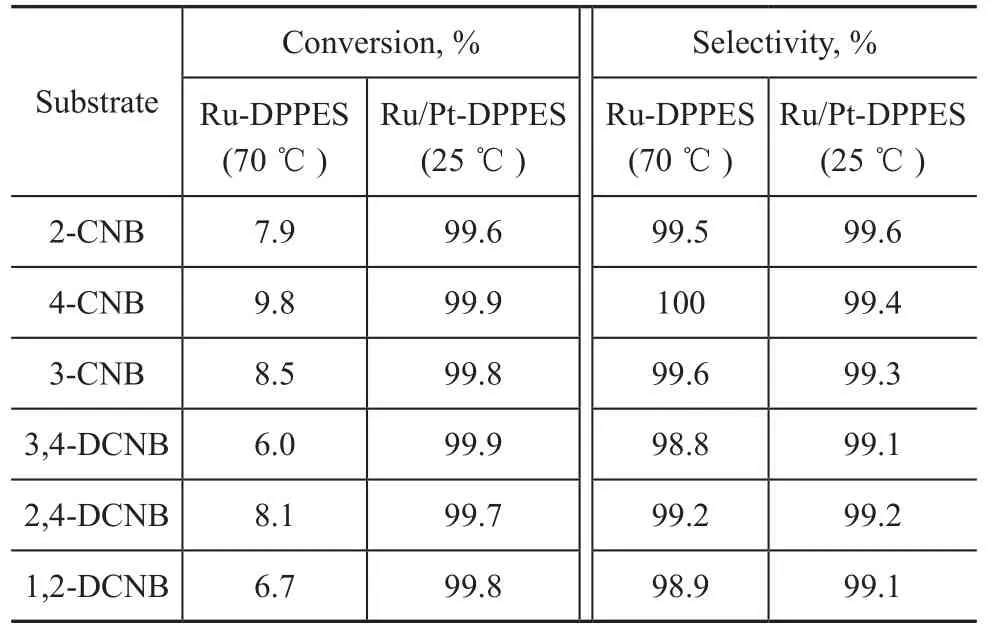
Table 4 Hydrogenation of different chloro- and dichloronitrobenzenes catalyzed by Ru-DPPES and Ru/Pt-DPPES catalysts
3.5 Recycle of the catalyst
The recycle of Ru/Pt-DPPES catalyst was investigated at ambient temperature (25 ℃) against the yield of the above-mentioned model reaction. After separation of the organic phase and water phase, the catalyst contained in water was reused in the next run without further purification. The data listed in Table 5 showed that the Ru/Pt-DPPES catalyst could be reused in the first and second run showing nearly no decrease of the catalytic activity, while a slight loss of the activity was observed in the third and fourth run, and especially in the fifth run. This might occur because of the gradual oxidation of catalyst during the separation process taking place in the air. However, the p-CNB still could be completely hydrogenated to p-CAN with extension of reaction time. Interestingly, the fresh catalyst showed a lower activity than that of the used catalysts. This phenomenon indicates that the catalytic system needs an induction time to form the active species of the catalyst. Compared with the heterogenous catalysts, the easy recycling performance is also an attractive advantage of the water-soluble catalysts in terms of the environmental protection and economic benefits.

Table 5 Recycle of the Ru/Pt-DPPES catalyst
4 Conclusions
The water-soluble Ru-DPPES and Ru/M-DPPES catalysts formed in-situ were used for the hydrogenation of p-CNB to p-CAN. The Ru/Pt-DPPES catalyst showed much higher activity than other bimetallic catalysts while maintaining good selectivity to p-CAN. Moreover, in a wide range of Pt molar ratio value, Ru/Pt-DPPES bimetallic catalyst exhibited a strong synergistic effect. The starting material p-CNB can be completely converted to p-CAN under mild conditions when the molar ratio of Pt in Ru/Pt-DPPES catalyst was 20%. In addition, the Ru/Pt-DPPES catalyst could be recycled five times. The Ru/Rt-DPPES catalyst also showed high activity and selectivity for the hydrogenation of other CNBs and DCNBs.
Acknowledgments: The authors are grateful for the financial supports of the Natural Science Foundation of China (No. 21303139), the Open Project of Chemical Synthesis and Pollution Control Key Laboratory of Sichuan Province (No. CSPC2013-1), the Key Fund Project of Educational Departmentof Sichuan Province (No. 14ZA0126) and the Doctoral Initiating Fund of China West Normal University (No. 10B010).
[1] Fernado C L, Santiago G Q, Mark A K. Clean production of chloroanilines by selective gas phase hydrogenation over supported Ni catalysts[J]. Appl Catal A: Gen, 2008, 334(1/2): 199-206
[2] Coq B, Tijani A, Figuéras F. Particle size effect on the kinetics of p-chloronitrobenzene hydrogenation over platinum/ alumina catalysts[J]. J Mol Catal, 1991, 68: 331-345
[3] Yang X, Liu H F. Influence of metal ions on hydrogenation of o-chloronitrobenzene over platinum colloidal clusters[J]. Appl Catal A: Gen, 1997, 164(1/2): 197-203
[4] Han X X, Zhou R X, Lai G H, et al. Influence of support and transition metal (Cr, Mn, Fe, Co, Ni and Cu) on the hydrogenation of p-chloronitrobenzene over supported platinum catalysts[J]. Catal Today, 2004, 93-95: 433-437
[5] Liang M H, Wang X D, Liu H Q, et al. Excellent catalytic properties over nanocomposite catalysts for selective hydrogenation of halonitrobenzenes[J]. J Catal, 2008, 255(2): 335-342
[6] Cheng H Y, Meng X C, Wang Q, et al. Fabrication of Co(OH)2coated Pt nanoparticles as an efficient catalyst for chemoselective hydrogenation of halonitrobenzenes[J]. J Colloid Interface Sci, 2012, 377: 322-327
[7] Claudio E, Laura A A, Maria B, et al. Chemoselective hydrogenation of halonitroaromatics over γ-Fe2O3-supported platinum nanoparticles: The role of the support on their catalytic activity and selectivity[J]. J Mol Catal A: Chem, 2013, 366: 288-293
[8] Cheng H Y, Meng X C, HelM, et al. Supported polyethylene glycol stabilized platinum nanoparticles for chemoselective hydrogenation of halonitrobenzenes in scCO2[J]. J Colloid Interface Sci, 2014, 415: 1-6
[9] Liu Y C, Chen Y W. Hydrogenation of p-chloronitrobenzene on lanthanum-promoted NiB nanometal catalysts[J]. Ind Eng Chem Res, 2006, 45(9): 2973-2980
[10] Yao N, Chen J X, Zhang J X, et al. Influence of support calcination temperature on properties of Ni/TiO2for catalytic hydrogenation of o-chloronitrobenzene to ochloroaniline[J]. Catal Commun, 2008, 9(6): 1510-1516
[11] Dutta D, Dutta D K. Selective and efficient hydrogenation of halonitrobenzene catalyzed by clay supported Nionanoparticles[J]. Appl Catal A: Gen, 2014, 487: 158-164
[12] Tijani A, Coq B, Figuéras F. Hydrogenation of parachloronitrobenzene over supported ruthenium-based catalysts[J]. Appl Catal, 1991, 76(2): 255-266
[13] Yan X P, Liu M H, Liu H F, et al. Metal complex effect on the hydrogenation of o-chloronitrobenzene over polymerstabilized colloidal ruthenium clusters[J]. J Mol Catal A: Chem, 2001, 170(1/2): 203-208
[14] Pietrowski M, Zieliński M, Wojciechowska M. Selective reduction of chloronitrobenzene to chloroaniline on Ru/ MgF2catalysts[J]. Catal Lett, 2009, 128(1/2): 31-35
[15] Kratky V, Kralik M, Mecarova M, et al. Effect of catalyst and substituents on the hydrogenation of chloronitrobenzenes[J]. Appl Catal A: Gen, 2002, 235(1/2): 225-231
[16] Vishwanathan V, Jayasri V, Basha P M, et al. Gas phase hydrogenation of ortho-chloronitrobenzene (O-CNB) to ortho-chloroaniline (O-CAN) over unpromoted and alkali metal promoted-alumina supported palladium catalysts[J]. Catal Commun, 2008, 9(3): 453-458
[17] He D P, Shi H, Wu Y, et al. Synthesis of chloroanilines: selective hydrogenation of the nitro in chloronitrobenzenes over zirconia-supported gold catalyst[J]. Green Chem, 2007, 9: 849-851
[18] Cárdenas-Lizana F, Gómez-Quero S, Keane M A. Ultraselective gas phase catalytic hydrogenation of aromatic nitro compounds over Au/Al2O3[J]. Catal Commun, 2008, 9(3): 475-481
[19] Chen Y Y, Wang C, Liu H Y, et al. Ag/SiO2: A novel catalyst with high activity and selectivity for hydrogenation of chloronitrobenzenes[J]. Chem Commun, 2005, 5298-5300
[20] Liu M H, Yu W Y, Liu H F, et al. Preparation and characterization of polymer-stabilized ruthenium-platinum and ruthenium-palladium bimetallic colloids and their catalytic properties for hydrogenation of o-chloronitrobenzene[J]. J Colloid Interface Sci, 1999, 214(2): 231-237
[21] Yang X L, Liu H F, Zhong H. Hydrogenation of o-chloronitrobenzene over polymer-stabilized palladium-platinum bimetallic colloidal clusters[J]. J Mol Catal A: Chem, 1999, 147(1/2): 55-62
[22] Cárdenas-Lizana F, Gómez-Quero S, Amorim C, et al. Gas phase hydrogenation of p-chloronitrobenzene over Pd-Ni/Al2O3[J]. Appl Catal A: Gen, 2014, 473: 41-50
[23] Mistri R, Llorca J, Ray BC, et al. Pd0.01Ru0.01Ce0.98O2-δ: Ahighly active and selective catalyst for the liquid phase hydrogenation of p-chloronitrobenzene under ambient conditions[J]. J Mol Catal A: Chem, 2013, 376: 111-119
[24] Gulyás H, Bényei A C, József B. Catalytic properties of water-soluble rhodium and iridium complexes: the influence of the ligand structure[J]. Inorg Chim Acta, 2004, 357: 3094-3098
[25] Li R X, Li X J, Wong N B, et al. Syntheses and characterizations of iridium complexes containing bidentate phosphine ligands and their catalytic hydrogenation reactions to α, β-unsaturated aldehydes[J]. J Mol Catal A: Chem, 2002, 178(1/2): 181-190
[26] Davis M E, Hanson B E, Hanson B E. Process for the hydroformulation of olefinically unsaturated organic reactants using a supported aqueous phase catalyst: US Patent Appl, US 4947003[P]. 1990-08-07
date: 2014-12-05; Accepted date: 2015-03-10.
Professor Zhou Yafen, Telephone: +86-817-2568081;E-mail: cwnuzyf@163.com.
杂志排行
中国炼油与石油化工的其它文章
- Pyrolysis Characteristics and Kinetics of Methyl Oleate Based on TG-FTIR Method
- Development of RSDS-III Technology for Ultra-Low-Sulfur Gasoline Production
- Effects of Fe2+, Co2+and Ni2+Ions on Biological Methane Production from Residual Heavy Oil
- Curing Mechanism of Condensed Polynuclear Aromatic Resin and Thermal Stability of Cured Resin
- A Novel Thermally Coupled Reactive Distillation Column for the Hydrolysis of Methyl Acetate
- Synthesis of Waterborne Polyurethane Modified by Nano-SiO2Silicone and Properties of the WPU Coated RDX
