Synthesis of Waterborne Polyurethane Modified by Nano-SiO2Silicone and Properties of the WPU Coated RDX
2015-06-22LiYangZhaoTianboQuXiaolingDingHongjingLiFengyan
Li Yang; Zhao Tianbo; Qu Xiaoling; Ding Hongjing; Li Fengyan
(1. School of Chemistry, Beijing Institute of Technology, Beijing 100081; 2. Department of Applied Chemistry, Beijing Institute of Petrochemical Technology, Beijing 102617)
Synthesis of Waterborne Polyurethane Modified by Nano-SiO2Silicone and Properties of the WPU Coated RDX
Li Yang1; Zhao Tianbo1; Qu Xiaoling1; Ding Hongjing1; Li Fengyan2
(1. School of Chemistry, Beijing Institute of Technology, Beijing 100081; 2. Department of Applied Chemistry, Beijing Institute of Petrochemical Technology, Beijing 102617)
A series of nano silica/silicone modified waterborne polyurethane (WPU) have been synthesized from polytetramethylene glycol and isophorone diisocyanate, dihydroxymethyl propionic acid and triethylamine, ethylenediamine, trimethylolpropane, nano-SiO2and the silane coupling agent KH550. The effect of the dosage of nano-SiO2on the WPU-Si membrane and the coated RDX (cyclotrimethylenetrinitramine) particles have been studied in terms of their surface properties, mechanical properties, and thermal stability. The results showed that with the increase of Si content, the stability of the emulsion reduced gradually. The material with more Si content displayed an increased thermodynamic stability, an increased high temperature resistance, an increased tensile strength and a decreased elongation at break. With the increase of Si content, the surface tension of the material decreased, the bibulous rate reduced, and the contact angle increased gradually, so that the surface tension of the polyurethane and RDX are close to each other which could improve the performance of coating.
nano-SiO2; organic silicon; waterborne polyurethane; coating of RDX
1 Introduction
Traditional PU (polyurethane) materials are short of meeting the requirements for heat resistance, water resistance, surface properties and dielectric properties, and naturally its application is limited in some areas[1-3]. The development of the new type modified polyurethane has become very important. At present, silicone materials in the deep processing and application is going to be a huge development domain, since the introduction of silicon materials is very necessary and urgent for the PU, so seeking new method of introducing and researching on the relationship between macro performance of material and its structure is of great significance[4-7].
Organic silicon compound is a special polymer compound, because it has the characteristics of both inorganic compounds and organic compounds. Thanks to its low temperature resistance, weather resistance, anti-aging ability, hydrophobicity, organic solvent resistance, radiation resistance and other excellent properties, the organic silicon compound has been widely used for polyurethane modification[8-13]. Modification can greatly improve the mechanical properties of waterborne polyurethane[14].
SiO2is a kind of porous inorganic particles featuringhigh surface area and low surface energy[15-18], capable of combining firmly with polymers. The presence of SiO2increases the liquidity of polyurethane molecular chain, which can improve the orderliness of polymer segments. The introduction of nano-silica can improve the polymer incompatibility between soft segment and hard segment, and promote the system phase separation[19-24]. In addition, the introduction of inorganic silicon can enhance the mechanical properties, thermodynamic performance and corrosion resistance of polymer[25-27].
In this paper both the organic silicon and nano-SiO2are introduced to waterborne polyurethane, and waterborne polyurethane with high hydrophobic material is prepared by means of synergistic reaction so that the effect of the polyurethane coated RDX was improved.
2 Experimental
2.1 Preparation of waterborne polyurethane
PTMG (polytetramethylene glycol, Mn= 1 000 g/mol)was dried for 2 h at 110 ℃ under vacuum before use. A three-necked round-bottomed separable flask, equipped with a mechanical stirrer, a thermometer, and a condenser with a drying tube, was used as the reactor. The reaction was carried out in a constant-temperature oil bath. IPDI (isophorone diisocyanate) and PTMG after having been mixed at first entered into reaction at 80 ℃ for 1 h. Then DMPA (dimethylol propionic acid) dissolved in DMF (dimethyl formamide) at a temperature of 80 ℃ was added for conducting the reaction for 10 min. An appropriate amount of acetone, the catalyst DBTDL (dibutyltin dilaurate) and TMP (trimethylolpropane) was added for entering into the reaction for 2 h. At the same time, the stirring speed was slightly improved. When the mixture was cooled down to 40 ℃, TEA (triethylamine) was added into the flask for entering into the reaction for 2 h. After adding a mixture of distilled water and ethylenediamine and having been subjected to shear emulsification for 1.5 h, acetone was then removed under vacuum. This synthesis process is shown in Scheme 1.
2.2 Modification of waterborne polyurethane
Aerosil 200, a type of hydrophilic pyrogenic nanosilica (nano-SiO2), with a primary particle size of 300 nm provided by Degussa was used as a filler in the polyurethane composites. Synthesized polyurethane films were dissolved in dimethylformamide with the addition of 0%, 1%, 2%, and 3% of nano-SiO2, respectively, and an appropriate amount of silane coupling agent (KH550), and then subsequently the mixture was homogenized by a magnetic stirrer (at a stirring rate of 500 r/min). The solution was cast onto the Teflon plate to reach a uniform thickness using a hand coater (designed to provide a certain film thickness). The samples were dried at room temperature to a constant weight, and were then put in an oven at 40 ℃ for 4 h prior to being heated to 80 ℃ for 2 h.
2.3 Coating according to demulsification method
The waterborne polyurethane emulsion and distilled water (at a mass ratio=1:8) were charged into a three-necked round-bottomed separable flask at room temperature. Then under a certain stirring rate to achieve a fully mixing suspension, the RDX to be coated was added at the same time and heated to 45 ℃. At a rate of 1 drop per second, a proper amount of 1.00% aqueous solution of alum was added (the mass ratio of demulsifier solution/ polyurethane=4:1). After breaking the emulsion as a result of demulsification precipitation, polyurethane was bonded on the surface of the material to be coated. The reaction process is shown in Figure 1.

Figure 1 Coating by demulsification method
2.4 Analysis and characterization
Water absorption: The film (2 cm × 2 cm) was dried to a constant weight in vacuum at 60 ℃ for 24 h before use. The swelling of the film in water was measured by immersing a film in water at room temperature until the film reached an equilibrium with water. The bibulous rate was calculated as follows:
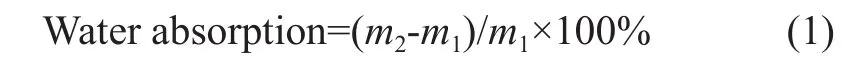
where m1and m2are the mass of the initial sample and the swelled sample, respectively.
Contact angle: The contact angles were measured by a JC2000 measurement instrument using distilled water as the medium, and the average value of at least 3 measurements was adopted.
Melting point: The melting point measurements were carried out on a Tektronix microscopic melting point meter X-6 operating in the range from 50 ℃ to 200 ℃.
Tensile strength and elongation at break: The tensile strength and elongation at break measurements were performed on a Instron 6022 material testing machine at a constant strain rate of 100 mm/min. During the measurements the samples were cut in dumbbell form with a required dimension (20 mm × 15 mm × 5 mm) according to the China’s National Standard GB 1040—1979.
Hardness: The hardness test was performed with a QHQ hardness tester (Tianjin, China) according to the standard method GB/T 6739—1996.
Thermal stability: The measurement was made using a Netzsch TG209 thermogravimetric analyzer. All measure-ments were performed in nitrogen atmosphere (at a flow rate of 50 cm3/min) at a heating rate of 20 ℃/min.

Scheme 1 Preparation of the silicon-containing polyurethane dispersion
Scanning electron microscopy (SEM): The morphologies of RDX samples coated by waterborne polyurethane were obtained by a scanning electron microscope (SEM, TM-1000; Hitachi Corp., Osaka, Japan).
3 Results and Discussion
3.1 The surface performance of waterborne polyurethane modified by nano-SiO2/silicone
The samples were synthesized under the same condition (at a ratio of -NCO/-OH=1.6), with a DMPA content of 5.5%, a TMP content of 2% and a neutralization degree of 100%. The surface performance of 4 groups named PUSi0, PUSi0.3, PUSi0.5, PUSi0.7, with addition of 0%, 1%, 2% and 3% (0 g, 0.3 g, 0.5 g, and 0.7 g, respectively) of nano-SiO2, respectively, are shown in Table 1.
As shown in Table 1, the use of silicone can enhance the surface properties of the polyurethane emulsion, such as reducing the water absorption, increasing the water contact angle, and lowering the surface tension, leading to improvements in wettability and spreadability on the surface of material. When the water contact angle of WPU is larger, it would be easier to separate polyurethane from the water, and at the same time, the contact angle relative to the RDX surface is smaller, so that it’s more easier to wet and spread on the surface of RDX and more suitable for coating RDX with the demulsification agent (Figure 2). Organic silicone KH550 plays a main role when the content of nano-SiO2is relatively low. Silicone polymer can provide the film with excellent softness and smooth silk feel, and in consequence makes the polyurethane film flexible, resulting in a decline in its melting point as shown in Table 1. However, when polyurethane molecules were doped with more nano-SiO2, the hard segment content was enhanced, so the hardness of thin film was gradually strengthened leading to a rising melting point (Table 1).With the increase of the content of nano-SiO2, the stability of polyurethane emulsion gradually reduced (Table 1). The introduction of the nanoparticles makes the size of polyurethane molecular increase while the big molecules cannot be stabilized in the water, which would cause obvious deterioration in the stability of the emulsion.

Table 1 Surface performance of waterborne polyurethane modified by nano-SiO2/silicone

Figure 2 The surface interaction of WPU used for coating RDX
3.2 Mechanical properties of waterborne polyurethane modified by nano-SiO2/silicone
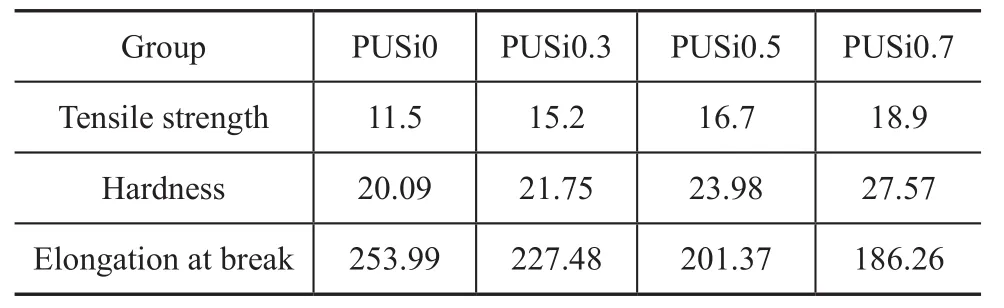
Table 2 Impact of nano-SiO2content on the mechanical properties of PU membrane
With the increase of nano-SiO2content, the tensile strength and the hardness of the polyurethane film increased (Table 2), because the intervention of inorganic nanoparticles enhanced internal degree of cross link-ing of polyurethane molecules. But at the same time the elongation at break decreased with the increase of nano-SiO2content (Table 2). Especially when the content of nano-SiO2was more than 0.5 g, the elongation at break decreased obviously. Because the intervention of excessive SiO2greatly increased the degree of cross linking of polyurethane, the entire performance of the polyurethane film was affected.
3.3 The thermal stability of waterborne polyurethane modified by nano-SiO2/silicone
The thermal degradation temperature of modified polyurethane film is between 150 ℃ and 450 ℃. The thermal degradation temperature of the polyurethane film increased with an increasing nano-SiO2content (Figure 3). This showed that thermal stability of the modified polyurethane is enhanced, which is determined by the internal structure of polyurethane. The phenomenon of sol-gel happened in the process of emulsification of Si modified waterborne polyurethane emulsion and -Si-O-Si- bonds were formed in this process. And then the cross-linking structure was formed in the internal structure of polyurethane molecules, which could limit the movement of the polyurethane molecular chain, resulting in an improved thermal stability. In addition, Si-O bonds were generated in the reaction between KH550 and nano-SiO2, whose bond energy was greater than that of C-O bonds, which could enhance the thermal stability of polyurethane. With the increase of nano-SiO2content, the heat resistance of polyurethane was greatly improved.

Figure 3 TGA curves of PUSi0, PUSi0.3, PUSi0.5 and PUSi0.7 nanocomposite films
3.4 SEM analysis of polyurethane emulsion
The microcosmic principle of coating RDX via the emulsion demulsification method is shown in Figure 4. As shown in Figure 5a, the demulsification effect of PU emulsion was not very good, and the phenomenon of reunion of polyurethane molecules appeared, resulting in a very bad coating effect that a large part of RDX was not coated. With the increase of Si content, the effect of demulsification was getting better and better, and the macromolecular reunion phenomenon was gradually weakened, while the effect of coating was improved (as shown in Figure 5).In order to obtain a high bonding strength, it is necessary for WPU emulsion to fully wet the surface of the particles. The closer the surface tension between WPU emulsion and RDX is, the better the effect of wetting would be. Organic silicon has an advantage of low surface energy so polyurethane can better spread out on the surface of RDX particles with the increase of silicon content.
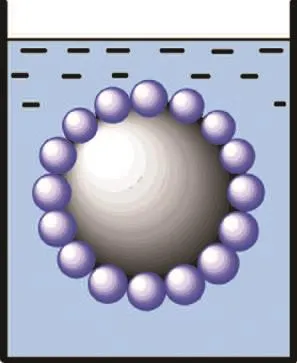
Figure 4 Microcosmic principle of coating RDX via emulsion demulsification
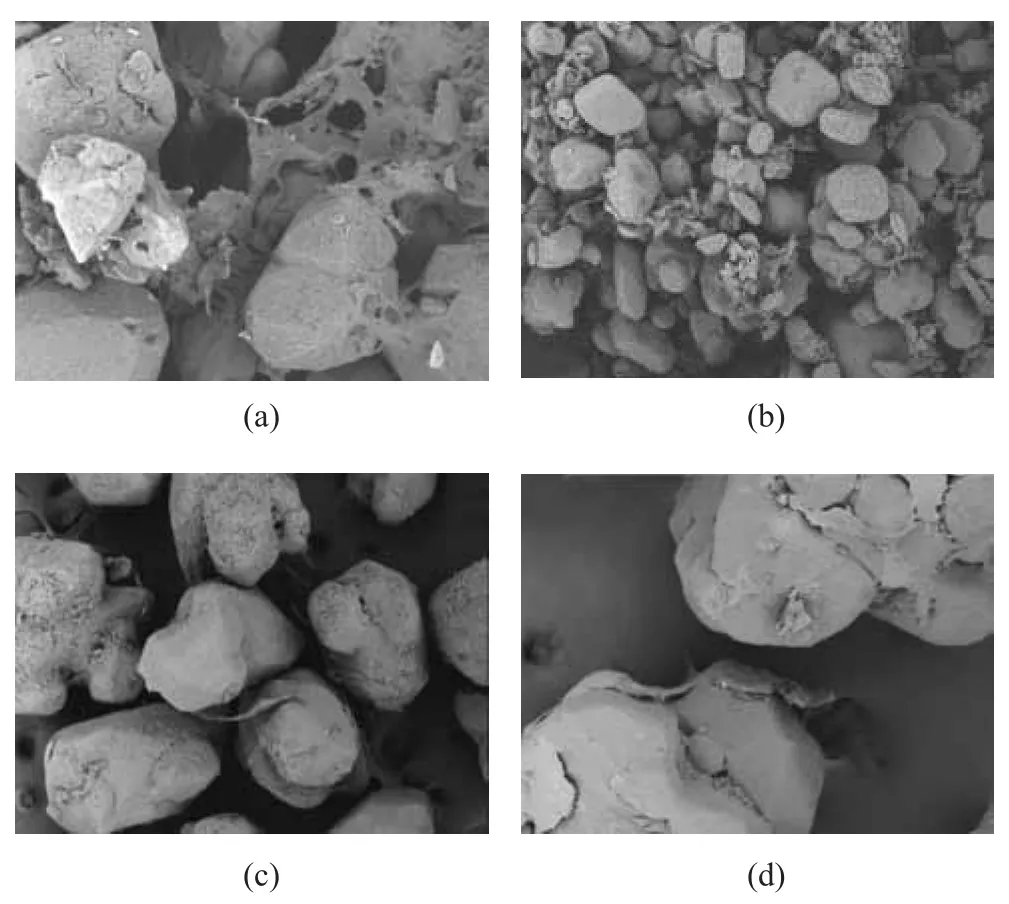
Figure 5 SEM of polyurethane emulsions
4 Conclusions
The samples were synthesized under the same condition (the ratio of -NCO/-OH=1.6), with a DMPA content of 5.5%, a TMP content of 2% and a neutralization degree of 100%. The main conclusions obtained by experiments are as follows:
(1) The water resistance and heat resistance of WPU modified by inorganic-organic silicon compounds were improved. The phenomenon of sol-gel happening in the process relating to Si modified waterborne polyurethane emulsion and -Si-O-Si- bonds was identified in this process. Nano-SiO2was evenly dispersed in polyurethane macromolecules system, and Si atoms migrated to the surface, so the surface tension of the polyurethane was decreased. In addition, the introduction of inorganic silicon compound improved the mechanical properties of the polymer, lowered the swelling degree and improved the thermodynamic performance.
(2) With the unique chemical structure and low surface energy, the organic siloxane provided polyurethane film with low surface energy and excellent water resistance. The nano-SiO2, a typical porous inorganic nanoparticle with high surface area and low surface energy, provided the material with surface roughness, and the mechanical properties, wear resistance, heat resistance and surface properties of materials were improved. The waterborne polyurethane with high hydrophobic ability were prepared.
(3) After polyurethane materials were modified by using inorganic-organic silicon compounds, the surface tension of the WPU emulsion and RDX particle became close and the effect of coating was improved.
Acknowledgements: The authors are pleased to acknowledge the financial support by the National Natural Science Foundation of China (No. 20973022 and No. 11472048)
[1] Tutti S, Levi M, Trombetta T. Process design of fluorinated polyurethane-urea anionomer aqueous dispersions[C]//Macromolecular Symposia. WILEY-VCH Verlag, 2004, 218(1): 29-38
[2] Kakn M, GrimmingerlC, Sogah D Y, et al. New fluorinated oxazoline block copolymer lowers the adhesion of playlets on polyurethane surfaces[J]. Journal of Polymer Science Part A: Polymer Chemistry, 1994, 32(11): 2187-2192
[3] Blackwell J, Nagarajan M R, Hoitink T B. The dynamic mechanism property and microstructure study of nanosegment polyurethane[J]. Polymer, 2007, 23(1): 950-954
[4] Enderle H F, Kilian H G, Heise B, et al. Irreversible deformation of semicrystalline PUR-elastomers—A novel concept[J]. Colloid and Polymer Science, 1986, 264(4): 305-322
[5] Gisselfalt K, Helgee B, Macromol A F M. Study of the nanostructure of quenched isotactic polypropylene[J]. Mater Eng, 2003, 288(1): 265-268
[6] Chirvase D, Parisi J, Hummelen J C, et al. Influence of nanomorphology on the photovoltaic action of polymerfullerene composites[J]. Nanotechnology, 2004, 15(9): 1317
[7] Ikeda Y, Kohjiya S, Yamashita S. Segmented Polyurethanes Based on Triblock Copolyether as Biomedical Materials[J]. Rubber World, 1989, 200(6): 21-27
[8] Gun X A, Hunter A D. Polyesters, polyearbonate, and polyurethanes from a novel monomer a,a,a'a'-tetramethyl-1,4-tetrafluorobenzenedimethon1[J]. Journal of Polymer Science Part A: Polymer Chemistry, 1993, 31(6): 1431-1439
[9] Jr Gaines G L. On the surface of block of copolymers[M]. Macromolecules, 1998, 14: 280-283.
[10] Fan Q L, Fang J L, Chen Q M, et al. Synthesis and properties of polyurethane modified with aminoethyl aminopropyl poly (dimethylsiloxane)[J]. Journal of Applied Polymer Science, 1999, 74(10): 2552-2558
[11] Figovsky O L, Shapovalov L, Axenov O. Advanced coatings based upon non-isocyanate polyurethanes for industrial applications[J]. Surface Coatings International Part B: Coatings Transactions, 2004, 87(2): 83-90
[12] Garipov R M, Sysoev V A, Mikheev V V, et al. Reactivity of Cyclocarbonate Groups in Modified Epoxy-Amine Compositions[C]//Doklady Physical Chemistry. MAIK Nauka/Interperiodica, 2003, 393(1): 289-292
[13] Lee J S, Shin J H, Kim B K, et al. Modification of aqueous polyurethanes by forming latex interpenetrating polymer networks with polystyrene[J]. Colloid and Polymer Science, 2001, 279(10): 959-965
[14] Kim B K, Seo J W, Jeong H M. Morphology and properties of waterborne polyurethane/clay nanocomposites[J]. European Polymer Journal, 2003, 39(1): 85-91
[15] Li F, Zuo J, Song D, et al. The design, synthesis and characterization of polyurethane with super macromolecular size[J]. European Polymer Journal, 2001, 37(1): 193-199
[16] Price D, Pyrah K, Hull T R, et al. Flame retarding poly (methyl methacrylate) with phosphorus-containing compounds: Comparison of an additive with a reactive approach[J]. Polymer Degradation and Stability, 2001, 74(3): 441-447
[17] Chuang F S, Tsi H Y, Chow J D, et al. Thermal degradation of poly (siloxane-urethane) copolymers[J]. Polymer Degradation and Stability, 2008, 93(10): 1753-1761
[18] Lai X, Shen Y, Wang L. Preparation and properties of selfcrosslinkable polyurethane/silane hybrid emulsion[J]. Journal of Polymer Research, 2011, 18(6): 2425-2433
[19] Shih Y F, Wang T Y, Jeng R J, et al. Biodegradable nanocomposites based on poly (butylene succinate)/ organoclay[J]. Journal of Polymers and the Environment, 2007, 15(2): 151-158
[20] Leibler L. Theory of microphase separation in block copolymers[J]. Macromolecules, 1980, 13(6): 1602-1617.
[21] Wang Z, Zhou Y, Yao Q, et al. Preparation, characterization and infrared emissivity study of helical polyurethane/ SiO2core-shell composite[J]. Applied Surface Science, 2009, 256(5): 1404-1408
[22] Gun’ko V M, MironyukiF, Zarko V I, et al. Morphology and surface properties of fumed silicas[J]. Journal of Colloid and Interface Science, 2005, 289(2): 427-445
[23] Wang Q, Chen S, Wang T, et al. Damping, thermal, and mechanical properties of polyurethane based on poly (tetramethylene glycol)/epoxy interpenetrating polymer networks: effects of composition and isocyanate index[J]. Applied Physics A, 2011, 104(1): 375-382.
[24] Bistričić L, Baranović G, Leskovac M, et al. Hydrogen bonding and mechanical properties of thin films of polyether-based polyurethane-silica nanocomposites[J]. European Polymer Journal, 2010, 46(10): 1975-1987
[25] Chen Y, Zhou S, Yang H, et al. Preparation and characterization of nanocomposite polyurethane[J]. Journal of Colloid and Interface Science, 2004, 279(2): 370-378
[26] Zhou H, Chen Y, Fan H, et al. The polyurethane/SiO2nano-hybrid membrane with temperature sensitivity for water vapor permeation[J]. Journal of Membrane Science, 2008, 318(1): 71-78
[27] Chen Y, Zhou S, Yang H, et al. Structure and properties of polyurethane/nanosilica composites[J]. Journal of Applied Polymer Science, 2005, 95(5): 1032-1039
date: 2014-11-30; Accepted date: 2015-04-07.
Prof. Zhao Tianbo, Telephone: +86-13522293506; E-mail: zhaotb@bit.edu.cn.
杂志排行
中国炼油与石油化工的其它文章
- Pyrolysis Characteristics and Kinetics of Methyl Oleate Based on TG-FTIR Method
- Development of RSDS-III Technology for Ultra-Low-Sulfur Gasoline Production
- A Highly Efficient and Selective Water-Soluble Bimetallic Catalyst for Hydrogenation of Chloronitrobenzene to Chloroaniline
- Curing Mechanism of Condensed Polynuclear Aromatic Resin and Thermal Stability of Cured Resin
- A Novel Thermally Coupled Reactive Distillation Column for the Hydrolysis of Methyl Acetate
- Effects of Fe2+, Co2+and Ni2+Ions on Biological Methane Production from Residual Heavy Oil
