Diester Derivatives from Chemically Modified Waste Cooking Oil as Substitute for Petroleum Based Lubricating Oils
2015-06-22XiangShuoChenLigongXuLanLiLiangYangXinZhuLiye
Xiang Shuo; Chen Ligong; Xu Lan; Li Liang; Yang Xin; Zhu Liye
(1. Department of Military Oil Application & Management Engineering, Logistical Engineering University of PLA, Chongqing 401311; 2. School of Chemistry and Chemical Engineering, Southwest University, Chongqing 400715; 3. Oil Technical Supervision Office, Logistical Department of Chengdu Military Area, Chengdu 610041)
Diester Derivatives from Chemically Modified Waste Cooking Oil as Substitute for Petroleum Based Lubricating Oils
Xiang Shuo1; Chen Ligong1; Xu Lan2; Li Liang1; Yang Xin1; Zhu Liye3
(1. Department of Military Oil Application & Management Engineering, Logistical Engineering University of PLA, Chongqing 401311; 2. School of Chemistry and Chemical Engineering, Southwest University, Chongqing 400715; 3. Oil Technical Supervision Office, Logistical Department of Chengdu Military Area, Chengdu 610041)
In order to provide a new way for waste cooking oil (WCO) resource utilization, several diester derivatives were obtained from WCO through a three-step chemical modifications, viz.: transesterification, epoxidation and oxirane ring opening with carboxylic acids. The effects of the chain length of side chain groups on the viscosity, acid value, low temperature fluidity, thermo-oxidative stability, tribological properties and surface tension of diester derivatives were investigated. The results showed that increasing the chain length of side chain groups had a positive influence on the viscosity, viscosity index, acid value, pour point, friction coefficient and wear scar diameter along with a negative influence on the oxidation onset temperature, volatile loss, insoluble deposit, maximum non-seizure load and surface tension. These diester derivatives exhibited improved physicochemical and tribological properties that make themselves promising environmentally friendly biolubricant basestocks.
waste cooking oil; diester derivative; lubricating oil; physicochemical property; tribological property
1 Introduction
The progressive depletion of world petroleum reserves and increased concerns on their environmental impact have given rise to increasing interests in the development of substitutes originating from renewable resources for petroleum-based lubricants[1-3]. Waste cooking oil (WCO) has been considered to be a sustainable and economical raw material for the production of biolubricants thanks to the suitable properties of its base materials (vegetable oils and animal fats), such as very low volatility, narrow range of viscosity changes, biodegradability and high lubricity[4-8]. In addition, WCO will result in environmental contamination if no proper disposal method is adopted. Therefore, an approach for utilizing WCO not only can offer benefits on environmental conservation but can also attain great significance for realizing the sustainable development.
Although the use of bio-lubricants looks promising based on the suitable properties of vegetable oils and animal fats, their shortcomings, such as poor low temperature fluidity resulted from the strong intermolecular interactions, insufficient thermo-oxidative stability due to the unsaturated double bonds, and hydrolytic instability arising from the susceptibility of the triglyceride to hydrolysis, are the critical barriers to their wide application in the lubricating oil industry[9-10]. Appropriate chemical modification of vegetable oils and animal fats is a sound alternative to allow for their direct use as lubricating base oil. Chemical modifications such as transesterification[11-13], epoxidation[14-16], oxirane opening with carboxylic acids[17-19], oxirane opening with anhydrides[20-22]and oxirane opening by alcoholysis[23-25]have been shown to improve the physicochemical and tribological properties of vegetable oils and animal fats based lubricants.
The main objective of this study is to provide a practical synthetic approach to convert WCO with high animal fats content into several ecologically friendly diester derivatives as substitute for the petroleum-based lubricating oil through a three-step chemical approach, including transesterification of WCO, epoxidation of fatty acid methyl ester (FAME) and oxirane ring opening with carboxylic acids, as shown in Figure 1. Subsequent evaluation of di-ester derivatives on their viscosity, low temperature fluidity, thermo-oxidative stability, tribological properties and surface tension are also reported. This research may aid in the effort of converting WCO into high value-added materials for application as substitute for petroleum products in the industry and elsewhere.
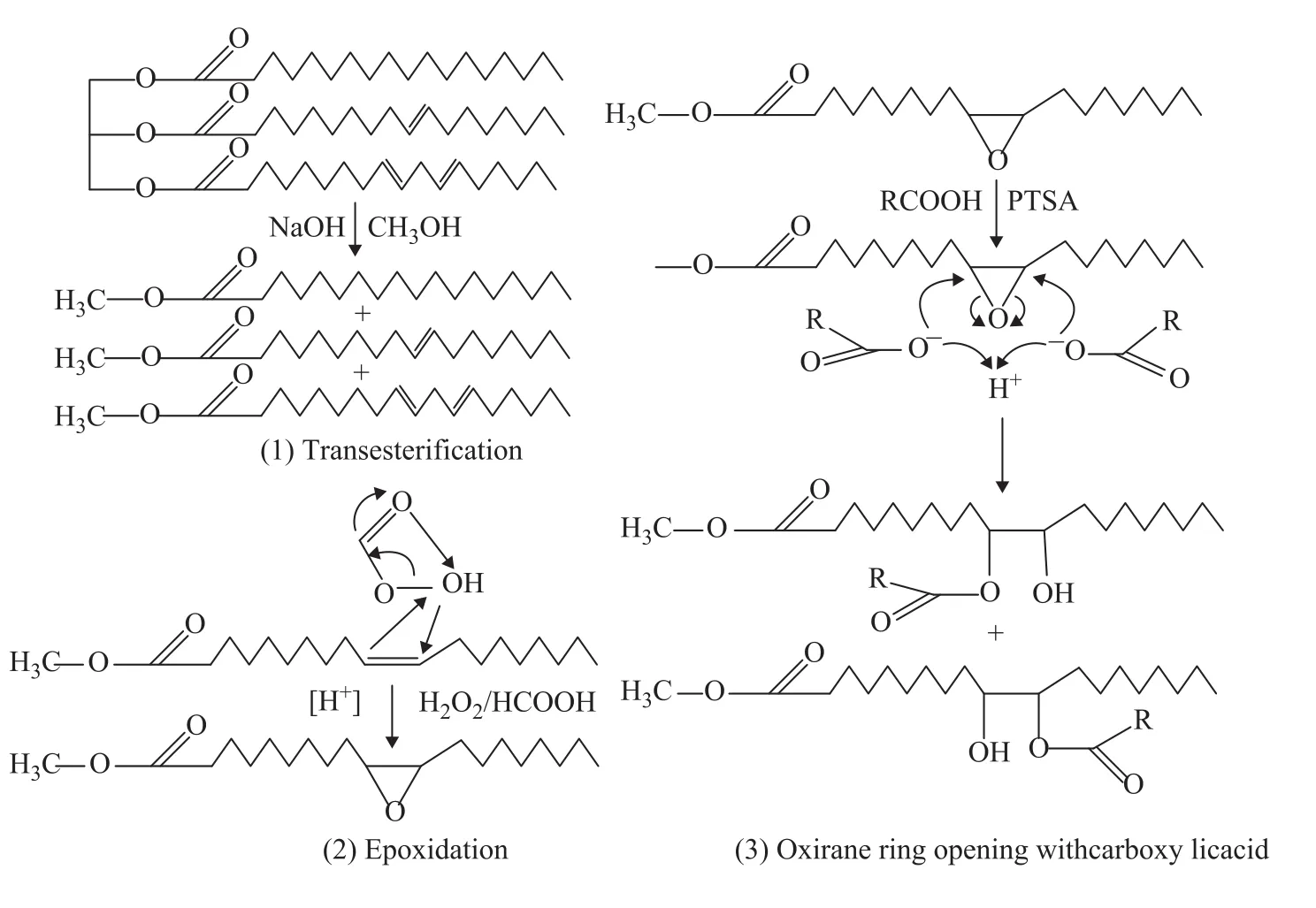
Figure 1 The reaction scheme for the formation of diester derivatives
2 Experimental
2.1 Materials
WCO with an acid value of 114.16 mgKOH/g was provided by the Chongqing Environment & Sanitation Holding (Group) Co., Ltd. (Chongqing, China) authorized by the local government. Impurities and moisture remaining in the WCO were removed through washing, filtration, evaporation, decolorization and degumming after settling. All other chemicals and reagents were obtained from the Chongqing Reagent Company. All materials were used without further purification and all solvents were dried with anhydrous sodium sulfate.
2.2 Synthesis
2.2.1 Transesterification
Fatty acid methyl ester (FAME) was prepared by transesterification of WCO with methanol (with the molar ratio of methanol to oil=8:1), under alkaline condition (in the presence of 1.3% of potassium hydroxide). The reaction was carried out for 131 min under reflux at 76℃ under agitation. After the termination of reaction, the mixture was left until a phase separation was observed, with the FAME settling in the upper phase and glycerol in the bottom phase. The upper phase containing FAME was harvested and rinsed several times with de-ionized water until the color of the rinsed water was clear. Finally, the obtained FAME was dried under vacuum at 50 ℃ for 24 h.
2.2.2 Epoxidation
Epoxidized fatty acid methyl ester (EFAME) was prepared by epoxidation of FAME with performic acid generated in situ, using benzene as a diluent of the organic phase. The chosen molar ratio of hydrogen peroxide/ formic acid/double bonds of the FAME was 1.1/0.5/1. After homogenizing the mixture for 30 min at 70 ℃, a predefined amount of 30% aqueous H2O2was placed in a dropping funnel and added to the mixture over a period of 1 h. The reactor temperature was then slowly raised to 50 ℃ in order to complete the reaction (about 7 h). The mixture was then washed with distilled water until the performic and formic acids were completely removedfrom the organic phase, and then a 5% NaCl solution was added to the liquid phase. Finally, water and benzene were separated from EFAME in a rotary evaporator.
2.2.3 Oxirane ring opening reaction
Diester derivatives were prepared by oxirane ring opening of EFAME with carboxylic acids, catalyzed by para-toluene sulphonic acid (PTSA). Then a 5% PTSA, 100 mL of toluene, and 40 mL of acetic acid were added to 60 mL of EFAME within 1 h in order to maintain the reaction mixture temperature at 70—80 ℃. The reaction mixture was subsequently heated to 100—110 ℃ and refluxed for 4 h. After the reaction mixture was cooled down to room temperature, the product was washed three times with 100 mL of 5% NaHCO3solution followed by 100 mL of brine solution, respectively. The organic layer was dried over anhydrous sodium sulfate and the solvent was removed using the vacuum evaporator to recover the diester derivative. The product (AWCO) was then stored under vacuum. The above procedure was repeated using propionic, butyric, i-butyric, and hexanoic acids, respectively, for preparation of diester derivatives PWCO, BWCO, i-BWCO, and HWCO, respectively
2.3 Methods
2.3.11H NMR and FTIR
The1H NMR spectra were recorded on a Bruker (Boston, MA) Avance 500 NMR spectrometer operated at 500.13 MHz using a 5-mm broadband inverse Z-gradient probe in CDCl3(Cambridge Isotope Laboratories, Andover, MA). The FTIR spectra were recorded on a Shimadzu FTIR-8400 spectrometer equipped with a KBr beam splitter. The diffuse reflectance system (DRS) was used for powder samples and the NaCl plate for liquid samples by the thin film deposition technique. A regular scanning range of 400—4 000 cm-1was used for 45 repeated scans at a spectral resolution of 4 cm-1.
2.3.2 Viscosity and acid value
Glass capillary viscometer tubes were used to determine the kinetic viscosity of diester derivatives. Measurements were run in a constant temperature bath set at 40.0 ℃ and 100.0 ℃. The kinetic viscosity was measured according to the Chinese standard method GB/T 265—1988. Duplicate measurements were made and the average values were reported. The viscosity index could be calculated by the Chinese standard method GB/T 1995—1998. The acid values were analyzed by the volumetric titration of samples with potassium hydroxide according to the Chinese standard method GB/T 5530—2005.
2.3.3 Pour point and cloud point
The pour point of a sample is defined as the lowest temperature when it remains pourable. The cloud point is the temperature at which dissolved solids, such as paraffin wax, are no longer completely soluble, and instead of precipitating as a second phase would give the fluid a cloudy appearance. This method has shown quite good consistency for determining the low temperature flow property of fluids. Pour points were measured as per the Chinese standard method GB/T 3535—2006. Each run was carried out at least in duplicate and the average value rounded to the nearest whole degree was reported.
2.3.4 Oxidation stability
Pressure differential scanning calorimetry (PDSC) experiments were accomplished using a computerized NETZSCH DSC 204 HP instrument to measure the oxidative stability of samples. The onset (OT) and signal maxima (SM) temperatures of oxidation were obtained from DSC exotherm. OT and SM are obtained from a plot of heat flow versus temperature that was generated by the exothermal reaction of oil sample. High OT and SM would suggest a high oxidative stability of samples. Thin film micro oxidation (TFMO) experiments were conducted by oxidizing a thin film of sample oil (25 μL) on the catalytic high carbon steel pan surface with a steady flow (20 cm3/ min) of dry air. The oxidation experiments were performed at 150, 175, 200, 225 ±1 ℃for 2 h by placing these pans on a thermally equilibrated aluminum slab, covered with a bottomless glass reactor. After oxidation, the pans with the oxidized oil sample were removed, cooled down for 2 h, and weighed to determine the volatile loss (or gain). The pans were immersed (30 min) in tetrahydrofuran (THF) solvent to dissolve the soluble portion of the oxidized oil. Finally, theses pans were dried and weighed to determine the remaining insoluble deposits.
2.3.5 Surface tension
Surface tension measurements were conducted with a BP100 dynamic surface tensiometer using the maximum bubble pressure method. Experiments were carried out at 23—60 ℃, and the bubbles were blown with purified nitrogen gas. A bubble lifetime of at least 4 sec was used so that dynamic effects were not a factor in the measurements.
2.3.6 Friction and wear tests
The anti-wear and friction-reducing properties of diester derivatives were evaluated using a four-ball friction and wear testers. The maximum non-seizure load (PB) was tested with an MQ-800 four-ball friction and wear tester following the procedures of Chinese standard method GB/T 3142—1982. The wear scar diameters (WSD) and friction coefficients were measured with an MMW-1P universal four-ball friction and wear tester. The SAE 52100 steel balls with a diameter of 12.5 mm and a hardness of 59—61 HRC were adopted to assemble the frictional pair. Prior to friction and wear tests, the steel balls and specimen holders were cleaned in an ultrasonic bath with petroleum ether for 10 min and then dried with a cold air flow. After the friction and wear tests, the worn steel balls were rinsed ultrasonically with petroleum ether for 10 min prior to surface analysis.
3 Results and Discussion
3.1 Characterization
In the FTIR spectra of EFAME, the characteristic peaks of an epoxy group at 822 cm-1and 842 cm-1were observed, while they disappeared in the FTIR spectra of diester derivatives (AWCO, PWCO, BWCO, i-BWCO, HWCO). This fact indicates that EFAME undergoes complete oxirane ring opening with carboxyl radical. In the FTIR spectra of diester derivatives, bands representing C=O groups (722 cm-1and 1 739 cm-1), CH3groups (1 365—1 470 cm-1), OH groups (3 442—3 475 cm-1) and also C-O-C bands in esters (998—1 100 cm-1) are clearly visible.
In the1HNMR spectra of diester derivatives, peaks represented the protons on the ester branched carbon of the fatty chain (4.84), the protons from methoxy group (3.67—3.69), the hydroxy protons (3.56—3.60), the protons on side chain’s α position to both carboxy group (2.30—2.32), the protons at the end of the side chain (1.15—1.18), and the protons at the end of the fatty chain (0.88—0.91) besides the unresolvable signals (1.27—1.68).
3.2 Viscometric properties and acid value
The physicochemical properties of diester derivatives (AWCO, PWCO, BWCO, i-BWCO, HWCO), are shown in Table 1. Kinematic viscosity (both at 40 ℃ and 100 ℃ ) of the prepared diester derivatives was higher than that of FAME and EFAME, suggesting that the induction of side chain at the site of unsaturation has a notable effect on the viscometric properties. The kinematic viscosity (KV) values of the prepared diester derivatives varied in the range between 20.4 mm2/s and 24.6 mm2/s at 40 ℃, and VI values were between 70 and 90. The KV and VI values increased with the increase in chain length of carboxylic acids, which might be attributed to the increase in the molecular weights and the change in the structure of their molecules on the side chain. The acid value of samples was related to the free fatty acid content in the samples and could be disadvantageous to the performance of lubricating base oil. It can be seen from Table 1 that FAME, EFAME and all diester derivatives show lower acid value than that of WCO (with an AV of 114.16 mgKOH/g), which indicates that chemical modification has a signif icant effect on removing FFA from WCO. AV decreases with the increase in the chain length of carboxylic acids.
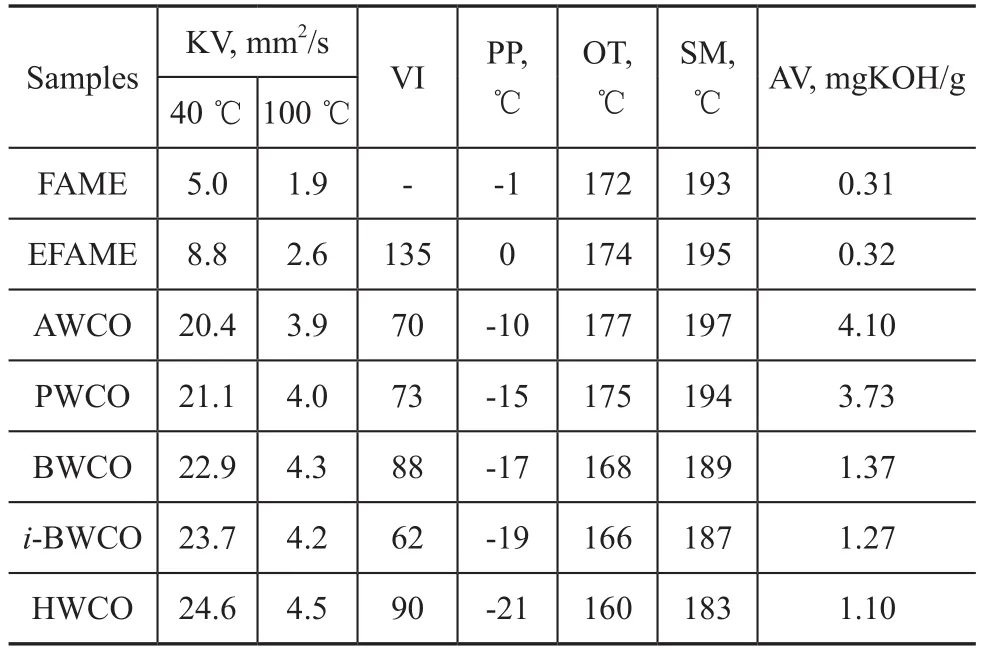
Table 1 Effect of the chain length increase of carboxylic acids on the kinematic viscosity (KV), viscosity index (VI), pour point (PP), onset temperature (OT), signal maximum temperature (SM)
3.3 Low temperature fluidity
Low temperature fluidity is the ability of a substance to remain liquid at low temperatures. Pour point is a good indicator for evaluating the low temperature fluidity of lubricants, surfactants, and fuels. Pour point values for FAME, EFAME, AWCO, PWCO, BWCO, i-BWCO, HWCO are given in Table 1. The pour point of FAME is -1 ℃, while that of EFAME is 0 ℃. Hence, the prepared diester derivatives, attached with various side chain groups at the double bond sites on the fatty acid methyl ester main chains, have much lower PP ranging from -10 ℃ to -21 ℃. HWCO exhibits an increased PP in comparison to AWCO. PP values decrease with the increase in the chain length of carboxylic acids, which may be attributed to the greater ability of the longer side chain groups that can disrupt the molecular symmetry to inhibit easy stacking of the individual molecules at reduced temperatures than that of shorter ones.
3.4 Thermo-oxidation stability
Thermo-oxidation stability is the ability of a substance to resist thermo-oxidative degradation, which plays an important role in its ageing, shelf-time and other performance characteristics. Pressurized differential scanning calorimetry (PDSC) test was used to measure the onset temperature of oxidation (OT) and the signal maximum temperature of oxidation (SM). OT is the temperature at which a rapid increase in the rate of oxidation is observed. A high OT indicates a high oxidative stability of a substance. SM is the temperature at which maximum heat output is observed during the oxidative degradation of sample. A high SM is irrelevant to oxidative stability of a material. The PDSC results relating to OT and SM of diester derivative samples are given in Table 1 (Note: OT and SM values are the average of three experiments with a standard error of ±1 ℃). The diester derivatives exhibited higher thermo-oxidation stability than FAME and EFAME, as evidenced by their higher OT and SM values. For diester derivatives, the thermo-oxidation stability decreases with the increase in chain length of carboxylic acids, which may be attributed to the longer side chain groups providing more accessible sites that can be readily oxidized making themselves more susceptible to cleavage than shorter ones. Thin film microoxidation (TFMO) test was carried out to study the volatility and depository tendencies of diester derivatives and EFAME. During the oxidation process, some small primary oxidation products are measured as volatile loss, while others, in the presence of excess oxygen, undergo oxidative polymerization to form oil insoluble deposits. The volatile loss and insoluble deposit can give an indication of useful lubricant lifetime for a lubricant. Figure 2 presents the effect of the increase in chain length of carboxylic acids on the volatile loss of diester derivatives and EFAME from 150 ℃ to 225 ℃through every 25 ℃ increment. The volatile loss of diester derivatives and EFAME increased with an increasing temperature, and as the chain length of carboxylic acids increased, a corresponding decrease in volatile loss of diester derivatives was observed, which took place because the longer side chain groups attached to EFAME at the site of epoxy carbons increased the possibility of oxidative degradation. Figure 3 shows the effect of the increase in chain length of carboxylic acids on the insoluble deposit of diester derivatives and EFAME from 150 ℃ to 225 ℃ through every 25 ℃ increment. It can be observed that the insoluble deposit of diester derivatives and EFAME could be negligible below 200 ℃ during the oxidation process, while the insoluble deposit of diester derivatives increased dramatically after 200 ℃. This indicates that oxidative polymerization occurred after 200 ℃, which might beascribed to the generation of reactive oxygen containing radicals. As regards the diester derivatives, the insoluble deposit increased with an increasing chain length of carboxylic acids, since the longer side chain groups would have more sites susceptible to possible polymerization reactions.
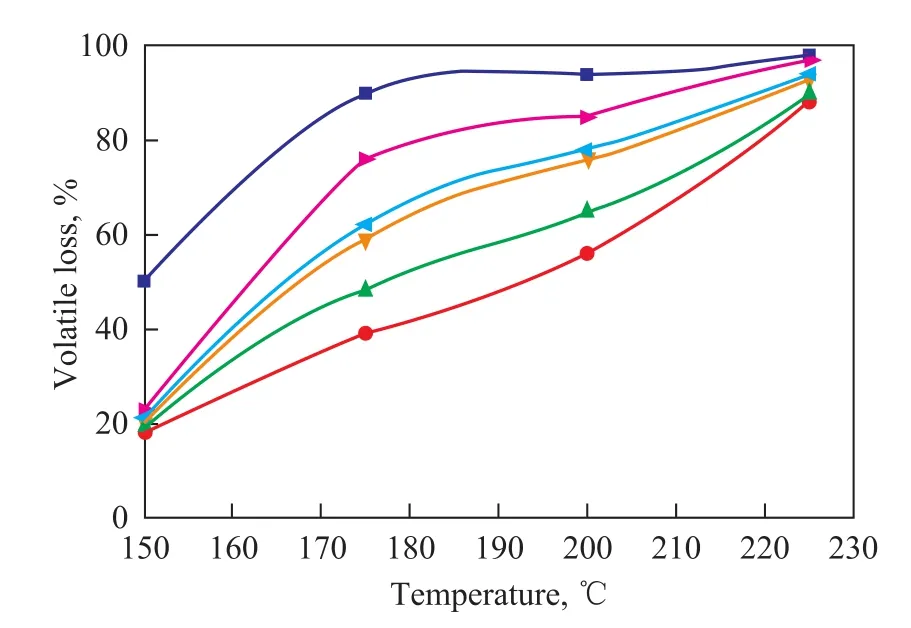
Figure 2 Effect of the increase in chain length of carboxylic acids on the volatile loss of diester derivatives and EFAMEat various temperatures

Figure 3 Effect of the increase in chain length of carboxylic acids on the insoluble deposit of diester derivatives and EFAME at various temperatures
3.5 Surface tension
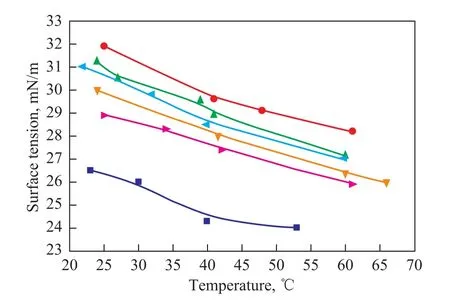
Figure 4 Effect of the increase in chain length of carboxylic acids on the surface tension of diester derivatives andEFAME at various temperatures
Surface tension has a considerable influence on the film formation process of lubricants, thus determining their physicochemical behaviors, such as flow and atomization properties. Figure 4 shows the effect of the increase in chain length of carboxylic acids on the surface tension of diester derivatives and EFAME from 23 ℃ to 66 ℃. The surface tension of diester derivatives and EFAME decreased with an increasing temperature as expected. Surface tension of all diester derivatives was greater than that of EFAME, indicating that introducing side chain on the epoxy groups had a positive effect on the surface tension. As for diester derivatives, the surface tension decreased significantly with the increase in the chain length of carboxylic acids, and this phenomenon could be attributed to the effect of larger side chain groups which disrupted the close packing of molecular structure.
3.6 Tribological properties
Maximum non-seizure load (PB) represents the loadcarrying capacity of a lubricant under boundary lubrication condition, which is the maximum load that can be sustained by the lubricant without failure of the sliding contact surfaces. The PBvalue of diester derivatives and 250BS were obtained using an MQ-800 four-ball friction and wear tester under the condition specifying a speed of 1 450 r/min for 10 sec at room temperature. It can be seen from Figure 5 that all diester derivatives were superior to 250BS in terms of their extreme pressure properties, while AWCO showed its load-carrying capacity as high as 865 N at room temperature, which was much higher than that of 250BS (650 N). As for diester derivatives, the PBvalue decreased gradually with the increase in chain length of carboxylic acids, which could be rationalized by the reduction of oxygen content in diester derivatives resulting in the decrease of af finity for metal surface. The friction-reduction properties of diester derivatives and 250BS were demonstrated by the friction coefficient obtained using a MQ-800 four-ball friction andwear tester under the conditions specifying a varying load ranging from 98 N to 294 N, and a speed of 1 200 r/min for 30 min at room temperature. The friction coefficient values were the average of three independent measurements and the standard deviation observed was ±0.02, as shown in Figure 6. The results indicate that all diester derivatives demonstrated better friction-reducing property than that of 250BS under whatever loads on the steel-steel contact. As regards diester derivatives, the friction coefficient decreased with the increase in chain length of carboxylic acids, leading to increased polarity in the ester functional groups of diester derivatives that could increase the strength of the lubricating oil film at the steel-steel contact zone and accordingly could enhance their frictionreduction properties.

Figure 5 Effect of increase in the chain length of carboxylic acids on PBof steel ball
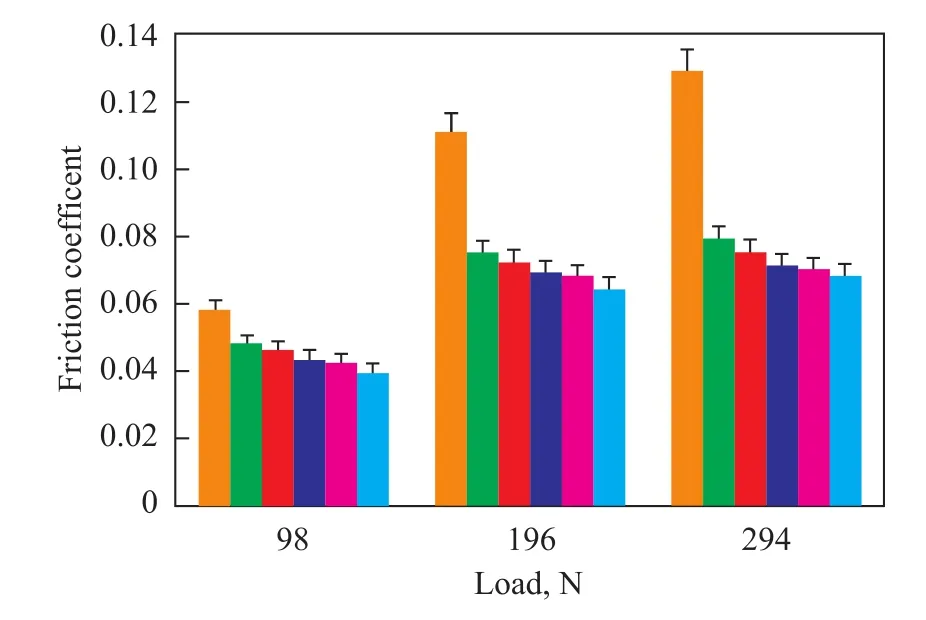
Figure 6 Effect of increase in the chain length of carboxylic acids on friction coefficient of steel ball
The anti-wear properties of diester derivatives and 250BS were demonstrated by the wear scar diameters (WSD) obtained using a MQ-800 four-ball friction and wear tester under the conditions including a load of 294 N and a speed of 1 200 r/min for 30 min at room temperature, as shown in Figure 7. It was observed that all diester derivatives demonstrated better antiwear property than that of 250BS under the given experimental condition. As for diester derivatives, when the chain length of carboxylic acids increased, the WSD decreased evidently, which might be ascribed to the increased viscosity originating from an increase in molecular weight as a result of long side chain groups.
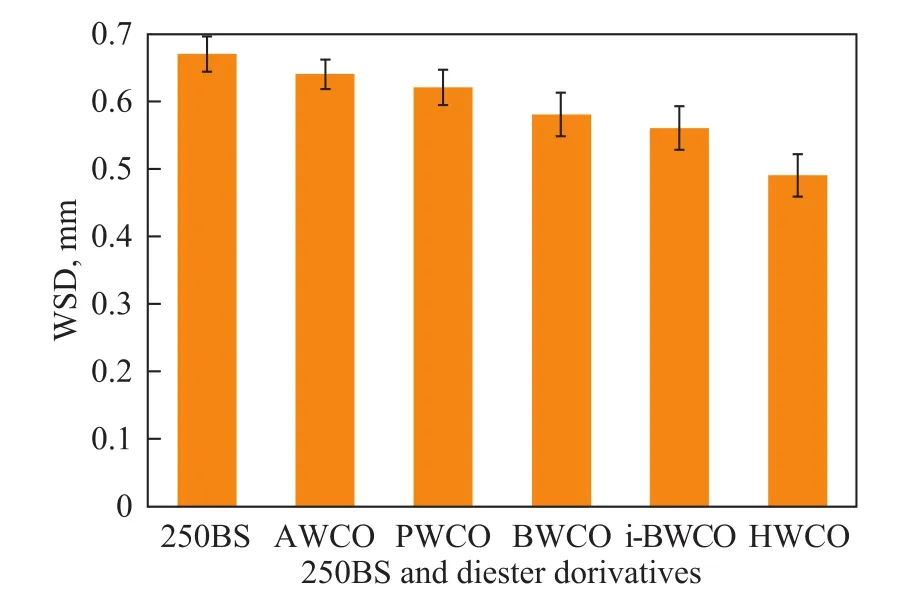
Figure 7 Effect of the increase in chain length of carboxylic acids on friction coefficient and WSD of steel ball
4 Conclusions
The effects of increase in the chain length of carboxylic acids on the viscosity, low temperature fluidity, thermooxidation stability, tribological properties and surface tension of diester derivatives are presented. With the increase in the chain length of carboxylic acids, the viscosity and viscosity index also increased, which was attributed to the increase in the molecular weights and the change in the structure of their molecules on the side chain, while PP decreased owing to the greater ability of the longer side chain groups to disrupt the molecular symmetry, and the thermo-oxidation stability decreased because of the longer side chain groups that were more susceptible to oxidative cleavage than the shorter ones. Meanwhile, the friction-reduction and anti-wear properties were improved because of the increased polarity in the functional groups of esters and the increased viscosity, the surface tension decreases because of the hindrance to close packing by larger side chain groups.
Acknowledgements: The authors gratefully acknowledge the financial support from the Natural Science Foundation of Chongqing (Project No. cstc2014jcyjA90013).
[1] Gorla G, Kour S M, Korlipara P V, et al. Novel acyl derivatives from Karanja oil: alternative renewable lubricant base stocks [J]. Industrial & Engineering Chemistry Research, 2014, 53 (21): 8685-8693
[2] Salimon J, Salih N, Yousif E. Biolubricants: raw materials, chemical modifications and environmental benefits [J]. European Journal of Lipid Science and Technology, 2010,112(5): 519-530
[3] Mobarak H M, Niza Mohamad E, Masjuki H H, et al. The prospects of biolubricants as alternatives in automotive applications [J]. Renewable and Sustainable Energy Reviews, 2014, 33: 34-43
[4] Fox N J, Stachowiak G W. Vegetable oil-based lubricants—a review of oxidation [J]. Tribology International, 2007, 40(7): 1035-1046
[5] Erhan S Z, Sharma B K, Perez J M. Oxidation and low temperature stability of vegetable oil-based lubricants [J]. Industrial Crops and Products, 2006, 24(3): 292-299
[6] Dmytryshyn S L, Dalai A K, Chaudhari S T, et al. Synthesis and characterization of vegetable oil derived esters: evaluation for their diesel additive properties [J]. Bioresource Technology, 2004, 92(1): 55-64
[7] QuinchialA, Delgado M A, Reddyhoff T, et al. Tribological studies of potential vegetable oil-based lubricants containing environmentally friendly viscosity modifiers [J]. Tribology International, 2014, 69: 110-117
[8] Cai Tianfeng, Li Huipeng, Zhao Hua, et al. Purification of crude glycerol from waste cooking oil based biodiesel production by orthogonal test method[J]. China Petroleum Processing and Petrochemical Technology, 2013, 15(1): 48-53
[9] Sharma B K, Rashid U, Anwar F, et al. Lubricant properties of Moringa oil using thermal and tribological techniques [J]. Journal of Thermal Analysis and Calorimetry, 2009, 96(3): 999-1008
[10] Serrano M, Oliveros R, Sánchez M, et al. Influence of blending vegetable oil methyl esters on biodiesel fuel properties: Oxidative stability and cold flow properties [J]. Energy, 2014, 65: 109-115
[11] Lam M K, Lee K T, Mohamed A R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: a review [J]. Biotechnology Advances, 2010, 28(4): 500-518
[12] K ouzu M, Hidaka J. Transesterification of vegetable oil into biodiesel catalyzed by CaO: a review [J]. Fuel, 2012, 93: 1-12
[13] C ai Jie, Zhang Qiuyun, Huang Congmin, et al. ZrOCl2•8H2O: An ef ficient and cheap catalyst for esterification of free fatty acids to methyl esters[J]. China Petroleum Processing and Petrochemical Technology, 2014, 16(1): 40-44
[14] L athi P S, Mattiasson B. Green approach for the preparation of biodegradable lubricant base stock from epoxidized vegetable oil [J]. Applied Catalysis B: Environmental, 2007, 69(3): 207-212
[15] Madankar C S, Dalai A K, Naik S N. Green synthesis of biolubricant base stock from canola oil [J]. Industrial Crops and Products, 2013, 44: 139-144
[16] Goud V V, Patwardhan A V, Pradhan N C. Kinetics of in situ epoxidation of natural unsaturated triglycerides catalyzed by acidic ion exchange resin [J]. Industrial & Engineering Chemistry Research, 2007, 46(10): 3078-3085
[17] Salimon J, Salih N, Yousif E. Chemically modified biolubricant basestocks from epoxidized oleic acid: Improved low temperature properties and oxidative stability [J]. Journal of Saudi Chemical Society, 2011, 15(3): 195-201
[18] Doll K M, Sharma B K, Erhan S Z. Synthesis of branched methyl hydroxy stearates including an ester from biobased levulinic acid [J]. Industrial & Engineering Chemistry Research, 2007, 46(11): 3513-3519
[19] Sharma B K, Doll K M, Erhan S Z. Ester hydroxy derivatives of methyl oleate: tribological, oxidation and low temperature properties [J]. Bioresource Technology, 2008, 99(15): 7333-7340
[20] Adhvaryu A, Erhan S Z, Perez J M. Tribological studies of thermally and chemically modified vegetable oils for use as environmentally friendly lubricants [J]. Wear, 2004, 257(3): 359-367
[21] Sharma B K, Liu Z, Adhvaryu A, et al. One-pot synthesis of chemically modified vegetable oils [J]. Journal of Agricultural and Food Chemistry, 2008, 56(9): 3049-3056
[22] Cermak S C, Isbell T A. Synthesis and physical properties of mono-estolides with varying chain lengths [J]. Industrial Crops and Products, 2009, 29(1): 205-213
[23] Hwang H S, Erhan S Z. Synthetic lubricant basestocks from epoxidized soybean oil and Guerbet alcohols [J]. Industrial Crops and Products, 2006, 23(3): 311-317
[24] Kulkarni R D, Deshpande P S, Mahajan S U, et al. Epoxidation of mustard oil and ring opening with 2-ethylhexanol for biolubricants with enhanced thermo-oxidative and cold flow characteristics [J]. Industrial Crops and Products, 2013, 49: 586-592
[25] Moser B R, Erhan S Z. Preparation and evaluation of a series of α-hydroxy ethers from 9, 10-epoxystearates [J]. European Journal of Lipid Science and Technology, 2007, 109(3): 206-213
date: 2014-12-19; Accepted date: 2015-04-10.
Professor Chen Ligong, Telephone: +86-023-86731421; E-mail: clg818@qq.com.
杂志排行
中国炼油与石油化工的其它文章
- Pyrolysis Characteristics and Kinetics of Methyl Oleate Based on TG-FTIR Method
- Synthesis of Waterborne Polyurethane Modified by Nano-SiO2Silicone and Properties of the WPU Coated RDX
- A Highly Efficient and Selective Water-Soluble Bimetallic Catalyst for Hydrogenation of Chloronitrobenzene to Chloroaniline
- Curing Mechanism of Condensed Polynuclear Aromatic Resin and Thermal Stability of Cured Resin
- A Novel Thermally Coupled Reactive Distillation Column for the Hydrolysis of Methyl Acetate
- Effects of Fe2+, Co2+and Ni2+Ions on Biological Methane Production from Residual Heavy Oil
