Synthesis of Polymethacrylates Used as Multifunctional Lubricating Oil Additives
2015-06-22
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Synthesis of Polymethacrylates Used as Multifunctional Lubricating Oil Additives
Liu Yinong; Fan Lichuang; Duan Qinghua
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
In this paper, four kinds of polymethacrylates (PMAs) used as multifunctional additives were synthesized from quaternary C1—C14methacrylate, among which sample 4 exhibited relatively better performance. According to the methacrylate ratio of sample 4, the optimized reaction conditions of PMA were explored by orthogonal experiments comprising 4 factors and 3 levels, and the optimized reaction conditions covered an initiator dosage of 0.8 %, a molecular weight regulator dosage of 0.4%, a reaction temperature of 95 ℃ and a reaction time of 8.5 h. When the optimized PMA samples were used to formulate the 75W/90 automotive gear base oil, they exhibited improved shear stability and good low temperature property. In comparison with foreign commercial polymethacrylate GP, the optimized PMA samples exhibited better thickening ability, similar shear stability and slightly weak low temperature property, with their performance being the same as GP’s on the whole. The slight difference in the performance between the optimized PMA and GP was attributed to the difference of chain length of copolymers and the distribution of relative molecular mass between them.
polymethacrylate, thickening ability, shear stability, low temperature property
1 Introduction
Viscosity index improvers, such as OCP (olefin copolymer), PIB (polyisobutene ), HSD (hydrogenated styrene isoprene) and PMA (polymethacrylate), have been making rapid development in recent years thanks to their good ability to improve the viscosity index of lubricating oil. Among them, OCP and HSD are widely formulated in diesel engine oil for their good thickening ability, better shear stability and reasonable price. PIB has certain applications in engine oil formulation for its better thickening ability and shear stability, but it has limited application in industrial oils because of its poor low temperature property. PMA, featuring good thickening ability, shear stability, low temperature property and excellent viscosity temperature property, has wide application in multigrade gasoline engine oil grades of 0W/30 and 5W/30, and automotive gear oil formulations of 75W/90 in recent years[1-4].
PMA is composed of copolymer molecules with a main chain and many alkyl side chains, in which the main chain is formed through polymerization of the double bonds of different methacrylate molecules, and the side chains are formed from alkyls of different methacrylates whose alkyls include shorter alkyl side chains of C1—C7groups, with the medium-length alkyl side chains consisting of C8—C13groups and longer side chains consisting of C14and higher alkyl radicals. The average carbon number of the side chains is a weighted average of all individual methacrylate molecules, and ranges from 8 to 12[5-7]. The performance of PMA is related to its molecular structures greatly; for example, the longer the main chain of copolymer is, the better the thickening ability would be. At the same time, the copolymer with more narrow distribution of relative molecular mass would exhibit a better shear stability, and the copolymer with more broad distribution of side chains would be beneficial to its low temperature property. In addition, the property of PMA could be upgraded by co-polymerization with other compounds; for instance, the anti-wear type PMA is synthesized by reaction of PMA with thiophosphate[8-9], the anti-oxidation type PMA is synthesized by introducing aromatic amine group in PMA[10-12], the dispersive type PMA is synthesized by reaction of nitrogen monomer, such as vinylpyridine, N-aminoethyl piperazine, N, N-dimethyl amine propylmethacrylamide with PMA, while the shear stability and thickening ability of PMA are also enhanced by reaction of α-olefin or olefin copolymer with PMA[13-20]. Up to now, it is estimated that thousand tons of PMA are formulated in domestic lubricating oil every year. Although many research results on PMA have been accomplished in domestic research institutions in recent years[21-23], the commercial PMA products are not available in domestic plant. Most of the PMA formulated in lubricating oil are imported from the Rohmax Co. of Germany because of its reasonable price and better performance in lubricating oil.
In order to improve the domestic lubricating oil quality and sharpen its competitive edge, the PMA with good thickening ability, good low temperature property and shear stability has been synthesized by the Research Institute of Petroleum and Processing (RIPP). The research results are referred to in this paper.
2 Experimental
2.1 Reaction routes

where, w, x, y, and z are the number of monomers, and R1, R2, R3, and R4are C1—C14alkyls
2.2 Raw materials
The C1—C14methacrylate monomers, polymerization initiator, molecular weight regulator are all commercial products with a purity of 99% or more. The HVI 150N base oil has a kinematic viscosity of 5.37 mm2/s at 100 ℃and 31.6 mm2/s at 40 ℃. The 220N base oil has a kinematic viscosity of 7.14 mm2/s at 100 ℃ and 43.89 mm2/s at 40 ℃. The 150BS base oil has a kinematic viscosity of 31.82 mm2/ s at 100 ℃ and 586.8 mm2/s at 40 ℃. The #120 solvent oil is a gasoline fraction with a boiling point of 80—120℃. GP is a commercial PMA product VISCOPLEX 0-120 produced by the Rohmax Co. of Germany.
2.3 Analytical instruments and methods
The structures of PMA were analyzed by a Nicolet 6700 Fourier transform infrared spectrometer manufactured by the Thermo Fisher Scientific Inc. of USA and an INOVA500 nuclear magnetic resonance spectrometer manufactured by the Varian Co. of USA. The molecular weight distribution of PMA was measured by the gel permeation chromatograph (model Water 1515) manufactured by the Waters Co. of USA, which was equipped with a 7725 manual injector, a 1515 isocratic pump, a 2414 differential refractive index detector, and a Waters STYRAGEL column, and was run by the tetrahydrofuran mobile phase at a flow rate of 1.0 mL/min at 40 ℃.
The properties of PMA were tested according to the test methods GB/T 265 (Petroleum products—determination of kinematic viscosity and calculation of dynamic viscosity), GB/T 3536 (Petroleum products—determination of pour point), GB/T 11145 (Automotive fluid lubricants—determination of low temperature viscosity-Brookfield viscometer method), SH/T 0034 (Measurement of active components in additives), SH/T 0103 (Standard test method for shear stability of polymer contained fluids using a European diesel injection apparatus) and SH/T 0566 (Measurement of thickening ablility of lubricating viscosity index improver), respectively.
2.4 Experimental process
PMA was synthesized in a 500-mL three-necked flask equipped with an electric stirrer, a thermometer and a reflux condenser. When the measured base oil and C1—C14PMA monomer were introduced into the three-necked flask, the flask was purged with nitrogen gas prior to addition of the polymerization initiator, and the molecular weight regulator, and then the reagents were heated to a specified temperature for a certain period of time, and when the dilute regents became a pale yellow and viscous copolymer, the reaction was finished.The thickening ability of PMA was evaluated by measuring the viscosity difference between the 1% PMA blended lubricating oil and the blank lubricating oil. The low temperature property of PMA was evaluated by measuring the pour point and the Brookfield viscosity of the PMA contained lubricating oil. The shear stability of PMA was evaluated by calculating the viscosity ratio before and after shear according to the standard method SH/T0103.
3 Results and Discussion
According to the literature information[1-8], PMA is a copolymer of methacrylate ester with different carbon chain alkyls at different ratios, the structures and properties of which are determined by the polymerization degree of monomers and structures of monomers. Since the PMA products with good shear stability, thickening ability and good low temperature property should have a reasonable main chain and side chains, it is generally recognized that the average carbon number of side chains of multifunctional PMA is about 10, and the side chains should include the shorter carbon chains, the medium-length carbon chains and the longer carbon chains. So, the mass ratios of different methacrylate esters need to be determined firstly.
3.1 Determination of mass ratio of different methacrylates
Four kinds of quaternary PMA copolymers with different mass ratios of C1—C14methacrylate esters were synthesized at a polymerization temperature of 90 ℃ and a reaction time of 8.5 h, when the dosage of initiator was 1.1% and the molecular weight regulator dosage was 0.2%. The results are listed in Table 1. The thickening ability and shear stability of four kinds of PMA samples were evaluated when PMA samples were added to 150 BS oil, with the results presented in Table 2 and Table 3. The low temperature property of four PMA samples were evaluated when they were blended to a base oil mixture containing 90% of 220N base oil and 10% of 150BS base oil, with the results listed in Table 4.
It can be seen from Table 1 that four kinds of PMA samples had similar average carbon number of side chains, and at the same time, their kinematic viscosity rangerd from 356.08 mm2/s to 529.31 mm2/s. It can be seen from Table 2 that quaternary copolymers of PMA had better thickening ability than that of GP, their thickening ability decreased with a decreasing weight average relative molecular mass and viscosity of PMA. Among the four kindsof PMA, the sample S3 had the best thickening ability. It also can be seen from Table 3 that the shear stability of PMA was as good as that of GP, and among them the performance of sample 4 was close to that of GP. It is clearly shown in Table 4 that pour point of quanternary copolymers of PMA was high than that of GP, which indicated that the low temperature property of PMA was not as good as GP, and the pour point of samples 1, 3, and 4 was comparatively close to that of GP. Upon considering the experminental results of thickening ability, shear stability and pour points, the sample 4 exhibited a relavtively better performance than the three other samples, so the sample 4 was selected as a candidate for optimization research.

Table 1 Kinematic viscosity and average carbon number of quaternary copolymers

Table 2 Thickening ability of quaternary copolymers

Table 3 Shear stability of quaternary copolymers
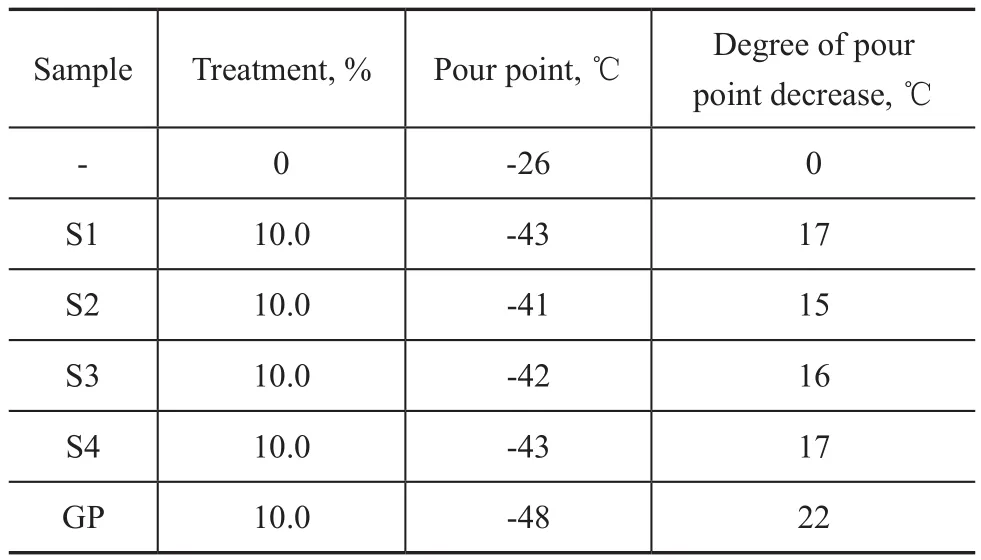
Table 4 Pour points of quaternary copolymers
3.2 Optimization method for synthesis of quaternary copolymer of sample 4
In order to determine the best conditions for synthesis of sample 4, an orthogonal experiment table comprising 4 factors and 3 levels was designed. The four factors covered the dosage of initiator, the dosage of molecular weight regulator, the polymerization temperature, and the reaction time. The thickening ability, shear stability index, and apparent viscosity at -40 ℃ were adopted as evaluation criteria. Factors of orthogonal experiments studied are shown in Table 5, and the experimental results are presented in Table 6, with the analytical results listed inTable 7.
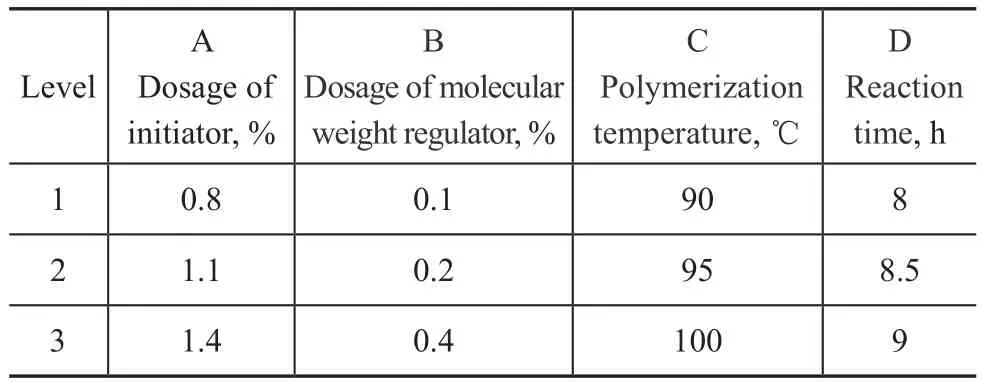
Table 5 Factors of orthogonal experiments studied
In Table 7, there are three influence orders of factors when shear stability, apparent viscosity and thickeningability were measured. As regards the shear stability, the influence order decreased according to B3>D2>A2>C2, which was obtained at a molecular weight regulator dosage of 0.4%, a reaction time of 8.5 h, an initiator dosage of 1.1% and a reaction temperature of 95 ℃. As regards the apparent viscosity, the influence order decreased according to C2>A1>B2>D3, which was obtained at a reaction temperature of 95 ℃, an initiator dosage of 0.8%, a molecular weight regulator dosage of 0.2%, and a reaction time of 9 h. As regards the thickening ability, the influence order decreased according to A1>B1>C1>D3, which was obtained at an initiator dosage of 0.8 %, a molecular weight regulator dosage of 0.1%, a reaction temperature of 90 ℃, and a reaction time of 9 h. According to the overall PMA performance requirements and the importance order covering the properties including the shear stability, the apparent viscosity and the thickening ability, the final optimization reaction conditions were determined to be A1, B3, C2, and D2, which specified an initiator dosage of 0.8 %, a molecular weight regulator dosage of 0.4%, a reaction temperature of 95 ℃ and a reaction time of 8.5 h. In order to verify the proposed conditions, the PMA samples were synthesized according to the above-mentioned optimized conditions, with the performance of PMA samples listed in Table 8.

Table 6 Results of orthogonal experiments on synthesis
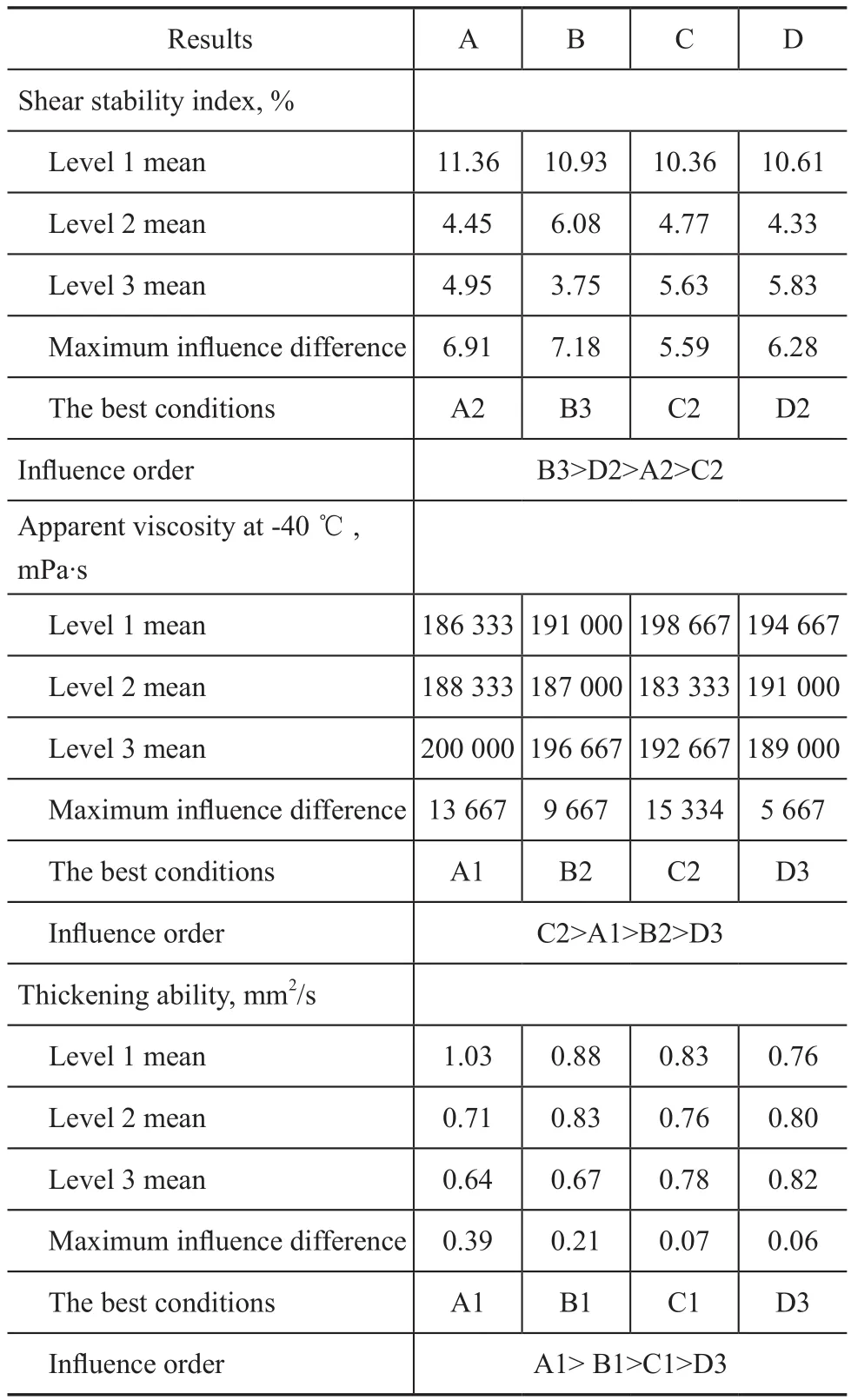
Table 7 Analysis of orthogonal experimental results
It can be seen from Table 8 that the average relative molecular mass of optimized PMA was smaller than that of GP, which might be attributed to the longer main chain of GP or the longer side chain of GP. The bigger value of the distribution of relative molecular mass of GP could be explained as that the molecular chain length of GP was more diversified than that of the optimized PMA.

Table 8 PMA samples synthesized according to the optimization reaction conditions
3.3 Performance of PMA in 75W/90 automotive gear base oil
The 75W/90 automotive gear oil formulation has very strict requirements for such performance items as the viscosity-temperature curve, the low temperature property, the antiwear, antirust and anti-oxidization properties. Most commercial 75W/90 oils are formulated with PMA to meet the viscosity-temperature and low temperature requirements. In order to evaluate the real performance of optimized PMA in oil, the 75W/90 base oil was formulated with 90% of 220N base oil and 10% of 150BS base oil; the thickening ability of PMA was evaluated by blending 1% of PMA in the 75W/90 base oil; and the low temperature ability and shear stability were evaluated by adding 10% of PMA to the 75W/90 base oil, with the experimental results listed in Table 9.
According to Table 9, the optimized PMA exhibited an improved shear stability and apparent viscosity than those depicted in Table 6, and at the same time, the thickening ability of PMA almost kept the same value. Compared with GP, the four optimized PMA samples showed good thickening ability, similar shear stability and slightly weak low temperature property, so their performance was comparable to that of foreign samples on the whole.
The performance of PMA is related to its molecular structure greatly, when the relative molecular mass of PMA increases, its thickening ability increases along with a decrease in its shear stability. The greater the distribution of relative molecular mass (Mw/Mn) of PMA is, the worse the shear stability and the better the low temperature property would be.
When the Mw of two kinds of PMA samples is close and the average carbon number of side chains of each one is the same, a broad distribution of relative molecular massof PMA may be related to two situations; one is that PMA exhibits a broad distribution of the main chain length and a narrow distribution of side chain length, while the another is that the PMA exhibits a narrow distribution of the main chain length and a broad distribution of the side chain length. As regards the first situation, the PMA sample would exhibit a better thickening ability because a longer chain of PMA is beneficial to the thickening ability, and the shear stability of the first PMA sample would be worse because the main chain of its molecule contributes to the shear stability more. As for the second situation, the PMA sample would exhibit a slightly weak thickening ability because the main chain of its molecule is shorter than that of the first one, while the shear stability of the second polymer would be better because the main chain of polymer is more even, which can contribute more to the shear stability than the side chain does. The second PMA also meet the demand of oil formulation for low temperature requirement as its variable molecular structures of side chain could match the different molecular structures of base oil, so the PMA could possess a better low temperature performance.

Table 9 Performance of optimized PMA in 75W/90 automotive gear base oil
As regards the optimized PMA and GP, although the Mw of GP is bigger than that of the optimized PMA, the main chain of GP should be shorter than that of the optimized PMA and the distribution of main chain length of GP should be narrower than that of the optimized PMA, so GP exhibits a slightly weak thickening ability and a better shear stability. A broad distribution of side chain length of GP is beneficial to formulation of lubricants meeting low temperature requirements.
According to the above analysis, the structures of PMA, in particular the chain length and the distribution of both the main chain and side chains are the main factors that can influence its performance. In the future, the PMA molecule having excellent performance may be designed by chemical calculation at first, and then can be synthesized by an efficient organic synthesis scheme.
4 Conclusions
(1) The sample S4 composed of the quaternary PMA has better thickening ability, shear stability and low temperature property among four kinds of PMA samples.
(2) The optimized preparation conditions of PMA were explored by orthogonal experiment comprising 4 factors and 3 levels, and the optimized reaction conditions covered an initiator dosage of 0.8 %, a molecular weight regulator dosage of 0.4%, a reaction temperature of 95 ℃, and a reaction time of 8.5 h.
(3) The optimized PMA exhibited improved shear stability and good low temperature property. Compared with GP, four optimized PMA samples had good thickening ability, similar shear stability and slightly weak low temperature property; their performance is comparable to that of GP on the whole.
(4) The slight performance differences between the optimized PMA and GP were ascribed to the difference in the chain length of copolymer and the distribution between both the main chain and the side chains.
[1] Yang D S, Hou Z M. The mechanism of viscosity index improvement of oil by viscosity modifiers [J]. Lubricating Oil, 2013, 28(2): 55-64 (in Chinese)
[2] Gao J M, Ma D L, Jia R T. Recent Progress on Viscosity Index Improver[J]. Lubricating Oil, 2011, 26(5): 1-6 (in Chinese)
[3] Fei J Q. The Structure, properties and preparation of PMAs[J]. Lubricating Oil, 2011, 26(6): 24-35 (in Chinese)
[4] Li Maosheng, Du Qungui. Study on tribological properties of CVT fluid containing inert and active functional elements[J]. China Petroleum Processing and Petrochemical Technology, 2014, 16(3): 92-98
[5] China Petrochemicals Corporation. A method for copolymer of N-alkanes acrylate: China Pat Appl, CN1011411[P]. 1991 (in Chinese)
[6] Morishita Y, Dong T S, Onuma E. Lubricating oil additives and compositions containing copolymer of acrylate thereof: China Pat Appl, CN1984981[P]. 2007 (in Chinese)
[7] Scher M, Delhi K, Aribert H. Application of poly(meth) acrylate in lubricating oil compositions: China Pat Appl, CN101142303[P]. 2008 (in Chinese)
[8] Pellegrini J P, Thayer H I. Lubricating oils containing dithiophosphorylated copolymers of aziridineethyl acrylates or methacrylates and alkyl acrylates or methacrylates: USA Pat Appl, US4136042[P]. 1979
[9] Pellegrini J P, Thayer H I. Dithiophosphorylated copolymers of aziridineethyl acrylates or methacrylates and alkyl acrylates or methacrylates: USA Pat Appl, US4148981[P]. 1979
[10] Benfaremo N, Kapuscinski M M, Nalesnik T E. Dispersant/antioxidant VII lubricant additive: USA Pat Appl, US4941985[P]. 1990
[11] Benfaremo N. Dispersant/antioxidant VII lubricant additive: USA Pat Appl, US5013468[P]. 1991
[12] Benfaremo N. Antioxidant VII lubricant additive: USA Pat Appl, US5013470[P]. 1991
[13] Liu C S, Hart W P, Kapuscinski M M. Lubricating oil containing dispersant viscosity index improver: USA Pat Appl, US4790948[P]. 1988
[14] Pennewiss H, Jost H, Knoell H. Shear stable multirange oils having an improved viscosity index: USA Pat Appl, US4822508[P]. 1989
[15] Hart W P, Mays D L. Lubricating oil containing VII pour depressant: USA Pat Appl, US4606834[P]. 1986
[16] Hart W P, Liu C S. Lubricating oil containing dispersant VII and pour depressant: USA Pat Appl, US4668412[P]. 1987
[17] Pennewiss H, Jost H, Knoell H. Additives for paraffinic lubricants: USA Pat Appl, US5043087[P]. 1991
[18] Bryant C P, Grisso B A, Cantiani R. Dispersant-viscosity improvers for lubricating oil compositions: USA Pat Appl, US6294628[P]. 2001
[19] Pennewiss H, Benda R, Jost H. Lubricating oil additives: USA Pat Appl, US4290925[P]. 1981
[20] Wenzel F, Schoedel U, Jost H. Lubricating oil additives: USA Pat Appl, US4149984[P]. 1979
[21] Guo Y H. Preparation and evaluation of multifunctional group viscosity index improver of automatic transmission fluids[J]. Acta Petrolei Sinica (Petroleum Processing Section ), 1997, 13(2): 93-104 (in Chinese)
[22] Mei L, Dong H J. Polymerization of methacrylate and alpha-olefin[J]. Synthetic Lubricants, 2004, 31(4):5-9 (in Chinese)
[23] Zhu H J, Wang G J. The effect of the VII structure on the performance of lube oil[J]. Lubricating Oil, 2000, 15(1): 51-56 (in Chinese)
date: 2014-11-24; Accepted date: 2015-04-29.
Liu Yinong, Telephone: +86-10-82368875; E-mail: liuyinong.ripp@sinopec.com.
杂志排行
中国炼油与石油化工的其它文章
- Pyrolysis Characteristics and Kinetics of Methyl Oleate Based on TG-FTIR Method
- Synthesis of Waterborne Polyurethane Modified by Nano-SiO2Silicone and Properties of the WPU Coated RDX
- A Highly Efficient and Selective Water-Soluble Bimetallic Catalyst for Hydrogenation of Chloronitrobenzene to Chloroaniline
- Curing Mechanism of Condensed Polynuclear Aromatic Resin and Thermal Stability of Cured Resin
- A Novel Thermally Coupled Reactive Distillation Column for the Hydrolysis of Methyl Acetate
- Effects of Fe2+, Co2+and Ni2+Ions on Biological Methane Production from Residual Heavy Oil
