Preparation of N-Doped TiO2-Loaded Halloysite Nanotubes and Its Photocatalytic Activity under Solar-Light Irradiation
2015-06-22
(College of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou 225002)
Preparation of N-Doped TiO2-Loaded Halloysite Nanotubes and Its Photocatalytic Activity under Solar-Light Irradiation
Cheng Zhilin; Sun Wei
(College of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou 225002)
The N-doped TiO2-loaded halloysite nanotubes (N-TiO2/HNTs) nanocomposites were prepared by using chemical vapor deposition method which was realized in autoclave. The photocatalytic activity of nanocomposites was evaluated by virtue of the decomposition of formaldehyde gas under solar-light irradiation. The XRD patterns verified that the anatase structured TiO2was deposited on HNTs. The TEM images showed that the surface of HNTs was covered with nanosized TiO2with a particle size of ca. 20 nm. The UV-vis spectra indicated that the N-TiO2/HNTs presented a significant absorption band in the visible region between 400 nm and 600 nm. Under solar-light irradiation, the highest degradation rate of formaldehyde gas attained 90% after 100 min of solar-light irradiation. The combination of the photocatalytic property of TiO2and the unique structure of halloysite would assert a promising perspective in degradation of organic pollutants.
photocatalytic activity; halloysite nanotubes; nitrogen-doped; titanium dioxide
1 Introduction
Titanium dioxide (TiO2) is an interesting and promising photocatalyst for purifying wastewater via decomposition of organic compounds and for hydrogen production via water splitting[1-4], thanks to its low cost, photostability, chemical inertness, non-toxicity, and high efficiency[5-8]. However, because of its large band gap (3.2 eV), pure TiO2can only be activated by ultraviolet (UV) light which merely accounts for 3%—4% in the whole radiant solar energy. There are many ways to sensitize TiO2in a much larger wavelength region of solar light. Many studies on nonmetal elements doping have been carried out to extend the spectral response of TiO2into the visible region and enhance its photocatalytic activity, such as N[9-12], C[13], S[14], P[15]and B[16]. Among them, the N-doped titania was reported to be the most effective in reducing the band gap.
So far, a variety of methods for preparation of N-doped TiO2(N-TiO2) have been developed, such as the ion implantation[17-20], sputtering[21-22], the chemical-vapor deposition[23-24]method, the sol-gel[25-26]method, and the decomposition of N-containing organometallic precursors[27-28]. However, TiO2nanoparticles are prone to aggregation, resulting in difficulty in separation and recovery of TiO2nanoparticles from the solution after degradation of pollutants. Therefore, the supporting technology was developed in order to prevent the aggregation of TiO2nanoparticles. To achieve pre-enrichment of pollutants and improve the separation of TiO2particles, immobilization of TiO2on an adsorbent or an inert support to create integrated photocatalytic adsorbents (IPAs) has been proposed[28]. Using IPAs, degradation of pollutants can be achieved by the simultaneous effects of physical adsorption by the adsorbent coupled with photochemical degradation by the immobilized TiO2.
To date, there has been great interest in preparation of the supported catalysts, such as carbon nanotubes-structured composites[29], magnetic composites[30], graphene composites[31], carbon fiber[32], etc.
HNTs structured nanomaterials have attracted great interest in their applications in different fields. Owing to their inherent hollow nanotube structure and silicon-aluminum composite structure, halloysite nanotubes (HNTs) have exhibited their promising performance as a catalyst support[33]. Compared to carbon nanotubes (CNTs), HNTs are an economically available nano-material with some unique characteristics[34]. Recently, Papoulis, et al.[35]have reported the fabrication of clay-supported TiO2compos-ites by coating TiO2sol solutions on halloysite nanotubes followed by subsequent hydrothermal treatment. Wang, et al.[36]have reported the TiO2/HNTs composite obtained by the solvothermal treatment with high photocatalytic activity on the degradation of methanol.
To improve the visible-light photocatalytic activity for TiO2/HNTs applications, the nonmetal element doping method could be simple and effective. Herein, a facile CVD method in this work was employed to prepare N-doped TiO2/HNTs nanocomposites with high solar-light photocatalytic activity. The integrated photocatalytic nanocomposites were evaluated in terms of their ability to remove formaldehyde gas.
2 Experimental
2.1 Materials and reagents
The halloysite nanotubes (HNTs) were obtained from Tianjin Linruide Science and Technology Co., Ltd., China. Titanic chloride and ammonium carbonate were purchased from the Shanghai Chemical Co., Ltd. All reagents were of analytically pure grade and used without further purification.
2.2 Preparation of N-TiO2/halloysite nanotube composites
The preparation of the N-TiO2/HNTs composites using the facile CVD method was shown in Figure 1. Firstly, one gram of HNTs and a proper amount of (NH4)2CO3serving as the doping N source were uniformly mixed by milling, and then the upside of bulkhead with many small holes was put into the autoclave. A certain amount of TiCl4was added to the bottom of the autoclave. The Ti/N molar ratio was adjusted by varying the mass of N source. Then the autoclave was transferred into the oven and treated at 100 ℃ for 12 h. At the end of reaction, the autoclave was cooled down to room temperature, and the powder was washed with deionized water at least five times and dried in a vacuum oven at 60 ℃ for 12 h. Finally, the samples were calcined at 500 ℃ for 6 h.
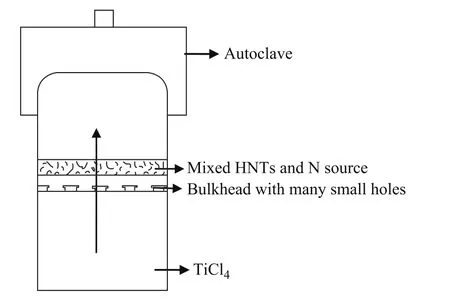
Figure 1 Sketch of the improved chemical vapor deposition device
2.3 Measurement of photocatalytic activity
The photocatalytic reaction was conducted in a 200-mL cylindrical glass vessel. 0.3 g of photocatalysts were evenly coated on a square glass substrate, 70 mm in length and 70 mm in width, and dried under vacuum. A 1000W Xe lamp was used as the simulated solar light source (with an UV portion of 8%). Formaldehyde gas (70 mg/m3) was used as a reactant to evaluate the catalytic activity of NTiO2/HNTs. The degradation rate was calculated by measuring the change in the concentration of formaldehyde before and after reaction by a gas chromatograph.
2.4 Characterization
The formation of zeolite membranes was confirmed by X-ray diffraction (XRD) using a Bruker-AXS D8 Advance powder diffractometer. The morphology of samples was observed by a transmission electron microscope (TEM, Tecnai 12, Philips Company). The XPS analysis was recorded by using an ESCALAB 250Xi XPS spectrometer (Thermo Scientific Company). The UV-vis light absorption spectra were obtained from a Varian Cary 5000 spectrophotometer equipped with an integrating sphere assembly
3 Results and Discussion
Figure 2 shows the XRD patterns of the N-TiO2/HNTs prepared with varied Ti/N molar ratios. Compared with the pure HNTs sample, all of the observed peaks are mainly consistent with the characteristic peaks of halloysite as shown in Figure 1E. However, the two new peaks at 2θ=48º and 53.9oand a stronger peak at 2θ=25.3ocan be observed when the Ti/N molar ratio was 1:1, 1:2 and 1:3, respectively, and meanwhile the reduction of the halloysite peaks after TiCl4vapor treatment was identified. According to JCPDS 21-1272, all of the characteristic peaks of TiO2can be ascribed to the (101), (004), (200), (211) planes of the anatase type TiO2. This indicates that the TiO2-loaded HNTs structured composite materials were successfully prepared. In addition, the sample witha Ti/N molar ratio of 1:0 indicated no characteristic peaks of TiO2on the XRD pattern, which could give an explanation that the NH3vapor released from the decomposition of N source accelerated the gas-phase hydrolysis of TiCl4under the heated condition, thus promoting the deposition of TiO2on the external surface of HNTs. Figure 3 shows the UV-vis optical absorption spectra obtained by diffuse reflectance of the N-TiO2/HNTs with different Ti/N molar ratios. The previously studied work indicated that only a strong absorption band in the UV region can be observed for pure TiO2[31]. However, the NTiO2/HNTs in this work present a significant absorption tail in the visible region between 400 nm and 600 nm, which is the typical absorption feature of N-TiO2. Furthermore, with a decreasing Ti/N molar ratio, the absorption band in the visible region is even more remarkable, which should be resulted from the increase of the N doping concentration in TiO2. Obviously, the modification of TiO2with nitrogen using the facile CVD method would result in a shift of the absorbance region towards longer wavelength, and even into the 600 nm region. The light absorbance of the N-TiO2/HNTs in the visible-light region is desirable for its practical application since it can be activated even by solar light.

Figure 2 XRD patterns of N-TiO2/ HNTs obtained with thevaried Ti/N molar ratios
As shown in Figure 4-A, a majority of HNTs consist of cylindrical tubes, 50 nm—70 nm in diameter and 0.2 μm—2 μm in length. After the facile CVD treatment, a large amount of N-TiO2nanoparticles can be clearly observed with a size of ca. 20 nm deposited on the outer surface of halloysite nanotubes (Figure 4-B). The result of XPS spectrum confirms that the N element has successfully doped into the crystal structure of TiO2.
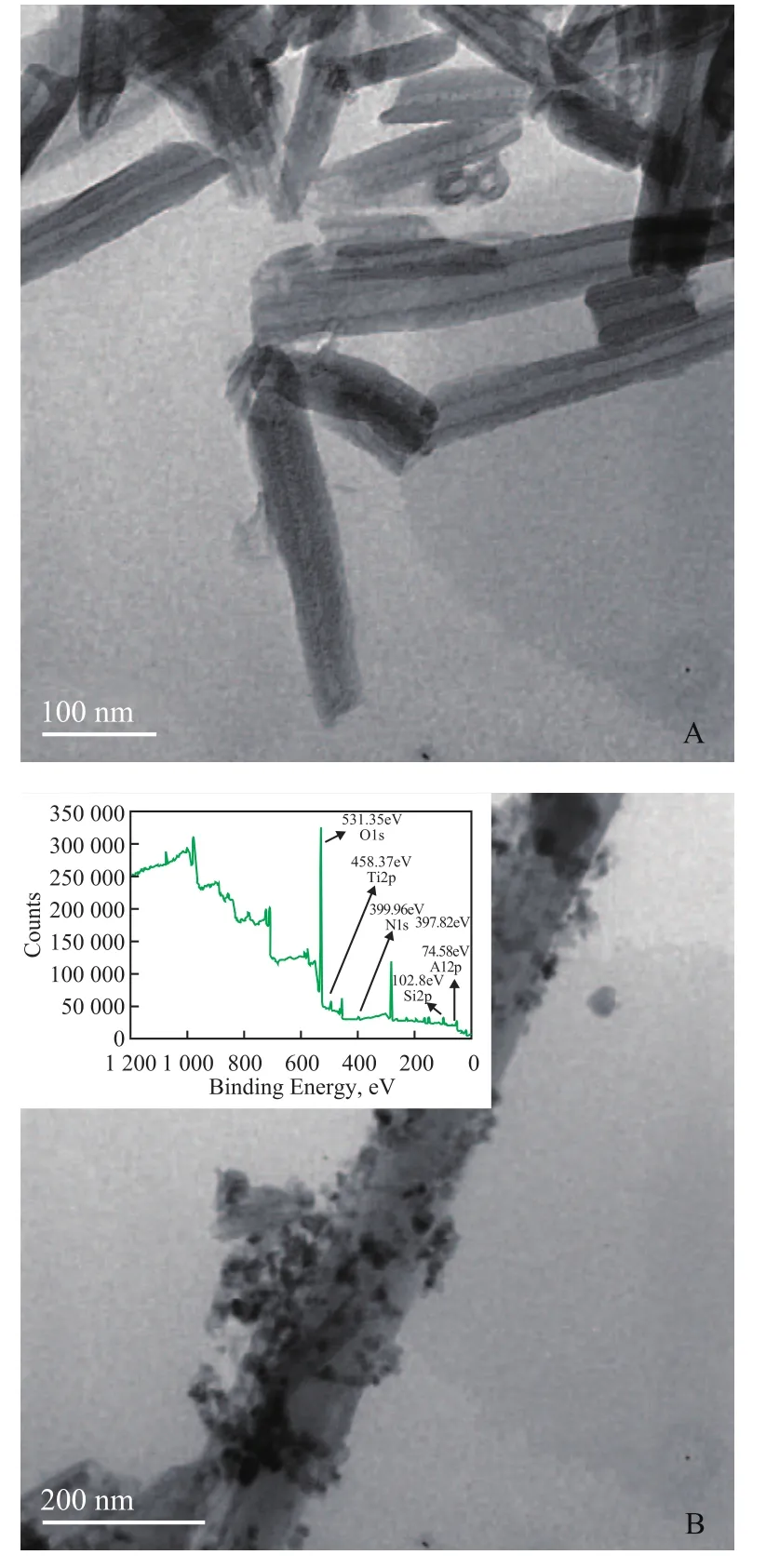
Figure 4 TEM micrographs of HNTs (A); N-TiO2/ HNTs at a Ti/N molar ratio of 1:3 (B); and XPS spectrum of N-TiO2/ HNTs (inset)
Figure 5 shows the dependence of the degradation rate offormaldehyde on HNTs and N-TiO2/HNTs under solarlight irradiation. With a decreasing Ti/N molar ratio in synthetic raw material, the solar-light-induced photocatalytic activity of the prepared sample was obviously enhanced. The maximum value of degradation rate on the N-TiO2/HNTs with a Ti/N molar ratio of 1:3 attained 90% with solar-light irradiation in 100 min, which occurred probably due to the increase of N atoms doped into TiO2. This result is consistent with the conclusion of UV-vis absorption spectrometric analysis. Because of the good absorption performance of HNTs, the photocatalytic activity of the HNTs that was close to 5% should be originated from the absorption of HNTs to formaldehyde, which therefore could keep the throughout stabilized value in the overall irradiation time.
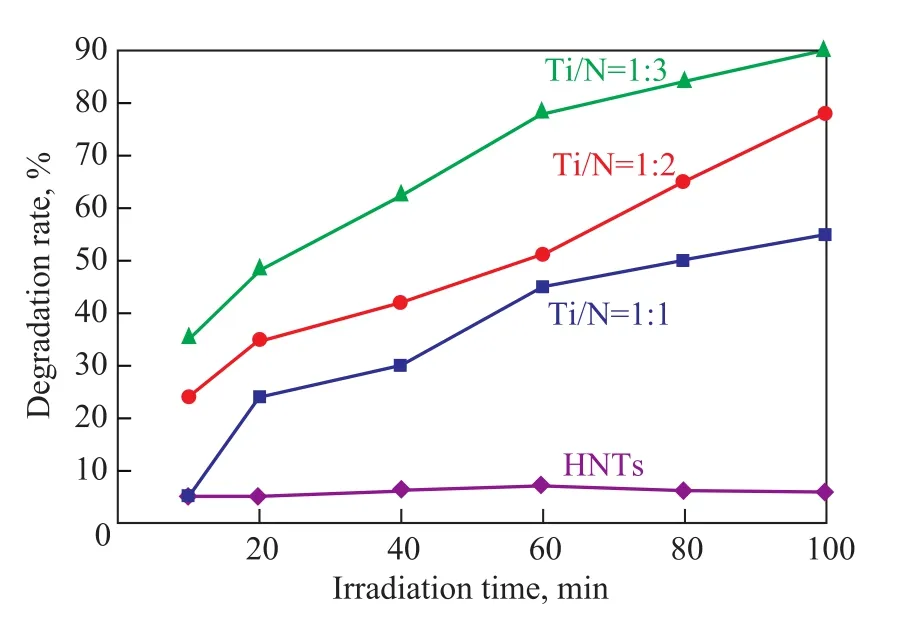
Figure 5 Degradation rate of formaldehyde on N-TiO2/ HNTs under solar-light irradiation
4 Conclusions
The N-doped TiO2-loaded halloysite nanotubes nanocomposites with solar-light photocatalytic activity were successfully prepared by using a facile chemical vapor deposition method. The method was realized via the vapor deposition of TiCl4assisted by decomposition of N source in autoclave at 100 ℃ for 12 h. When the Ti/N molar ratio in raw material was 1:3, the N-doped TiO2/HNTs exhibited a good solar-light photocatalytic oxidation activity for formaldehyde.
Acknowledgment: This work was supported by the Talent Introduction Fund of Yangzhou University, the Jiangsu Social Development Project (BE2014613) and the Six Talent Peaks of Jiangsu province (2014-XCL-013). The authors also acknowledge the Project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions. The data of this paper originated from the Test Center of Yangzhou University.
[1] Fujishima A, Honda K. TiO2photoelectrochemistry and photocatalysis[J]. Nature, 1972, 238(5358): 37-38
[2] Park J H, Kim S, Bard A J. Novel carbon-doped TiO2nanotube arrays with high aspect ratios for efficient solar water splitting[J]. Nano Lett, 2006, 6(1): 234-28
[3] Khan S U M, Al-Shahry M, Ingler W. B. Efficient photochemical water splitting by a chemically modified N-TiO2[J]. Science, 2002, 297(5589): 2243-2245
[4] Cermenati L, Pichat P, Guillard C, et al. TiO2photocatalytic mechanisms in water purification by use of quinoline, photo-Fenton generated OH radicals and superoxide dismutase[J]. J Phys Chem B, 1997, 101(14): 2650-2658
[5] Yang H, Zhu S, Pan N, Studying the mechanisms of titanium dioxide as ultraviolet blocking additive for films and fabrics by an improved scheme[J]. J Appl Polym Sci, 2004, 92(5): 3201-3210
[6] Linsebigler A L, Lu G, Yates J T. Photocatalysis on TiO2surfaces: principles, mechanisms, and selected results[J]. Chem Rev, 1999, 95(3): 735-758
[7] Su C, Hong B Y, Tseng C M. Sol-gel preparation and photocatalysis of titanium dioxide [J]. Catal Today, 2004, 96(3): 119-126
[8] Sakthivel S, Janczarek M, Kish H. Visible light activity and photoelectrochemical properties of nitrogen-doped TiO2[J]. J Phys Chem B, 2004, 108(50): 19384-19387
[9] Asahi R, Morikawa T, Ohwaki T, et al. Visible-light photocatalysis in nitrogen-doped titanium oxides[J]. Science, 2001, 293(5528): 269-271
[10] Cong Y, Zhang J, Chen F, et al. Synthesis and characterization of nitrogen doped TiO2nanophotocatalyst with high visible light activity[J]. J Phys Chem C, 2007, 111(19): 6976-6982
[11] Xing M, Zhang J, Chen F, New approaches to prepare nitrogen-doped TiO2photocatalysts and study on their photocatalytic activities in visible light[J]. Appl Catal B: Environ, 2009, 89(3/4): 563-569
[12] Ma Y F, Zhang J L, Tian B Z, et al. Synthesis and characterization of thermally stable Sm, N co-doped TiO2with highly visible light activity[J], J. Hazard. Mater, 2010, 182 (1/3): 386-393
[13] Sakthivel S, Kisch H, Daylight photocatalysis by carbonmodified titanium dioxide[J]. Angew Chem Int Ed, 2003,42 (40): 4908-4911
[14] Wang Y, Huang Y, Ho W, et al. Biomolecule-controlled hydrothermal synthesis of C-N-S-tridoped TiO2nanocrystalline photocatalysts for NO removal under simulated solar light irradiation[J]. J Hazard Mater, 2009, 169(1/3): 77-87
[15] Lin L, Lin W, Xie J L, et al. Photocatalytic properties of phosphor-doped titania nanoparticles[J], Appl Catal B: Environ, 2007, 75(1/2): 52-58
[16] Xing M, Wu Y, Zhang J, et al. Effect of synergy on the visible light activity of B, N and Fe co-doped TiO2for the degradation of MO[J]. Nanoscale, 2010, 2(7): 1233-1239
[17] Ghicov A, Macak J M, Tsuchiya H, et al. TiO2nanotube layers: Dose effects during nitrogen doping by ion implantation[J]. Chem Phys Lett, 2006, 419(4/6): 426-429
[18] Zhou M H, Yu J G. Preparation and enhanced daylightinduced photocatalytic activity of C, N, S-tridoped titanium dioxide powders[J]. J Hazard Mater, 2008,152(3): 1229-1236
[19] Diwald O, Thompson T L, Goralski E G, et al. The effect of nitrogen ion implantation on the photoactivity of TiO2rutile single crystals[J]. J Phys Chem B, 2004, 108(1): 52-57
[20] Chen S Z, Zhang P Y, Zhuang D M, et al. Investigation of nitrogen doped TiO2photocatalytic films prepared by reactive magnetron sputtering[J]. Catal Commun, 2004, 5(11) :677-680
[21] Premkumar J. Development of super-hydrophilicity on nitrogen-doped TiO2thin film surface by photoelectrochemical method under visible light[J]. Chem Mater, 2004, 16(21): 3980-3981
[22] Diwald O, Thompson T L, Zubkov T, et al. Photochemical activity of nitrogen-doped rutile TiO2(111) in visible light[J]. J Phys Chem B, 2004, 108(19): 6004-6008
[23] Tachikawa T, Takai Y, Tojo S, et al. Visible light-induced degradation of ethylene glycol on nitrogen-doped TiO2powders[J]. J Phys Chem B, 2006, 110(26): 13158-13165
[24] Imao T, Horiuchi T, Noma N, et al. Preparation of new photosensitive TiO2gel films using chemical additives including nitrogen and their patterning[J]. J Sol-Gel Sci Technol, 2006, 39(2): 119-122
[25] Venkatachalam N, Vinu A, Anandan S, et al. Visible light active photocatalytic degradation of bisphenol-A using nitrogen doped TiO2[J]. J Nanosci Nanotechnol, 2006, 6(8) : 2499-2507
[26] Belver C, Bellod R, Fuerte A, et al. Nitrogen-containing TiO2photocatalysts: Part 2. Photocatalytic behavior under sunlight excitation[J]. Appl Catal B: Environ, 2006, 65(3/4): 301-308
[27] Sano T, Negishi N, Koike K, et al. Preparation of a visible light-responsive photocatalyst from a complex of Ti4+with a nitrogen-containing ligand[J]. J Mater Chem, 2004, 14(3): 380-384
[28] Shan A Y, Ghazi TiM, Rashid S A, Immobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis: A review[J]. Appl Cat A: Gen, 2010, 389 (1/2): 1-8
[29] Peng T, Zeng P, Ke D, et al. Hydrothermal preparation of MWCNTs/CdS nanocomposite and its efficient photocatalytic hydrogen production under visible light irradiation[J]. Energy Fuels, 2011, 25: 2203-2210
[30] Li S K, Huang F Z, Wang Y, et al. Magnetic Fe3O4@C@ Cu2O composites with bean-like core/shell nanostructures: Synthesis, properties and application in recyclable photocatalytic degradation of dye pollutants[J]. J Mater Chem, 2011, 21(20): 7459-7466
[31] Li Q, Guo B D, Yu J G, et al. Highly efficient visible-lightdriven photocatalytic hydrogen production of CdS-clusterdecorated graphene nanosheets[J]. J Am Chem Soc, 2011, 133(28): 10878-10884
[32] Wang X D, Zhang K, Guo X L, et al. Synthesis and characterization of N-doped TiO2loaded onto activated carbon fiber with enhanced visible-light photocatalytic activity[J]. New J Chem, 2014, 38(12): 6139-6146
[33] Zhai R, Zhang B, Liu L, et al. Immobilization of enzyme biocatalyst on natural halloysite nanotubes[J]. Catal Commun, 2010, 12(4): 259-263
[34] Wang J H, Zhang X, Zhang B, et al. Rapid adsorption of Cr (VI) on modified halloysite nanotubes[J]. Desalination, 2010, 259(1/3): 22-28
[35] Papoulis D, Komarneni S, Nikolopoulou A, et al. Palygorskite and halloysite-TiO2nanocomposites: Synthesis and photocatalytic activity[J]. Appl Clay Sci, 2010, 50(1): 118-124
[36] Wang R, Jiang G, Ding Y, et al. Photocatalytic activity of heterostructures based on TiO2and halloysite nanotubes[J]. ACS Appl Mater Interfaces, 2011, 3(10): 4154-4158
[37] Peng F, CailF, Huang L, et al. Visible-light photocatalytic activity using a facile hydrothermal method[J]. J Phys Chem Sol, 2008, 69(7): 1657-1664
date: 2014-11-28; Accepted date: 2015-02-05.
Prof. Cheng Shilin, Telephone: +86-13820878380; E-mail: zlcheng224@126.com.
杂志排行
中国炼油与石油化工的其它文章
- Pyrolysis Characteristics and Kinetics of Methyl Oleate Based on TG-FTIR Method
- Synthesis of Waterborne Polyurethane Modified by Nano-SiO2Silicone and Properties of the WPU Coated RDX
- A Highly Efficient and Selective Water-Soluble Bimetallic Catalyst for Hydrogenation of Chloronitrobenzene to Chloroaniline
- Curing Mechanism of Condensed Polynuclear Aromatic Resin and Thermal Stability of Cured Resin
- A Novel Thermally Coupled Reactive Distillation Column for the Hydrolysis of Methyl Acetate
- Effects of Fe2+, Co2+and Ni2+Ions on Biological Methane Production from Residual Heavy Oil
