Investigation of the Ion-Exchange Behavior of Zeolite Y in the Presence of Resin
2015-06-22
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Investigation of the Ion-Exchange Behavior of Zeolite Y in the Presence of Resin
Zhang Yi; Zheng Jinyu; Liu Zhongqing; Gao Xiuzhi; Luo Yibin; Zong Baoning
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Ion-exchange process of zeolite Y using ammonium-type resin as an exchange reagent was successfully carried out. The effect of temperature, space velocity and ion concentration on the breakthrough curves was carefully investigated. At the first exchange section, the maximum proportion of qualified zeolites (QR) was obtained at a temperature of 70 ℃, a weight hourly space velocity of 0.61 h-1, and an ion concentration of 197 mg/L. The minimum length of mass-transfer zone (MTZ) of the resin bed was achieved at a temperature of 70 ℃, a space velocity of 0.61 h-1, and an ion concentration of 423 mg/L. At the second exchange section, the length of MTZ of the resin bed was significantly increased, and the exchange of Na+ions contained in zeolite Y was more difficult than that achieved at the first exchange section. In both the first and the second exchange sections, the zeolite Y subjected to ion exchange with the resin maintained the similar physical and chemical properties as compared to those exchanged by the conventional approaches, but the zeolite Y, which was obtained after ion exchange, contained a significantly lower content of Na2O.
zeolite Y; resin; ion-exchange
1 Introduction
Zeolite Y is one kind of zeolites which are the most widely used in catalytic reaction areas. The acidic active sites of zeolite Y are usually created by the substitution of Na+ions with other cations such as H+ions during the preparation process. For zeolites with high Si/Al ratio such as ZSM-5 and zeolite β, the H-form zeolites could be easily obtained by direct exchange with acid solution[1-2]. But HY cannot be obtained by the same method due to the hydrolysis of Al-O-Si bonds in the presence of an excessive acid. Therefore, HY is usually prepared by exchange with ammonium salt followed by the further thermal decomposition of ammonium cations[3].
Different catalytic reactions require different Na+contents in zeolite Y. For example, HY used in hydrocracking reaction has to be exchanged by ammonium salt for at least 5 times until the Na2O content is lower than 0.2%. One ton of zeolite will need 2 tons of ammonium salts. USY used in catalytic cracking reaction should be exchanged by ammonium salt until its Na2O content is lower than 1.5%, which means that at least 0.8 tons of ammonium salt would be used for 1 ton of zeolite. Therefore a large amount of ammonium salt is consumed in the ionexchange process and vast quantity of wastewater is produced[4]. The conventional methods for NH4+ion removal in wastewater include biological treatment[5]and air stripping method[6], which usually suffer from huge operating cost and high energy consumption[7].
As the national standards for pollutants discharge are becoming increasingly stringent, the wastewater containing large amount of ammonium salt turns to be a bottleneck for the development of catalyst production enterprises in China[8], and many studies[9-12]have been proceeded to reduce the emission of the wastewater by developing new exchange methods for zeolite. Chu P. and Dwyer F. G.[13]firstly introduced a kind of H-form resin as an exchange reagent for the ion exchange of zeolites. In their studies, an in finite dilute acidic medium and 7—10 days of equilibrium time have to be used, which limits its application in industrial process.
A new ion-exchange method for zeolites by using the ammonium-form resin as the exchange reagent was successfully developed in our lab. The amount of ammonium salts consumed in this process is approximately equiva-lent to the theoretical consumption value. Taking the HY zeolite for hydrocracking as an example, producing 1 ton of HY zeolite would just need 0.2 tons of ammonium salt. Combined with the electrodialysis technology[14], the water used for regeneration and rinse of resin could be recycled for several months. This will bring about great economic value to the catalyst manufacturing enterprises along with great significance for environmental protection.
In this work, the ammonium-type resin was employed in the ion exchange of NaY zeolite in the fixed bed. The HY zeolites were prepared through ion exchange with the NH4-form resin, followed by calcination, and then the second exchange with the NH4-form resin was carried out. The optimal operating conditions for obtaining a maximum proportion of qualified zeolite (QR) and a shortest length of mass-transfer zone (MTZ) of resin bed were realized in the first exchange section. The length of MTZ at the operating conditions required in the first exchange section was also obtained at the second exchange section.
2 Experimental
2.1 Materials
The NaY zeolite used in this experiment was a commercial product. The properties of the NaY zeolite sample are shown in Table 1. The ion-exchange resin used in experiments was purchased from the Chemical Plant of Nankai University, with the characteristics of this resin listed in Table 2. Before being used, the resin was firstly converted into H-form by flushing with 1 mol/L HCl solution, then into NH4-form by eluting with excessive 1 mol/L ammonia water, and finally washed with water until ammonium ions could not be detected in the eluate. It was used as a invariant condition in order to study the effect of Na+ion concentration of the zeolite slurry. Ammonium hydroxide (25%—28%), hydrochloric acid (36%—38%), deionized water (C(Na+) <1 mg/L), and sodium chloride (99.5%) were all purchased from the Beijing Chemical Works.
2.2 Ion-exchange equipment
The ion-exchange unit is shown in Figure 1. The exchange column consists of a glass tube, 1 cm in inside diameter and 100 cm in length, and is filled with 33 mL of resin. It is equipped with a heating jacket to maintain a stable temperature.
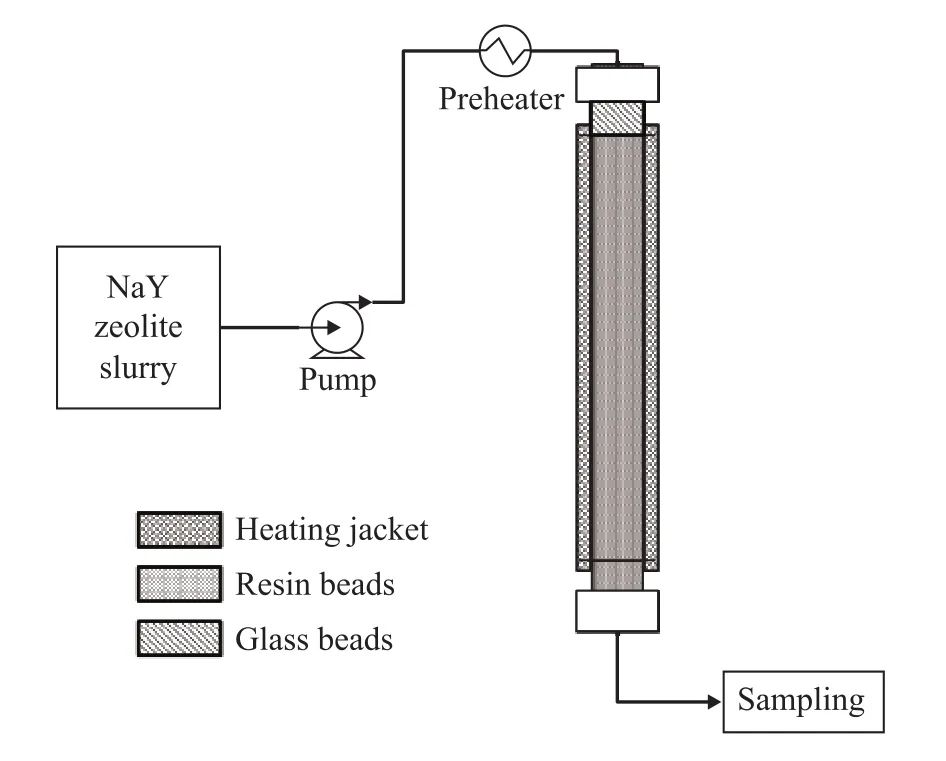
Figure 1 Flow diagram for fixed-bed ion-exchange process
2.3 Ion-exchange process
Zeolite was mixed with the deionized water to reach a H2O/zeolite mass ratio of 10. Then the obtained slurry of zeolite was stirred continuously to maintain a uniform condition. The Na+concentration of the initial slurry of zeolite was 6.4 mg/L. Then NaCl was added to the slurry at different concentrations to adjust the required ratio ofexchange ions in water.

Table 1 Physical properties of NaY zeolite
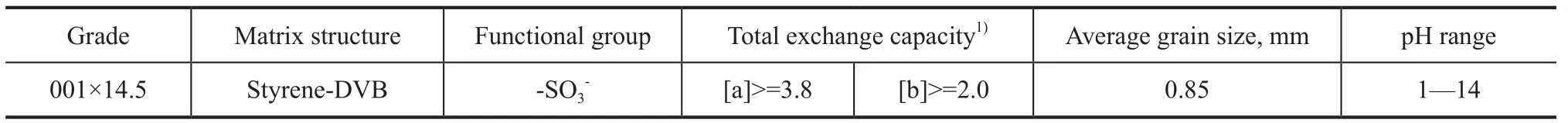
Table 2 Characteristics of the resin
The resin bed was firstly rinsed via pumping deionized water upwards through the column until no air bubbles were found. Then the slurry of zeolite was pumped downwards at a certain space velocity and temperature. The zeolite samples were collected at the column outlet until the Na2O content at the outlet was equal to that at the inlet. The collected samples were washed with distilled water and filtered. The content of Na2O and the relative crystallinity of the samples were measured by X-ray fluorescence spectrometry and X-ray diffractometry analysis, respectively.
All the breakthrough curves are plotted in terms of the Na2O content of effluent samples as a function of the run time.
2.4 Characterization techniques
X-ray fluorescence spectrometry (XRF) was used to analyze the chemical composition of the samples. The samples were measured on a Rigaku Primus II XRF spectrograph using a Rh target X-ray tube operating at 4 kW. X-ray diffraction (XRD) analysis was conducted on a Philips X'Pert Pro PW 3050 X-ray diffractometer, which was recorded on a X' Celerator diffractometer using the graphite filtered Cu Kα radiation (λ=1.5405 Å) at a voltage of 40 kV and a tube current of 40 mA, and the scanning speed was 1.2 (°)/min.
The samples were observed under a FEI Quanta 200 environmental scanning electron microscope (SEM) equipped with an EDX detector, while a gold coating was used to prevent charging effects.
The Na+ion concentration in zeolite slurry was measured on a PE 5300DV ICP-AES (inductively coupled plasmaatomic emission spectrometry) spectrograph.
2.5 Mass transfer parameters

The Na2O content in zeolite samples was set as the evaluation factor. After the first exchange process the Na2O content (YNa2O) of qualified sample was assumed as less than 4%. Therefore the mass of qualified zeolite (mQ) and the total mass of exchanged zeolite (mT) is defined by Eq. (1) and Eq. (2), respectively: where Q is the flow rate of zeolite slurry; C0is the mass fraction of dried zeolite in the slurry; tYNa2O=4%and tYNa2O=11.4%are the elapsed time expressed in minutes when Na2O content of the effluent samples is 4% and 11.4%, respectively; mQand mTare expressed in g. The proportion of qualified zeolite (QR) is defined by Eq. (3):

When the resin bed reaches equilibrium, the usable capacity of the resin bed (tu) and the saturation capacity of the resin bed (ts)[15]are defined by Eq. (4) and Eq. (5), respectively:

in which 11.4% is the initial Na2O content of the NaY zeolite, and tbis the time of break-point. The ratio tu/tsis the effective utilization ratio of resin bed[16]. So the length of mass-transfer zone (MTZ) of resin bed is defined by Eq. (6):

in which HTrepresents the total height of the resin bed.
MTZ decreases with the decline of mass transfer resistance. So a small MTZ is highly required for the exchange experiment. Under the ideal situation of no mass transfer resistance, the MTZ is zero and the breakthrough curve forms a step curve. The ideal situation is impossible to be completely realized, however the breakthrough curves can be close to a step curve by adjusting the operating conditions appropriately.
3 Results and Discussion
3.1 The first exchange section
In order to obtain the maximum QR values and the minimum MTZ values, the space velocity, temperature, and Na+concentration were investigated in the first exchange section.
3.1.1 Effect of temperature
The breakthrough curves of the first exchange stage in the ammonium-form resin packed bed are shown in Figure 2, in which the temperature of ion-exchange process was inthe range of 25—70 ℃, while the Na+ion concentration of the initial slurry was 6.4 mg/L and the space velocity was 0.66 h-1.
The mass of qualified zeolite (mQ) is 0 g, 9.7 g and 15.4 g, respectively, at different temperatures, and the QR of these processes increases from 0 % to 36.5 % with an increasing temperature (as shown in Table 3). It is noticeable from the experimental results that the amount of exchanged Na+ions in zeolite increases rapidly with an increasing temperature, which indicates that the exchange rate of Na+ions from zeolite to resin is accelerated with the rising temperature. It occurs because a higher temperature can lead to a more active thermal motion of the cations in liquid phase, and the molecular collision frequency is greatly enhanced, so the higher the temperature is, the faster the diffusion velocity of Na+from zeolite to resin would be. But if the temperature is too high, the adsorption strength of cations on the surface of resin will be greatly reduced, and the thermal stability of the resin will also be affected. So, a temperature of 70 ℃ is considered to be the optimum temperature.

Figure 2 Change of breakthrough curves with the increase of temperature
3.1.2 Effect of Na+concentration
The breakthrough curves of zeolite slurry with different initial Na+ion concentrations (6.4—700 mg/L) are shown in Figure 3, which were obtained at a space velocity of 0.61 h-1and a temperature of 70 ℃.
It can be seen from Figure 3(a) that at a relatively low Na+ion concentration (6.4—197 mg/L), the inflection points of the breakthrough curves are gradually obvious with the increase of Na+ion concentration. When the Na+ion concentration is between 197—423 mg/L (Figure 3-b), the curves are almost overlapped before inflection points. At a much higher Na+ion concentration (423—700 mg/L), there has been a small increase in Na2O content of zeolite and the inflection points of the breakthrough curves would appear earlier. The experimental results showed that the ion-exchange rate between zeolite and resin increased with an increasing Na+ion concentration, because a higher Na+ion concentration in liquid phase could results in more opportunities for Na+ions to keep in contact with the resin and the zeolite, and ultimately would improve the ion-exchange velocity between the zeolite andthe resin. But if the Na+ion concentration is too high, the saturation time of resin will be shortened.
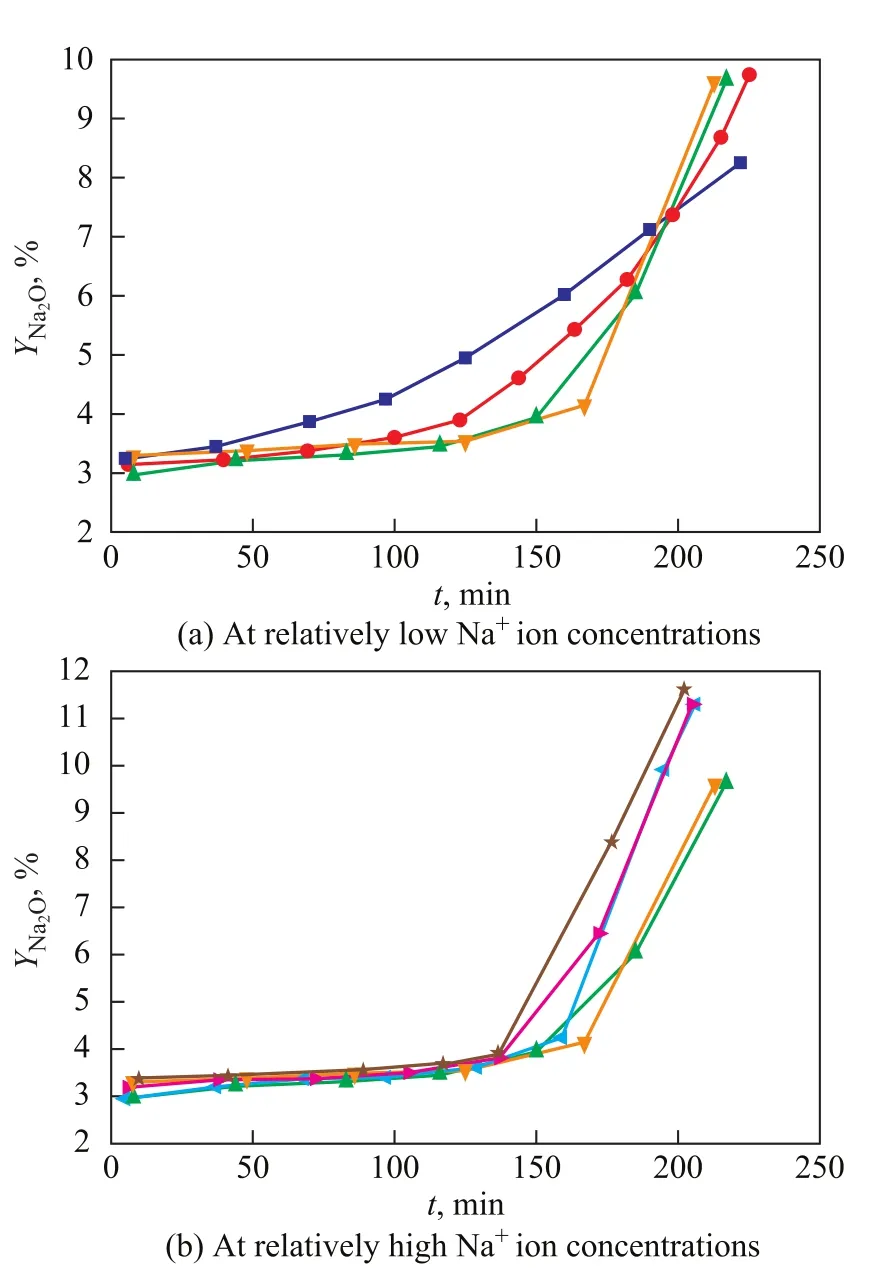
Figure 3 The breakthrough curves at different Na+ion concentrations
The mass transfer results are shown in Table 4. It is obvious that the QR of the zeolite increased from 30.9% to 78.9%, and then decreased to 71.1% with an increasing Na+ion concentration. The highest QR was 78.9% obtained at a Na+ion concentration of 197 mg/L. The MTZ decreased dramatically with the increase of Na+ion concentration, and the smallest MTZ was 5.5 cm at a Na+ion concentration of 423 mg/L. The effective utilization ratio of resin bed (tu/ts) rose quickly with the increase of Na+ion concentration and it could reach more than 82% when the Na+ion concentration was higher than 423 mg/L.
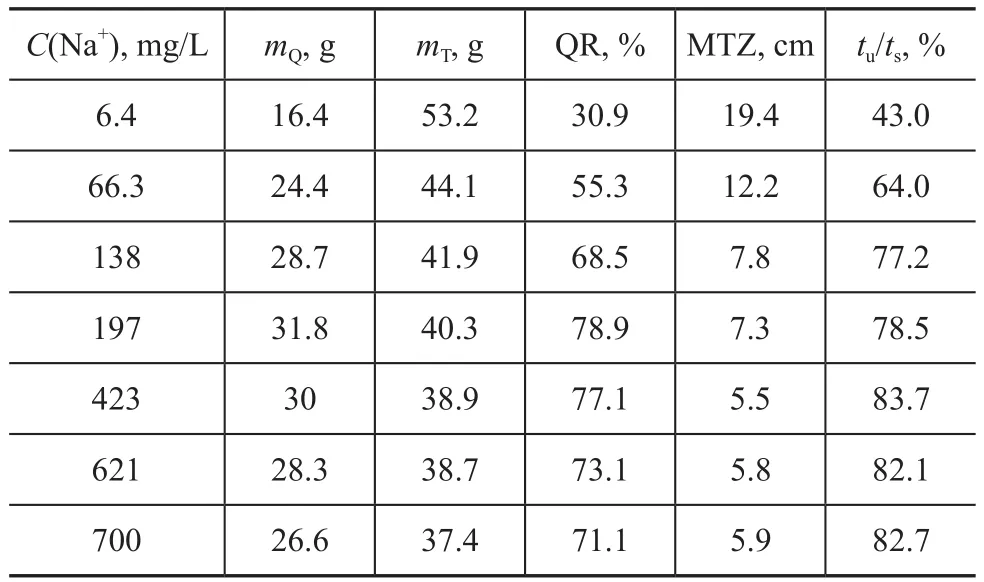
Table 4 Mass transfer parameters determined at different Na+concentrations
3.1.3 Effect of space velocity
The breakthrough curves of different space velocity and different Na+ion concentrations are shown in Figure 4. The ion-exchange processes were all run at 70℃. The inflection points of curves appear earlier with the increase of space velocity, which illustrates that the space velocity plays an important role on the breakthrough time and the saturation time of the resin column. The breakthrough curves almost have the same gradient at different space velocity, which indicates that the space velocity basically has no effect on the mass transfer resistance. At the same space velocity, the mQdecreases with the increase of Na+ion concentration (as shown in Table 5), since the Na+ions in slurry occupy a part of resin capacity. Higher Na+ion concentration can result in a shorter saturation time of resin and a decrease of mQ.
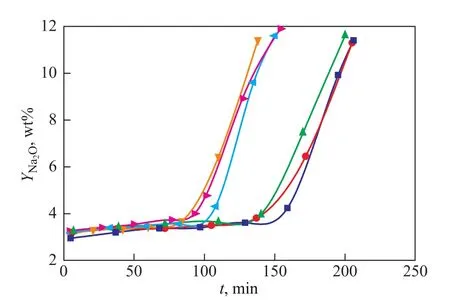
Figure 4 The breakthrough curves obtained at different space velocity values
3.2 The second exchange section
The sample of qualified zeolite obtained from the first exchange section was calcined at 500 ℃, and then it was subjected to ion exchange in a resin bed which was one meter long. The breakthrough curves of the ion exchange process are shown in Figure 5, which was run at the optimal operating condition according to the afore-mentioned result (a space velocity of 1 h-1, a Na+ion concentration of 700 mg/L and a temperature of 70 ℃). It can be seen from Figure 5 that the curve has two slopes. The slope of curve between 0—75 minute is bigger than the slope of the latter part, which illustrates that Na cations in zeoliteY have different locations and the difficulty for exchange of Na cations at different locations is different. The whole resin bed is the mass-transfer zone (1 m) at this exchange section. It is surprising to note that the MTZ at the second exchange section is much bigger than that of first exchange section (5.5 cm), which indicates that the exchange of Na+ions is more difficult at the second exchange section after the calcination treatment. Table 6 shows the physical properties of samples treated by resin exchange or by traditional method. The samples treated by resin exchange were obtained at a space velocity of 1 h-1, a Na+ion concentration of 700 mg/L and a temperature of 70 ℃. The traditional sample was treated by NH4Cl aqueous solution at a mass ratio of 1:0.4:10 (zeolite/NH4Cl/H2O) at 85 ℃. In the first exchange section, the samples which were subjected to ion exchange with resin had a lower Na2O content (3.61%) and a highest crystallinity (88%). And the percentage of Na+ions removed during the resin exchange process reached 68.3%, which was much higher than that achieved by the traditional method (58.9%). In the second exchange section, the samples which were subjected to ion exchange by resin had the lowest Na2O content (0.12%), which is much lower than that achieved by the traditional exchange method (1.28%). It can be seen from Figure 6 that the crystal morphology of the zeolite obtained by two different processes is similar to each other.
Table 5 Mass transfer parameters obtained at different space velocity values

Figure 5 The breakthrough curves in the second exchange section
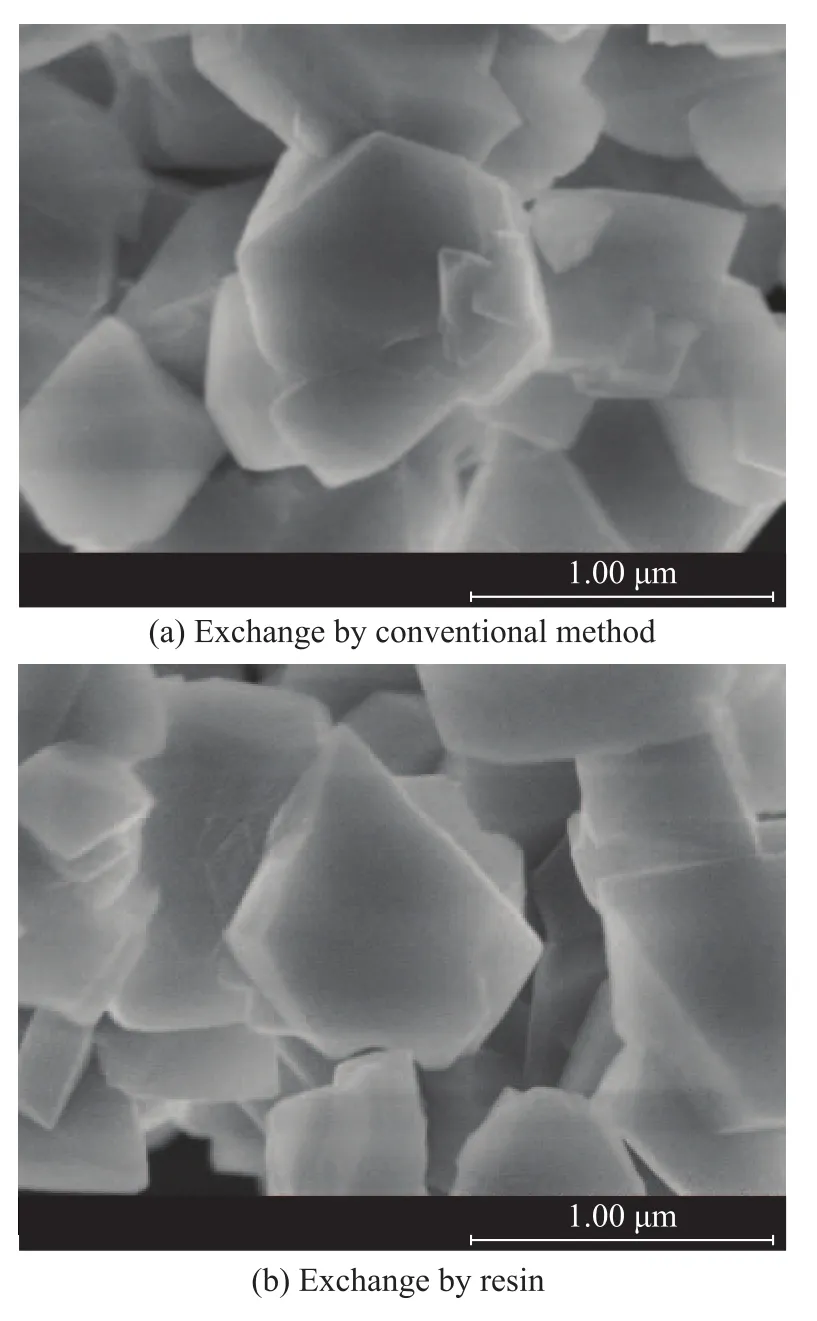
Figure 6 SEM images of exchanged zeolite
4 Conclusions
The technological conditions for exchange of Na+ions of zeolite Y by the NH4-form resin were investigated in a fixed-bed column. At the first exchange section, a maximum QR of 78.9% could be obtained at a space velocity of 0.61 h-1, a Na+ion concentration of 197 mg/L and a temperature of 70 ℃. The smallest MTZ of 5.5 cm could be achieved at a space velocity of 0.61 h-1, a Na+ion concentration of 423 mg/L and a temperature of 70 ℃. Themaximum effective utilization ratio of resin bed could reach more than 82%. At the second exchange section, the whole resin bed is the mass-transfer zone and the exchange of Na+ions in zeolite Y is more difficult even after the calcination treatment. The Na2O content of the samples exchanged by the resin is significantly lower than the sample exchanged by traditional exchange method in both the first and the second exchange sections. Moreover, the physical properties of zeolite obtained by resin exchange are similar to those obtained from the traditional exchange method. Based upon the experimental data, the method for ion-exchange of Na+ions from zeolite Y by the NH4-form resin is completely feasible.

Table 6 Physical properties of exchanged zeolite samples
Acknowledgements: The authors are pleased to acknowledge the financial support by the State Key Development Program for Basic Research of China (Grant No. 2012CB224800).
[1] Barrer R M, Makkr B M. Molecular sieve sorbents from clinoptilolite[J]. Canadian Journal of Chemistry, 1964, 42(6): 1481-1487
[2] Keough A H, SandlB. A new intracrystalline catalyst[J]. J Am Chem Soc, 1961, 83(16): 3536-3537
[3] Zhu H L, SonglJ, Gao X. Study on the selective adsorptive desulfurization of FCC naphtha by modified zeolite Y[J]. Petroleum Processing and Petrochemicals, 2009, 40(9): 37-41(in Chinese)
[4] Guo H S. Routes and countermeasures for solving ammonia nitrogen contamination of wastewater[J]. Petroleum Processing and Petrochemicals, 2001, 32(6): 47-50(in Chinese)
[5] Carrera J, Baeza J A, Vicent T. Biological nitrogen removal of high-strength ammonium industrial wastewater with twosludge system[J]. Water Research, 2003, 37(17): 4211-4221
[6] Liao P H, Chen A, Lo K V. Removal of nitrogen from swine manure wastewater by ammonia stripping[J]. Bioresource Technology, 1995, 54(1): 17-20
[7] Yu G Y, Yang Z S, Hu S B. Electrodialysis treatment of ammonia nitrogen wastewater[J]. Acta Agric Bor-Occid Sin, 2008, 17( 3): 332-335
[8] Liu Jian, Li Zhe. The treatment technology of ammonia nitrogen wastewater and its development[J]. Mining and Metallurgical Engineering, 2007, 27(4): 54-60
[9] Scherzer J, Humphries A. Dealumination of faujasite-type zeolites using ion exchange resins: US Patent, 4512961 [P]. 1983-08-22
[10] Hideo Y, Gorou S. Preparation of zeolite stable to water and heat: JP Patent, 58167420 [P]. 1982-03-23
[11] Tong Y C, Li Y L, Zhu C F, et al. An ion exchange method of zeolite: CN Patent, 102020288 [P]. 2009-09-09
[12] Zhao X T, Gao X H, Li Y, et al. A modified method of NaY zeolite: CN Patent, 101570334 [P], 2008-04-30
[13] Chu P, Dwyer F G. Hydronium ion-exchange of silica-rich zeolites using resin[J]. Zeolites, 1983, 3(1): 72-76
[14] Maria G B. Membrane processes for a sustainable industrial growth[J]. RSC Adv, 2013(3): 5694-5740
[15] Geankoplis C J. Transport Processes and Unit Operations[M]. USA: PTR Prentice Hall, 1993: 42-47
[16] Gazola F C, Pereira M R. Removal of Cr3+in fixed bed using zeolite NaY[J]. J Chem Eng, 2006, 117(3): 253-261
date: 2014-11-02; Accepted date: 2015-02-09.
Zhang Yi, Telephone: +86-10-82369282; E-mail: zhangyi.ripp@sinopec.com.
杂志排行
中国炼油与石油化工的其它文章
- Pyrolysis Characteristics and Kinetics of Methyl Oleate Based on TG-FTIR Method
- Synthesis of Waterborne Polyurethane Modified by Nano-SiO2Silicone and Properties of the WPU Coated RDX
- A Highly Efficient and Selective Water-Soluble Bimetallic Catalyst for Hydrogenation of Chloronitrobenzene to Chloroaniline
- Curing Mechanism of Condensed Polynuclear Aromatic Resin and Thermal Stability of Cured Resin
- A Novel Thermally Coupled Reactive Distillation Column for the Hydrolysis of Methyl Acetate
- Effects of Fe2+, Co2+and Ni2+Ions on Biological Methane Production from Residual Heavy Oil
