Effect of ginger-partitioned moxibustion on immunocytokines in patients with chronic nonbacterial prostatitis
2015-06-19LiGuodong李国栋LiShuyi李树义
Li Guo-dong (李国栋), Li Shu-yi (李树义)
1 Maternity and Child Care Centers in Lunan District, Tangshan City, Hebei 063000, China
2 The Ninth Hospital of Tangshan, Hebei 063000, China
Effect of ginger-partitioned moxibustion on immunocytokines in patients with chronic nonbacterial prostatitis
Li Guo-dong (李国栋)1, Li Shu-yi (李树义)2
1 Maternity and Child Care Centers in Lunan District, Tangshan City, Hebei 063000, China
2 The Ninth Hospital of Tangshan, Hebei 063000, China
Objective:To observe the effect of ginger-partitioned moxibustion on immunocytokines in patients with chronic nonbacterial prostatitis (CNP).
Methods:A total of 80 CNP patients were randomly allocated into two groups according to their visiting sequence, 40 cases in each group. Cases in the observation group were treated with oral Tamsulosin Hydrochloride Sustained Release Capsules (Harnal) (0.2 mg for each dose, one dose a day) and ginger-partitioned moxibustion at Qihai (CV 6), Guanyuan (CV 4), Zhongji (CV 3) and bilateral Zusanli (ST 36), Sanyinjiao (SP 6), Pangguangshu (BL 28), Shangliao (BL 31), Ciliao (BL 32), Zhongliao (BL 33) and Xialiao (BL 34) (once a day). Cases in the control group were treated with the oral Western medication alone (same administration as those in the observation group). Cases in both groups were treated for 28 d. Before and after treatment, the CD3+, CD4+, CD8+, CD4+CD25+, CD4+CD25+Foxp3+, transforming growth factor (TGF )-β1, immunoglobulin (Ig) A, IgE, IgG and IgM were detected and scored using National Institutes of Health chronic prostatitis symptom index (NIH-CPSI).
Results:The total effective rate was 90.0% in the observation group, versus 72.5% in the control group, showing a statistical difference (P<0.05). After treatment, the CD3+, CD4+, CD8+, CD4+CD25+, CD4+CD25+Foxp3+, TGF-β1, IgA, IgG and IgM were significantly increased in both groups, showing statistical differences (P<0.05). There were between-group statistical differences in CD3+, CD4+, CD8+, CD4+CD25+, CD4+CD25+Foxp3+, TGF-β1, IgA, IgG, IgE, IgM, total NIH-CPSI score and pain and discomfort score (P<0.05).
Conclusion:Ginger-partitioned moxibustion can improve clinical symptoms of CNP patients by improving their immune function.
Prostatitis; Moxibustion Therapy; Indirect Moxibustion; Ginger-partitioned Moxibustion; Immunity
Chronic nonbacterial prostatitis (CNP) is a major type of chronic prostatitis. It’s mainly characterized by pain, urgency and frequency of urination, coupled with soreness, pain and discomfort in the lower back and abdomen. These symptoms can affect the patient’s quality of life. Chinese medicine, especially acupuncture can help alleviate some CNP symptoms. To explore the action mechanism of ginger-partitioned moxibustion on CNP, we’ve observed changes of inflammatory cytokines that are closely associated with CNP[1-3]and compared with oral Western medication. The results are now summarized as follows.
1 Clinical Data
1.1 Diagnostic criteria
This was based on Chinese Diagnosis and Treatment Guidelines for Urological Conditions (2009 edition)[4]: pain mainly in the pelvic area but also in the lower abdomen, lumbosacral region, perineum, testis, penis or above the pubic bone; urgency, frequency or dribbling of urination, presence of white secretion after urination and difficult urination.
The symptom scores were evaluated according to the National Institutes of Health chronic prostatitis symptom index (NIH-CPSI).
1.2 Inclusion criteria
Those who met the above diagnostic criteria; aged between 40 and 60 years; CNP duration lasted 6-12 months.
1.3 Exclusion criteria
Having complications of urinary tract infection, inflammation involving the reproductive system, varicocele, irritable bowel syndrome, urogenital tuberculosis, urinary stones, urethral stricture or neurogenic cystitis; having complications of prostate cancer or hyperplasia.
1.4 Statistical methods
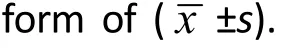
1.5 General materials
A total of 80 CNP cases treated at our Center between January 2012 and April 2014 were randomly allocated into an observation group and a control group according to their visiting sequence. There were no between-group statistical differences in age, duration and NIH-CPSI score (P<0.05), indicating that the two groups were comparable (Table 1).

Table 1. Between-group comparison of general materials
2 Treatment Methods
2.1 Observation group
2.1.1 Western medication
Patients took 0.2 mg of Tamsulosin Hydrochloride Sustained Release Capsules (Harnal) manufactured by Astellas Pharma Inc. (China, China Food and Drug Administration approval number: 20000681) for each dose, 1 dose a day for 28 d.
2.1.2 Ginger-partitioned moxibustion
Points: Qihai (CV 6), Guanyuan (CV 4), Zhongji (CV 3), bilateral Zusanli (ST 36), Sanyinjiao (SP 6), Pangguangshu (BL 28), Shangliao (BL 31), Ciliao (BL 32), Zhongliao (BL 33) and Xialiao (BL 34).
Method: The patient took a supine lying position. The practitioner placed the ginger powder (round shape of 3 cm in thickness) over Qihai (CV 6), Guanyuan (CV 4), Zhongji (CV 3), and bilateral Zusanli (ST 36) and Sanyinjiao (SP 6). Then, placed a moxa cone (manufactured by Hubei Li Shizhen Genuine Materia Medica Co., Ltd.) of 1.8 cm in diameter and 2.6 cm in height above the ginger powder on each point. Ignited the moxa cone and removed ashes and ginger powder when the moxa cones were burnt out. After this, the patient was changed to a prone lying position, the practitioner applied same method to bilateral Pangguangshu (BL 28), Shangliao (BL 31), Ciliao (BL 32), Zhongliao (BL 33) and Xialiao (BL 34). The treatment was done once a day, for 28 d.
2.2 Control group
3 Results Observation
3.1 Measurement parameters
3.1.1 T cell subsets
A total of 10 mL venous blood was extracted before and the next morning after treatment and then stored at a temperature of -20℃ after serum separation. Then the CD3+, CD4+and CD8+were detected using direct MCAB-A-E method. The regents were provided by Beijing Bole Life Science Development Co., Ltd.
3.1.2 Detection of CD4+CD25+, CD4+CD25+Foxp3 and TGF-β1
Some of the collected samples were made into single-cell suspension. Then the cell counts of CD4+CD25+and CD4+CD25+Foxp3+were detected using the flow cytometer. The rest of the samples were conducted anti-freezing management, followed by centrifugation for 10-minute at 2 000 r/min to separate plasma. Then, placed the plasma into an epikote (EP) tube and stored at refrigerator (-70℃). The TGF-β1 concentration was detected using enzyme-linked immunosorbent assay (ELISA) kit.
3.1.3 Detection of immuneglobulin (Ig)
The IgA, IgE, IgG and IgM levels in both groups were detected using ELISA method before and after treatment. The ELISA kits were manufactured by Dongxiyi (Beijing) Science & Technology Co., Ltd.
3.2 Therapeutic efficacy evaluation
The symptoms were scored using the NIH-CPSI. Then the therapeutic efficacy was evaluated by the NIH-CPSI scores.
Clinical recovery: Alleviation of clinical symptoms or NIH-CPSI score was decreased by ≥90%.
Marked effect: The NIH-CPSI score was decreased by≥50% but <90%.
Improvement: The NIH-CPSI score was decreased by≥25% but <50%.
Failure: The decrease of NIH-CPSI score was <25%.
3.3 Results
3.3.1 Clinical effects
The total effective rate was 90.0% in the observation group, versus 72.5% in the control group, showing a statistical differences (P<0.05) and a better effect in the observation group than that in the control group (Table 2).
3.3.2 Changes of CD3+, CD4+and CD8+in peripheral blood
After treatment, the CD3+, CD4+and CD8+in peripheral blood T cells in both groups were significantly increased (P<0.05). There were betweengroup statistical differences in CD3+, CD4+and CD8+(P<0.05), showing a more significant effect in the observation group than that in the control group (Table 3).
3.3.3 Changes of CD4+CD25+, CD4+CD25+Foxp3+and TGF-β1 contents
After treatment, the CD4+CD25+, CD4+CD25+Foxp3+and TGF-β1 contents in peripheral blood T cells were significantly increased in both groups (P<0.05). There were between-group statistical differences in CD4+D25+, CD4+CD25+Foxp3+and TGF-β1 contents (P<0.05), showing a better effect in the observation group than that in the control group (Table 4).
3.3.4 Changes of serum IgA, IgE, IgG and IgM
After treatment, the contents of IgA, IgG and IgM in both groups were significantly increased and the IgE content in both groups was significantly decreased (P<0.05). There were between-group statistical differences in IgA, IgE, IgG and IgM (P<0.05), showing a more significant change in the observation group than that in the control group (Table 5).
3.3.5 Changes of NIH-CPSI scores
After treatment, the total NIH-CPSI scores and pain discomfort scores in both groups were significantly decreased (P<0.05). There were between-group statistical differences in total NIH-CPSI score and pain and discomfort score (P<0.05), showing a more significant effect in the observation group than that in the control group (Table 6).

Table 2. Between-group comparison of clinical effects (case)
Table 3. Between-group comparison of T-cell subsets in peripheral blood

Table 3. Between-group comparison of T-cell subsets in peripheral blood
Note: Intra-group comparison before and after treatment, 1) P<0.05; inter-group comparison after treatment, 2) P<0.05
Group n Time CD3+CD4+CD8+Observation 40 Before treatment 31.31±6.04 23.49±6.05 18.73±4.66 After treatment 60.60±12.001)2)60.30±12.771)2)50.20±10.761)2)Control 40 Before treatment 30.74±5.96 23.07±6.15 19.08±5.53 After treatment 47.55±11.71)49.20±10.221)35.70±8.571)
Table 4. Between-group comparison of CD4+CD25+, CD4+CD25+Foxp3+and TGF-β1 contents

Table 4. Between-group comparison of CD4+CD25+, CD4+CD25+Foxp3+and TGF-β1 contents
Note: Intra-group comparison before and after treatment, 1) P<0.05; inter-group comparison after treatment, 2) P<0.05
Group n Time CD4+CD25+(%) CD4+CD25+Foxp3+(%) TGF-β1 (pg/mL) Observation 40 Before treatment 2.49±0.86 2.76±0.92 66.90±20.80 After treatment 5.11±1.081)2)4.84±1.521)2)162.70±39.201)2)Control 40 Before treatment 2.45±0.93 2.69±0.78 70.80±19.30 After treatment 3.88±1.131)3.61±1.441)127.1±40.11)
Table 5. Between-group comparison of IgA, IgE, IgG and IgM contents
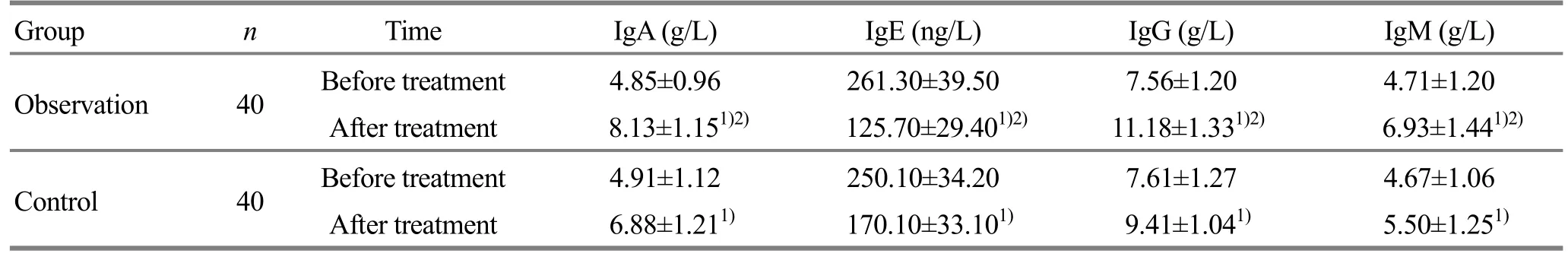
Table 5. Between-group comparison of IgA, IgE, IgG and IgM contents
Note: Intra-group comparison before and after treatment, 1) P<0.05; inter-group comparison after treatment, 2) P<0.05
Group n Time IgA (g/L) IgE (ng/L) IgG (g/L) IgM (g/L) Observation 40 Before treatment 4.85±0.96 261.30±39.50 7.56±1.20 4.71±1.20 After treatment 8.13±1.151)2)125.70±29.401)2)11.18±1.331)2)6.93±1.441)2)Control 40 Before treatment 4.91±1.12 250.10±34.20 7.61±1.27 4.67±1.06 After treatment 6.88±1.211)170.10±33.101)9.41±1.041)5.50±1.251)
Table 6. Between-group comparison of NIH-CPSI scores

Table 6. Between-group comparison of NIH-CPSI scores
Note: Intra-group comparison before and after treatment, 1) P<0.05; inter-group comparison after treatment, 2) P<0.05
Group n Total score Pain and discomfort score Before treatment After treatment Before treatment After treatment Observation 40 21.32±5.14 11.19±5.411)2)15.92±3.46 7.20±3.411)2)Control 40 22.94±7.52 15.62±3.601)16.13±4.20 10.54±5.091)
4 Discussion
The development of CNP is closely associated with lymphocyte regulation. T cell plays a central role in cell-mediated immunity. It’s known that CD4+cells can synthesize interleukin (IL)-4 and IL-13 and increase the IgE synthesis of B lymphocytes[5-6]; CD8+cells can synthesize interferon (IFN)-γ, inhibit IgE synthesis induced by IL-4 and IL-13 and result in imbalance of T-cells and their subsets in early CNP[7-8]. Experimental studies have shown that CNP patients have significantly lower transforming growth factor (TGF)-β. TGF-β can induce Foxp3 (CD4+CD25+regulatory T cells) positive and prevent allergic reaction. Studies have proven that hormone can up-regulate Foxp3, increase differentiation of CD4+CD25+and regulatory T cells and thus achieve therapeutic effects. Evidently, abnormal Foxp3 (CD4+CD25+regulatory T cells) is associated with the onset of CNP[9-11]. In addition, low levels of IgA, IgG and IgM in CNP patients may cause a poor resistance of the respiratory tract mucosa to virus, bacteria or other pathogenic microorganisms and make the patients susceptible to infection[12-14].
Numerous studies have confirmed the role of moxibustion in the prevention and treatment of prostatitis[15-18]. In this study, Guanyuan (CV 4) is the origin of Conception, Governor and Thoroughfare Vessels. It is the house of Yuan-Primordial qi where the seminal fluids are stored. In addition, this point is a crossing point of the Conception Vessel with the Liver, Spleen and Kidney Meridians as well as the Front-Mu point of the small intestine. As a key point for deficiency and consumption, Guanyuan (CV 4) can be used to treat frequency of urination, enuresis, urine retention, bloody urine, nocturnal emissions, impotence, gonorrhea and pain or blood stasis in the lower abdomen. Zhongji (CV 3), the Front-Mu point of the urinary bladder, is combined to cultivate Yuan-Primordial qi, resolve dampness and clear heat; Pangguangshu (BL 28) to harmonize meridian qi and blood; Shangliao (BL 31), Ciliao (BL 32), Zhongliao (BL 33) and Xialiao (BL 34) to tonify kidney yang and relieve water retention; Zusanli (ST 36) to supplement anti-pathogenic qi; and Sanyinjiao (SP 6) to regulate and facilitate qi of three yin meridians of foot. The above points combined can tonify the spleen and kidney, resolve dampness and stasis, clear heat and invigorate blood[19-21].
The study findings have shown that gingerpartitioned moxibustion can increase the levels of CD3+, CD4+, CD8+, CD4+CD25+, CD4+CD25+Foxp3 and TGF-β1 as well as the contents of IgA, IgG and IgM and decrease the IgE content. This indirectly proves that gingerpartitioned moxibustion can boost the immune response, control chronic infection and thus prevent CNP. In addition, the convenient ginger-partitioned moxibustion has no adverse reactions and is worthy of further clinical application.
Conflict of Interest
The authors declared that there was no conflict of interest in this article.
Acknowledgments
This work was supported by Hebei Tangshan Science & Technology Program (河北省唐山市科技计划项目, No. 121302118b).
Statement of Informed Consent
Informed consent was obtained from all individual participants included in this study.
Received: 28 September 2014/Accepted: 12 November 2014
[1] Liu HC. Detection of IFN-γ and COX-2 in patients with chronic prostatitis/chronic pelvic pain syndrome and their clinical significance. Zhongguo Weisheng Jianyan Zazhi, 2013, 23 (13): 2847-2848.
[2] Ye HY, Hou SK, Bai WJ, Deng QP. IL-6 and IL-8 levels in expressed prostate secretion of chronic prostatitis. Chin J Urol, 2003, 24(4): 279-281.
[3] Yuan SY, Tan Z, Yi WQ, Li SG, Liu DS, Zhang ZL, Wang WG. Regulating effect of Chinese medicine on IFN-γ, IL-2, IL-6 and IL-18 levels and their association with clinical symptoms. Guangming Zhongyi, 2010, 25(8): 1376-1378.
[4] Na YQ. Chinese Diagnosis and Treatment Guidelines for Urological Conditions. Beijing: People’s Medical Publishing House, 2009: 49-50.
[5] Liu CD, Wang HZ, Wei C. Effects of total glucosides of pacony on the expression of CD4+lymphocytes and IL-6 in rats with chronic nonbacterial prostatitis. Zhongguo Yaofang, 2009, 20(12): 891-893.
[6] Wei C, Liu CD, Wang HZ. Role of CD4+cells and IL-6 in chronic nonbacterial prostatitis. Zhongguo Xiandai Yixue Zazhi, 2009, 19(3): 395-397.
[7] Liu AG, Li HZ, Yan XK, He TY, Kan LL, Wang JY, Dong LL. Effect of electroacupuncture at “Sanyin” acupoints on cellular immune function in rats with chronic nonbacterial prostatitis. Zhen Ci Yan Jiu, 2013, 38(3): 192-197.
[8] Ren JG, Wang JY, Li JM, Li HH, Jiang LJ, Liu JX. Effects of Qianlieshutong capsule on T lymphocyte subsets in estradiol-induced non-bacterial prostatitis rats. Xiandai Miniao Waike Zahzi, 2013, 18(3): 233-236.
[9] Yu QJ. Measurement of androgen receptor and CD4+CD25+regulatory T cells in patients with chronic nonbacterial prostatitis/chronic pelvic pain syndrome. Master thesis of Anhui Medical University, 2007: 26-36.
[10] Cui Y, Chen JW, Shen WC, Feng Y, Hu YH, Huang FX. Effect of Qianliejian on TGF-β1 and CTGF expression in rats with chronic nonbacterial prostatitis. Zhongguo Zhongyiyao Keji, 2012, 19(6): 491-493.
[11] Wang YM, Lu SL, Zhao ZQ, Zhao YH, Wang N, Jing DS, Dong YC. Expressions of transforming growth factor-beta (1) and Smad4 in rat models of chronic nonbacterial prostatitis and their clinical significance. Zhonghua Nankexue Zazhi, 2010, 16(6): 490-494.
[12] Zhou Y. Clinical significance of detecting serum CRP and immunoglobulin in patients with chronic prostatitis before and after treatment. Fangshe Mianyixue Zazhi, 2007, 20(6): 591-593.
[13] Cheng SJ, Qu PB. Diagnostic value of immunoglobulin detection in prostatic fluid for chronic prostatitis. Xiandai Miniao Waike Zazhi, 2010, 15(4): 318-319.
[14] Zhong J, Jin ZX, Bian WX. Clinical observation on Wenglitong combined with Cardural XL for type-III prostatitis. Xiandai Zhongxiyi Jiehe Zazhi, 2012, 21 (33): 3676-3677.
[15] Fu Y, Zhang HF, Zhang B, Li L, Chen RX. Comparative study on thermosensitive state of Mingmen (GV 4) measured by moxibustion and infrared method. Jiangxi Zhongyi, 2012, 43(3): 52-53.
[16] Li SC, Zhao L. Therapeutic efficacy analysis on acupuncture combined with moxibustion for chronic prostatitis. Sichuan Zhongyi, 2011, 29(11): 112-113.
[17] Chen WY, Li GS, You YD, Zhang CD, Du XQ, Shen LP, Yang X, Du HY. Therapy and nursing of the box moxibustion for chronic nonbacterial prostatitis. Zhongguo Zhongyi Jichu Yixue Zazhi, 2011, 17(4): 427-429.
[18] Ma WJ, Hu QL. Clinical study on medicated oil moxibustion for chronic nonbacterial prostatitis. Zhongyiyao Linchuang Zazhi, 2012, 24(12): 1176-1177.
[19] Wang FS, Ma NC, Li Y. Clinical observation on moxibustion at Guanyuan (CV 4) for chronic nonbacterial prostatitis. Zhongguo Zhongyi Jichu Yixue Zazhi, 2009, 15(8): 617-618.
[20] Wu LH, Liu YB. Clinical observation on proximate needling on Zhongji (CV 3) and Zhibian (BL 54) for chronic prostatitis in 100 cases. Zhenjiu Linchuang Zazhi, 1999, 15(5): 26-27.
[21] Jiang L. Comparative observation on needling Guanyuan (CV 4) and Sanyinjiao (SP 6) for chronic nonbacterial prostatitis. Master’s thesis of Shandong University of Traditional Chinese Medicine, 2012: 23-24.
Translator: Han Chou-ping (韩丑萍)
隔姜灸对慢性非细菌性前列腺炎患者免疫细胞因子的影响
目的:观察隔姜灸对慢性非细菌性前列腺炎患者免疫细胞因子的影响。方法:将慢性非细菌性前列腺炎患者80例,按就诊顺序随机分为两组,每组40例。观察组予口服盐酸坦索罗辛缓释胶囊(哈乐) (Tamsulosin Hydrochloride Sustained Release Capsules, Harnal),每次0.2 mg,每日 1 次;隔姜灸气海、关元、中极及双侧足三里、三阴交、膀胱俞、上髎、次髎、中髎和下髎治疗, 每日1次。对照组仅口服盐酸坦索罗辛缓释胶囊,剂量及服用方法与观察组相同。两组均治疗28 d。治疗前、后检测患者外周血中CD3+、CD4+、CD8+、CD4+CD25+、CD4+CD25+Foxp3+、转化生长因子-β1 (transforming growth factor-β1, TGF-β1)、免疫球蛋白(immuneglobulin, Ig) A、IgE、IgG和IgM,并进行美国国立卫生研究院慢性前列腺炎症状指数(National Institutes of Health chronic prostatitis symptom index, NIH-CPSI)评分。结果:观察组总有效率90.0%;对照组总有效率72.5%,两组总有效率差异有统计学意义(P<0.05)。治疗后,两组患者CD3+、CD4+、CD8+、CD4+CD25+、CD4+CD25+Foxp3+、TGF-β1、IgA 、IgG及IgM均明显提高,与本组治疗前差异有统计学意义(P<0.05);观察组CD3+、CD4+、CD8+、CD4+CD25+、CD4+CD25+Foxp3+、TGF-β1、IgA、IgG、IgE、IgM、NIH-CPSI总分及疼痛与不适评分与对照组有统计学差异(P<0.05)。结论:隔姜灸可通过提高免疫功能改善慢性非细菌性前列腺炎患者的临床症状。
前列腺炎; 灸法; 间接灸; 隔姜灸; 免疫
R245.8 【
】A
in the control group only
oral Western medication (same dose and treatment course as those in the observation group).
Author: Li Guo-dong, attending physician
Li Shu-yi, master of medicine, vice chief analyst, attending physician.
E-mail: liqi19801211@163.com
猜你喜欢
杂志排行
Journal of Acupuncture and Tuina Science的其它文章
- Therapeutic effect of tuina combined with Jin Gui Shen Qi Decoction on lumbar spinal stenosis
- Clinical observation of warm needling moxibustion for rheumatoid arthritis
- Electroacupuncture down-regulates the expressions of colonic NGF and NGFR in visceral hypersensitivity rats
- Summary of Professor Jin Yi-cheng’s academic thoughts on pediatric tuina therapy
- Triple needling plus moxibustion and Tanbo-plucking tender points for the third lumbar vertebra transverse process syndrome
- Effect of row needling in muscle regions combined with seven-star needle tapping on cognitive function and quality of life in patients with post-stroke upper limb spasticity
