选择吸附性活性炭颗粒的制备及性能
2015-06-05舒羚安毅王晓晗秦卫普杨琳刘向东
舒羚,安毅,王晓晗,秦卫普,杨琳,刘向东
(1.浙江理工大学材料与纺织学院,杭州310018;2.哈尔滨卷烟厂,哈尔滨150001)
选择吸附性活性炭颗粒的制备及性能
舒羚1†,安毅2†,王晓晗2,秦卫普2,杨琳2,刘向东1*
(1.浙江理工大学材料与纺织学院,杭州310018;2.哈尔滨卷烟厂,哈尔滨150001)
根据非溶剂致相分离的原理,使用醋酸纤维素(cellulose acetate,CA)/丙酮/水3组分溶液于活性炭颗粒表面被覆多孔CA膜,并对改性后活性炭颗粒的吸附性能进行了研究.结果表明,这种多孔CA改性活性炭颗粒对气化状态和气溶胶状态的芘、肉桂腈、苯酚等有害物质的吸附量均高于活性炭原样,而对苯甲酸、麝香草酚等香味成分的吸附量均低于活性炭原样,表现出较好的选择吸附性.其中,对气化状态苯酚的吸附量相对于活性炭原样提高了17%,对苯甲酸的吸附量降低了60%;而对气溶胶状态苯酚的吸附量相对于活性炭原样提高了78%,对麝香草酚的吸附量降低了88%.
活性炭;醋酸纤维素;非溶剂致相分离;吸附
SummaryActivated carbons(AC)are effective adsorbents for removal of a wide variety of organic pollutants in aqueous media or gaseous environments.Their high adsorption capacity largely depends on the well-developed internal pore structure,surface area and special surface reactivity.However,general AC have not selective adsorption ability,which are often required in many practical uses such as reducing certain harmful chemicals from mainstream cigarette smoke. Therefore,modification to endow AC with selective adsorptivity is important and attractive.
Based on the findings of nonsolvent induced phase separation from a cellulose acetate(CA)ternary solution in a mixed solvent of acetone and water,we successfully covered a CA porous film on the surface of AC particles. Scanning electron microscopy(SEM)was used to observe the surface morphology of the CA films,and Brunner-Emmett-Teller(BET)measurement was used to characterize the surface area of the modified AC particles.The experimental results showed that the coverage rate of the porous CA film with an average pore size about 426 nm on the AC particles was over 80%,and the surface area of the modified AC particles(672 m2/g)was lower than that of the original AC(834 m2/g)used.
To investigate the selective adsorption property,pyrene,cinnamonitrile,benzene,phenol,benzoic acid,thymol and vanillin,which are the typical chemicals in the mainstream cigarette smoke,were selected as the model compounds in the simulating adsorption tests.For the gas adsorption experiments,the chemicals(each 0.1 g)were dissolved in chloroform(1.5 m L).Sixty millilitres of the mixed solution was dropped to a ball of cotton fiber(0.1 g),and put in a closed container after the chloroform had been volatilized.The control sample of the original AC and the modified one(each 0.2 g)were put in the closed container with the same distance(10 cm)to the cotton ball for 24 hours.For the aerosol adsorption experiments,the chemicals(each 0.1 g)were dissolved in acetone(15 m L).One point five millilitres of the mixed solution was uniformly sprayed to the tobacco(0.55 g)taken from a cigarette product,and burned in the closed container.Similar AC adsorption tests were carried out for 12 hours. These two tests were all repeated five times and the obtained samples were put together respectively.All adsorption quantities of the AC samples were analyzed by high performance liquid chromatography(HPLC)method.
The adsorption results,both in gas and aerosol conditions,indicated that the modified AC particles could absorb more harmful substances,i.e.,pyrene,cinnamonitrile,phenol,but much fewer aroma components,i.e.,benzoic acid,thymol,than the original AC.Contrasted with the original AC in gas adsorption test,the adsorption capacity of the modified AC increased about 17%for phenol but reduced about 60%for benzoic acid.In aerosol condition,it increased about 78%for phenol but decreased 88%for thymol.
In conclusion,this study provides a novel surface modification method for AC by covering CA porous film on the surface of AC following the nonsolvent induced phase separation mechanism.It demonstrates that the modified AC have improved the selective adsorption property.One of the potential applications of the modified AC particles is the use as a cigarette filter additive to remove harmful chemicals with little loss on the flavor of the cigarette products.
活性炭具有特殊的微晶结构,孔隙丰富,比表面积大,吸附能力强、速度快,饱和后可再生,能够吸附气体、胶态物质以及有机色素等[1-3].作为一种优良吸附剂,活性炭已广泛应用于制糖、医药、食品、化工、国防、农业以及人们的衣食住行中[4-8].
活性炭的吸附性能取决于其表面的物理化学性质.物理性质包括比表面积和孔隙结构,主要影响活性炭的吸附容量;化学性质即活性炭表面负载的官能团种类,主要影响活性炭与极性或非极性吸附质之间的相互作用.活性炭的物理化学性质与原材料、生产工艺、后处理技术等密切相关.市售活性炭的吸附性能受到原料和生产工艺的制约[9-10],在实际应用中具有一定的局限性.因此,对活性炭的改性尤为重要.
目前,活性炭的改性技术主要有物理改性和化学改性2类方法.物理改性主要是通过高温加热,改变活性炭的比表面积、孔径、孔容等物理特性,从而提高其吸附效率.Attia等[11]对活性炭分别进行400℃和600℃高温处理,改性后的活性炭比表面积增加,总孔容增加,但活性炭表面化学特性没有显著变化.Rangel-Mendez等[12]采用蒸汽或蒸汽与甲烷混合气体高温(1 000℃)改性处理活性炭,活性炭微孔孔容增加50%~70%,中孔孔容增加65%~ 90%.Dawson等[13]以椰壳为原料,用氢氧化钠水溶液预处理,使木质素部分溶解,然后再经炭化、活化后制得具有一定比例的微孔和中孔的活性炭.在单支卷烟中加入60 mg该活性炭,主流烟中挥发性有机物质的平均去除率相对普通活性炭提高了44%.左宋林等[14]以固-固混合的方式,用氢氧化钾活化石油焦制备了比表面积达3 000 m2/g、孔容达1.8 cm3/g、中孔孔容占31%的高比表面积和高孔容活性炭,其吸附性能是普通活性炭的2~3倍.田生友等[15]采用微波辐射改性活性炭研究其对CS2吸附性能的影响,结果表明,在微波辐射功率400 W、辐射时间3 min的条件下,改性活性炭产品对CS2的静、动态吸附量分别达到0.564 g/g和0.542 g/g,并发现改性后的活性炭发生缩孔,微孔数量增加,微孔孔容增加了28%,且活性炭表面碱性基团数量增多,酸性基团数量减少.
化学改性主要是通过酸改性、碱改性、负载改性、等离子体改性等方法来改变活性炭表面的化学结构,使其对某种物质的吸附性能得到提高,从而达到选择吸附效果[16].Girgis等[17]采用磷酸对活性炭进行改性,使活性炭表面形成含氧官能团,改性后的活性炭对Pb2+的吸附量可达299 mg/g,约为改性前的2倍.Shafeeyan等[18]采用氧化预处理、800℃高温氨改性的方式对活性炭进行改性,改性后活性炭微孔结构增加、表面酸性含氧官能团减少、表面碱性增强,对二氧化碳的吸附量高达73.5 mg/g,约为改性前的1.5倍.Agarwal等[19]对活性炭进行氯化铁负载改性,研究其对废水中苯酚和氰化物的去除效果,结果表明,负载处理后的活性炭对苯酚的去除率从73%增加到92%,对氰化物的去除率从76%增加到96%.Adhoum等[20]采用Ag和Ni负载改性活性炭去除水溶液中的氰化物,结果表明,Ag负载改性的活性炭对氰化物的去除量达到27 mg/g,Ni负载改性的活性炭对氰化物的去除量达到16.5 mg/g,分别为改性前的4倍和2倍.Qu等[21]采用介电阻挡放电等离子体改性处理活性炭,比较不同载气(O2、N2)等离子体改性活性炭的表面特性及其对五氯苯酚吸附能力的差异,结果表明,等离子体的作用可去除活性炭表面微粒,使其表面变得光滑. O2等离子体改性后的活性炭比表面积增加了6.2%,对五氯苯酚的吸附率提高了约10%;N2等离子体改性后的活性炭比表面积降低了6.6%,对五氯苯酚的吸附率减少了约15%.
吸烟固然有害健康,但加强宣传并推动众多烟民戒烟是一项长期工作,短时间内不可能产生显著效果.香烟作为一种嗜好商品,在我国仍具有深厚市场基础.为了减轻吸烟对现有吸烟嗜好者身体的危害,研究和推广香烟产品的降焦减害技术具有重大的健康和卫生意义.
本研究提出一种新的活性炭改性方法,该方法采用非溶剂致相分离法,使用醋酸纤维素(cellulose acetate,CA)/丙酮/水3组分溶液,制备了CA多孔薄膜[22],并将这种CA多孔膜成功被覆在活性炭表面,得到一种表面具有多孔CA覆盖膜的新型活性炭多孔颗粒.以苯、芘、肉桂腈、苯酚等有害物质和香草醛、苯甲酸、麝香草酚等香味成分对这种多孔颗粒的吸附性能进行模拟测试,结果表明,这种多孔颗粒对有害物质的吸附量高于活性炭颗粒,而对香味成分的吸附量则小于活性炭颗粒.这种新型多孔颗粒可以应用到卷烟滤嘴中,能够在较小影响卷烟吸烟口感的前提下去除主流烟气中的有害物质.
1 材料与方法
1.1 材料
醋酸纤维素(摩尔质量30 000 g/mol,乙酰含量39.8%)购自上海晶纯化学品有限公司.丙酮(纯度99%)购自上海化学试剂有限公司,用作溶剂.去离子水用作非溶剂.活性炭由厂家提供(椰壳炭,80目,松密度0.431 g/cm3,比表面积833.82 m2/g).苯、芘、肉桂腈、苯酚、香草醛、苯甲酸、麝香草酚等均为分析纯,购自上海晶纯化学品有限公司.烟丝来自利群牌卷烟.其他化学试剂均购自杭州米克化学试剂有限公司,规格均为分析纯.
1.2 性能表征
活性炭颗粒样品表面形貌通过场发射扫描电镜观察(FE-SEM,ULTRA-55,牛津,英国),加速电压2 k V,样品镀金处理.平均气孔尺寸通过统计扫描电子显微镜(scanning electron microscopy,SEM)照片上不特定区域超过100个孔的尺寸得到.样品颗粒的比表面积通过Brunner-Emmett-Teller(BET)比表面积测试仪测试(3H-2000PSI,贝士德仪器科技有限公司,北京).以氮气为吸附质,吸附温度-196℃,样品质量700 g,脱气温度200℃,脱气时间120 min.松密度通过测量50 m L样品获得,取3次测量的平均值.
1.3 高效液相色谱法测试
活性炭颗粒样品对各种物质的吸附量通过高效液相色谱法(high performance liquid chromatograph,HPLC)测定(HP1100,美国安捷伦科技公司,美国),色谱柱型号为ZORBAXSB-C18,粒径5μm,规格4.6 mm×150 mm.HPLC测试时根据标样的出峰位置来判断样品的位置.标样的配制按不同测试方法分为4组:苯酚、苯甲酸、麝香草酚为一组(Ⅰ),均为100 mg/L;芘(10 mg/L)为一组(Ⅱ);香草醛(100 mg/L)为一组(Ⅲ);苯(600 mg/L)和肉桂腈(100 mg/L)为一组(Ⅳ).
色谱条件:Ⅰ的流动相为水和甲醇,水与甲醇体积比由4∶1到1∶4的梯度测试时间6 min,检测波长215 nm.Ⅱ的流动相为水(10%)和甲醇(90%),测试时间6 min,检测波长220 nm.Ⅲ的流动相为水(70%)和甲醇(30%),测试时间12 min,检测波长210 nm.Ⅳ的流动相为水(30%)和甲醇(70%),测试时间10 min,检测波长215 nm.各组流速均为1 m L/min,柱温均为30℃.
1.4 制备多孔CA膜
CA(0.1 g)溶解于丙酮(1.6 m L)中,50℃超声波处理、反复搅拌,使其完全溶解得到澄清醋酸纤维素丙酮溶液.继续加入去离子水0.14 g,产生白色沉淀,50℃超声波处理、搅拌均匀使该沉淀溶解直至得到澄清溶液.取适量所得3组分溶液旋涂于玻璃板上(2 000 r/min,30 s),得到均匀的多孔CA膜,面积约为2.3 cm2.
1.2 制备表面被覆多孔CA膜的活性炭颗粒
CA(7 g)溶解于丙酮(109 m L)中,磁力搅拌,使其完全溶解.加入去离子水10 m L作为非溶剂搅拌均匀,得到m(CA)∶m(丙酮)∶m(水)=7∶83∶10这3组分溶液.取活性炭颗粒100 g,加入水20 m L进行湿润预处理,机械搅拌(200 r/min)至均匀.加入上述3组分溶液70 m L,机械搅拌至均匀,平铺在托盘上,室温条件静置6 h,再于60℃真空烘箱内干燥10 h,得到CA多孔膜被覆活性炭颗粒.
1.6 气体吸附实验
苯甲酸、苯酚、麝香草酚、芘、肉桂腈、苯、香草醛(图1)各0.1 g溶解于1.5 m L三氯甲烷中,搅拌至完全溶解.滴少量该溶液(0.06 m L)于棉花团(0.1 g)中心,通风干燥2 h,使三氯甲烷挥发.将棉花团放于密闭容器中心,取活性炭和CA改性活性炭样品颗粒各0.2 g放入密闭容器中,各样品与棉花团距离相等(10 cm),静置24 h.上述实验重复5次,将全部颗粒样品混合均匀.各颗粒样品吸附的物质通过索氏萃取器以甲醇为溶剂萃取后定容,使用HPLC定量分析.

图1 用于吸附实验的化合物分子结构式Fig.1 Chemical structures of the chemicals used in the adsorption experiment
1.7 气溶胶吸附实验
苯甲酸、苯酚、麝香草酚、芘、肉桂腈、苯、香草醛各0.1 g溶解于15 m L丙酮中,搅拌至完全溶解.取1.5 m L该溶液与烟丝(0.55 g)混合,搅拌均匀,通风干燥5 h,使丙酮挥发.将燃烧的烟丝放置于密闭容器中心,取活性炭和CA改性活性炭样品颗粒各2.0 g放入密闭容器中,各样品与烟丝距离相等(10 cm),静置12 h(图2).上述实验重复5次,将全部颗粒样品混合均匀.各颗粒样品吸附的物质通过索氏萃取器以甲醇为溶剂萃取后定容,使用HPLC定量分析.

图2 吸附试验装置示意图Fig.2 Sketch image of the experimental device used in the adsorption tests
2 结果与分析
2.1 CA改性活性炭的表面形貌与物理性质
图3A所示为m(CA)∶m(丙酮)∶m(水)= 7∶83∶10三元溶液在玻璃板上制备CA膜的SEM照片.从中可以看出,在CA膜上形成了连续的多孔网状结构,孔层相互交错,平均孔径大小约为280 nm,这与我们先前的研究报告结果一致[22].
在制备表面被覆多孔CA覆盖膜的活性炭颗粒过程中,我们发现在加入CA/丙酮/水3组分溶液搅拌前必须先加入一定量的水与活性炭颗粒搅拌均匀进行湿润预处理,否则不能成孔或成孔效果不好.从图3B中可以看出,未使用水湿润预处理的样品表面形成的是致密的CA膜,无多孔网状结构.这可能是因为活性炭表面本身存在多孔结构,与CA/丙酮/水3组分溶液混合时,吸附了体系中的部分水,改变了CA/丙酮/水3组分溶液的组成,使其不能形成多孔结构.
图3C和图3D所示为被覆多孔CA覆盖膜的活性炭颗粒表面形态的SEM照片.这种样品在制备过程中预先加入20 m L水与活性炭颗粒搅拌均匀进行湿润预处理.从图3C中可以看出,覆盖膜表面形成了连续的多孔网状结构,平均孔径大小约为430 nm.图3D为放大倍数为200倍时的覆盖膜照片,从中可以看出,活性炭表面基本被多孔CA膜覆盖,覆盖效果很好,覆盖面积大于80%.其中,凹面的CA膜覆盖效果最好.

图3 各种醋酸纤维素多孔薄膜扫描电镜图片Fig.3 Scanning electron microscopy images of porous films ofvarious cellulose acetate
表1列出了活性炭原样和CA改性活性炭样品的物理性质.从中可以看出,覆盖膜的孔径大于活性炭原样,BET法测出活性炭原样平均孔径为7 nm,而CA改性活性炭样品平均孔径为10 nm.此外,由SEM照片统计的CA改性活性炭样品的平均孔径为426 nm.被覆多孔CA膜的活性炭颗粒比表面积相对于活性炭原样有所减少,活性炭原样比表面积约为834 m2/g,而CA改性活性炭样品比表面积约为672 m2/g.

表1 改性活性炭的物理性质Table 1 Physical properties of the modified AC samples
2.2 气体吸附性能
表2列出了各活性炭颗粒样品对部分气化状态下有害物质和香味成分的吸附量.从中可以看出,被覆CA多孔覆盖膜的活性炭颗粒对芘、肉桂腈、苯、苯酚等有害物质的吸附量高于活性炭原样,而对苯甲酸、麝香草酚等香味成分的吸附量低于活性炭原样,表现出较好的选择吸附性.此外,这2种颗粒样品对苯酚、苯甲酸和麝香草酚的吸附量相对于其他化合物都较高,这是由于本实验是在密闭容器中进行的,样品吸附的是混合物自然挥发出的气体.若化合物易挥发,则分散在密闭容器中的气体浓度高;反之,则分散在密闭容器中的气体浓度低.这时,在颗粒的吸附过程中存在吸附竞争,气体浓度越高越容易被吸附.苯甲酸、苯酚等物质沸点相对较低,较易挥发,在密闭容器中气化的浓度相对较高,因此被吸附的量多;而肉桂腈、芘等物质沸点相对较高,不易挥发,在密闭容器中气化的浓度相对较低,所以被吸附的量相对较少.

表2 改性活性炭对气化状态化合物的吸附效果Table 2 Adsorption effects of the modified AC particles for the gasified chemicals
图4为活性炭原样和CA改性活性炭颗粒样品对部分气化状态下有害物质和香味成分的吸附对比(以活性炭原样的吸附量为100%).从中可以看出,被覆CA多孔覆盖膜的活性炭样品对有害物质的吸附量高于活性炭原样,尤其是对苯的吸附,CA改性活性炭颗粒样品对苯的吸附量相对于活性炭原样增加了25%.而对香味成分的吸附量,CA改性活性炭颗粒样品低于活性炭原样,特别是对苯甲酸的吸附,其吸附量不到活性炭原样的一半.

图4 改性活性炭和活性炭原样对气化状态化合物的吸附对比Fig.4 Comparison on the adsorptivity of the modified activated carbon(AC)particles and the original AC ones for the gasified chemicals
一般而言,比表面积越大,样品对气体的吸附量越大[23].在本研究中虽然被覆CA多孔覆盖膜的活性炭样品比表面积比活性炭原样小(表1),但CA改性活性炭吸附的有害物质的量比活性炭原样高.这可能是因为CA多孔覆盖膜本身也可以吸附有害物质,并且CA多孔膜的孔径较大,对气体的传输性能好,可以充当气体的传输孔,有利于对气体的吸附,因而提高了其对有害成分的吸附量[23].
2.3 气溶胶吸附性能
烟丝在燃烧时除部分物质被氧化成CO2、CO、H2O外,其他物质在800~900℃的高温区产生气态物质.随着温度迅速下降,挥发性较低的各种成分随温度急剧下降而开始冷凝.悬浮在气流中的因烟草燃烧所形成的很小的炭粒以及有机物微小的碎片、灰分、离子化的分子等组成微粒.挥发性低的蒸汽便以此为核心凝结,形成由气、液、固三相共存的烟雾体系,即气溶胶[24].
在本试验中,苯甲酸、苯酚、麝香草酚、芘、肉桂腈、苯、香草醛等物质均匀地附着在烟丝表面.随着烟丝的燃烧,这些化合物与烟气一并形成气溶胶分散在密闭容器中.表3列出了各活性炭颗粒样品对部分气溶胶状态下有害物质和香味成分的吸附量.从中可以看出,被覆CA多孔覆盖膜的活性炭颗粒对气溶胶状的有害物质的吸附量也是基本高于活性炭原样,只有对苯的吸附量低于活性炭原样.而对苯甲酸、麝香草酚等香味成分的吸附量也低于活性炭原样,这与表2的结果一致,也表现出较好的选择吸附性.
图5为活性炭原样和CA改性活性炭颗粒样品对部分气溶胶状态下有害物质和香味成分的吸附对比(以活性炭原样的吸附量为100%).从中可以看出,被覆CA多孔覆盖膜的活性炭样品对有害物质的吸附量高于活性炭原样,尤其是对苯酚的吸附,CA改性活性炭颗粒样品对苯酚的吸附量相对于活性炭原样提高了78%.而对香味成分的吸附量,CA改性活性炭颗粒样品低于活性炭原样,特别是对麝香草酚的吸附,其吸附量仅为活性炭原样的12%.
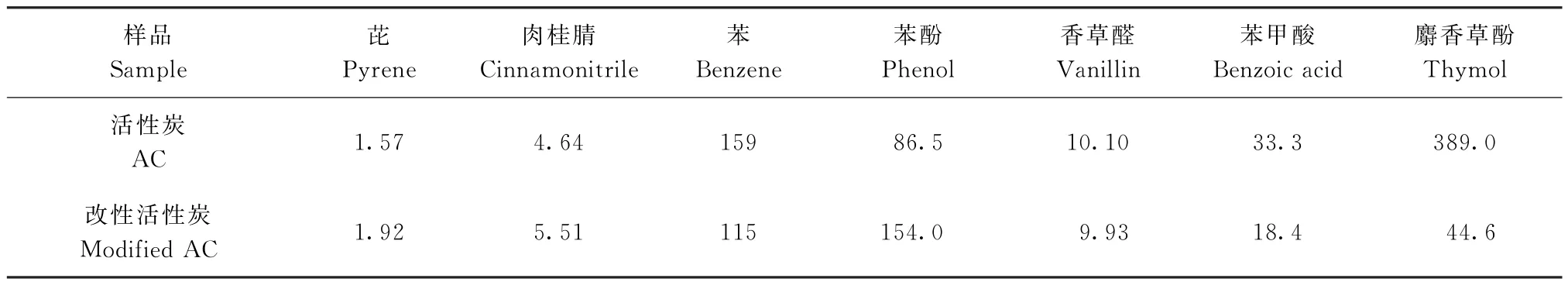
表3 改性活性炭对气溶胶状态化合物的吸附效果Table 3 Adsorption effects of the modified AC particles for the aerosol chemicals μg/g

图5 改性活性炭和活性炭原样对气溶胶状态化合物的吸附对比Fig.5 Comparison on the adsorptivity of the modified activated carbon(AC)particles and the original AC ones for the aerosol chemicals
在气溶胶混合物中,有害物质沸点相对较高,挥发性较低,较易凝结,分布在颗粒较大的焦油上;而香味成分沸点相对较低,挥发性较高,分布在较小的颗粒上.当较大的焦油颗粒被CA改性活性炭颗粒吸附时,CA改性活性炭颗粒表面的孔被堵住,从而含有香味成分的小颗粒物质不被吸附.因此,被覆CA多孔覆盖膜的活性炭颗粒吸附的香味成分较活性炭原样低,而对有害物质的吸附量较活性炭原样高.
3 结论
本研究采用非溶剂致相分离法,通过配制一定质量比的CA/丙酮/水三元溶液,制备得到多孔CA薄膜,并被覆在活性炭颗粒上,制备了表面具有多孔CA覆盖膜的新型活性炭颗粒.该改性活性炭颗粒的CA覆盖膜的覆盖率大于80%,比表面积672 m2/g,平均孔径约426 nm.模拟气体吸附试验结果表明,CA改性活性炭对气化状态下的芘、肉桂腈、苯、苯酚等有害物质的吸附量高于活性炭原样,对苯甲酸、麝香草酚等香味成分的吸附量低于活性炭原样.模拟气溶胶吸附试验结果表明,CA改性活性炭对气溶胶状态下的芘、肉桂腈、苯酚等有害物质的吸附量高于活性炭原样,对苯甲酸、麝香草酚的吸附量低于活性炭原样.这种改性的新型多孔活性炭颗粒可以应用在卷烟滤嘴中,在去除主流烟气中有害物质的同时又不显著影响卷烟的口感.
(References):
[1] Alslaibi T M,Abustan I,Ahmad M A,et al.A review:Production of activated carbon from agricultural byproducts via conventional and microwave heating.Journal of Chemical Technology and Biotechnology,2013,88(7):1183-1190.
[2] Chingombe P,Saha B,Wakeman R.Surface modification and characterisation of a coal-based activated carbon. Carbon,2005,43(15):3132-3143.
[3] 张乐忠,胡家鹏,赵升云,等.活性炭改性研究新进展.材料导报:纳米与新材料专辑,2009,23(14):435-438.
Zhang L Z,Hu J P,Zhao S Y,et al.Recent advances of research on modified activated carbon.Materials Review:Nano and New Materials,2009,23(14):435-438.(in Chinese with English abstract)
[4] Wigmans T.Industrial aspects of production and use of activated carbons.Carbon,1989,27(1):13-22.
[5] Ioannidou O,Zabaniotou A.Agricultural residues as precursors for activated carbon production:A review. Renewable and Sustainable Energy Reviews,2007,11(9):1966-2005.
[6] Kubota M,Hata A,Matsuda H.Preparation of activated carbon from phenolic resin by KOH chemical activation under microwave heating.Carbon,2009,47(12):2805-2811.
[7] Liu Y,Hu Z,Xu K,et al.Surface modification and performance of activated carbon electrode material.Acta Physico-Chimica Sinica,2008,24(7):1143-1148.
[8] Pereira M F R,Soares S F,Órfão J J,et al.Adsorption of dyes on activated carbons:Influence of surface chemical groups.Carbon,2003,41(4):811-821.
[9] 杨四娥,林建清.活性炭的改性技术及其应用研究进展.安徽农业科学,2014,42(9):2712-2715. Yang S E,Lin J Q.Review of modification technology of activated carbon and its application.Journal of Anhui Agriculture Sciences,2014,42(9):2712-2715.(in Chinese with English abstract)
[10] Ogata F,Iwata Y,Kawasaki N.Lead(Ⅱ)adsorption on chemically modified activated carbon in aqueous solution.e-Journal of Surface Science and Nanotechnology,2013,11:93-98.
[11] Attia A A,Rashwan W E,Khedr S A.Capacity of activated carbon in the removal of acid dyes subsequent to its thermal treatment.Dyes and Pigments,2006,69(3):128-136.
[12] Rangel-Mendez J,Cannon F.Improved activated carbon by thermal treatment in methane and steam:Physicochemical influences on MIB sorption capacity.Carbon,2005,43(3):467-479.
[13] Dawson E A,Parkes G M,Branton P.Synthesis of vegetable-based activated carbons with mixed micro-and mesoporosity for use in cigarette filters.Adsorption Science&Technology,2012,30(10):859-866.
[14] 左宋林,滕勇升.KOH活化石油焦制备工艺对活性炭吸附性能的影响.南京林业大学学报:自然科学版,2008,32(3):48-52. Zuo S L,Teng Y S.Effects of technological parameters on the adsorption properties of activated carbons from petroleum coke by KOH activation.Journal of Nanjing Forestry University:Natural Sciences Edition,2008,32(3):48-52.(in Chinese with English abstract)
[1 5] 田生友,梅华,吴文艳.微波-浸渍联合改性活性炭对CS 2吸附性能的研究.石油化工,2011,40(12):1348-1354. Tian S Y,Mei H,Wu W Y.Adsorbility of CS2on modified activated carbon by impregnation-microwave.Petrochemical Technology,2011,40(12):1348-1354.(in Chinese with English abstract)
[16] Zhao N,Wei N,Li J,et al.Surface properties of chemically modified activated carbons for adsorption rate of Cr(Ⅵ). Chemical Engineering Journal,2005,115(1):133-138.
[17] Girgis B S,Attia A A,Fathy N A.Modification in adsorption characteristics of activated carbon produced by H3PO4under flowing gases.Colloids and Surfaces A:Physicochemical and Engineering Aspects,2007,299(1):79-87.
[18] Shafeeyan M S,Daud W M A W,Houshmand A,et al. Ammonia modification of activated carbon to enhance carbon dioxide adsorption:Effect of pre-oxidation.Applied Surface Science,2011,257(9):3936-3942.
[19] Agarwal B,Thakur P,Balomajumder C.Use of ironimpregnated granular activated carbon for Co-adsorptive removal of phenol and cyanide:Insight into equilibrium and kinetics.Chemical Engineering Communications,2013,200(9):1278-1292.
[20] Adhoum N,Monser L.Removal of cyanide from aqueous solution using impregnated activated carbon.Chemical Engineering and Processing:Process Intensification,2002,41(1):17-21.
[21] Qu G Z,Li J,Liang D L,et al.Surface modification of a granular activated carbon by dielectric barrier discharge plasma and its effects on pentachlorophenol adsorption. Journal of Electrostatics,2013,71(4):689-694.
[22] Wang C,An Y,Li Q,et al.Nonsolvent effects on morphology of cellulose acetate films prepared by dry-cast process.Journal of Macromolecular Science:Part B,2012,51(11):2266-2275.
[23] Branton P,Lu A H,Schüth F.The effect of carbon pore structure on the adsorption of cigarette smoke vapour phase compounds.Carbon,2009,47(4):1005-1011.
[24] 金闻博,李承忠,戴亚.烟气胶体化学.合肥:安徽教育出版社,1990:26-38. Jin W B,Li C Z,Dai Y.The Cigarette Smoke Colloid Chemistry.Hefei:Anhui Education Press,1990:26-38.(in Chinese)
Simple preparation method and selective adsorption performance of novel activated carbon particles.
Journal of Zhej iang Universi ty(Agric.&Li fe Sci.),2015,41(1):111-118
Shu Ling1†,An Yi2†,Wang Xiaohan2,Qin Weipu2,Yang Lin2,Liu Xiangdong1*(1.College of Materials and Textile,Zhejiang Sci-Tech University,Hangzhou 310018,China;2.Harbin Cigarette Factory,Harbin 150001, China)
activated carbon;cellulose acetate;nonsolvent induced phase separation;adsorption
TQ 424
A
10.3785/j.issn.1008-9209.2014.07.281
中国烟草实业发展中心科技项目(ZYSY-2012-16);浙江省自然科学基金资助项目(LY12E03007).
刘向东,E-mail:liuxd@zstu.edu.cn
联系方式:舒羚,E-mail:shuling2029@163.com;安毅,E-mail:easeme1201@163.com.†为共同第一作者
2014 07 28;接受日期(Accepted):2014 09 28;
日期(Published online):2015 01 19 URL:http://www.cnki.net/kcms/detail/33.1247.S.20150119.1708.010.html
