王浆主蛋白(MRJPs)对张氏肝细胞的促增殖作用及其机制
2015-06-05于张颖谌迪王一然沈立荣
于张颖,谌迪,王一然,沈立荣
(浙江大学生物系统工程与食品科学学院,馥莉食品科学研究院,浙江省农产品加工技术研究重点实验室,杭州310058)
王浆主蛋白(MRJPs)对张氏肝细胞的促增殖作用及其机制
于张颖,谌迪,王一然,沈立荣*
(浙江大学生物系统工程与食品科学学院,馥莉食品科学研究院,浙江省农产品加工技术研究重点实验室,杭州310058)
将王浆主蛋白(major royal jelly proteins,MRJPs)添加到细胞培养基中,用MTT法比较纯MRJPs与胎牛血清(fetal bovine serum,FBS)、不同MRJPs/FBS比例对张氏肝细胞增殖的影响,用流式细胞仪检测MRJPs各处理对细胞周期的影响.结果表明:仅添加MRJPs不能促进细胞增殖;完全培养基(10%FBS)中MRJPs的最佳添加量为0.5 mg/m L;MRJPs(5 mg/m L)/FBS的添加比例为60/40时混合培养基对细胞的促增殖效果与完全培养基相比差异无统计学意义(P>0.05).流式细胞仪检测结果显示,MRJPs与FBS配合使用可促使细胞S期及G0/G1期比例增大;推测MRJPs的作用可能与促进DNA合成、前期RNA和核糖体合成有关.
王浆主蛋白;张氏肝细胞;胎牛血清;促增殖;替代;细胞周期
Yu Zhangying,Chen Di,Wang Yiran,Shen Lirong*(Zhejiang Key Laboratory for Agro-Food Processing,Fuli Institute of Food Science,School of Biosystems Engineering and Food Science,Zhejiang University,Hangzhou 310058,China)
SummaryMajor royal jelly proteins(MRJPs)are the soluble proteins in royal jelly,which account for 82% 90%of total royal jelly proteins.MRJPs are consisted of ten members,i.e.MRJP1 MRJP9 and MRJPψ,whose biological functions include enhancing cell proliferation,inducing differentiation,modulating immune responses,accelerating wound healing,etc.MRJP1 with 57 ku in molecular mass was found possessing proliferation-promoting activities,enhancing proliferation of primary cultured rat hepatocytes and increasing albumin production in the absence of serum.MRJPs could significantly stimulate the proliferation and growth of endothelial progenitor cells(EPC).The recombinant MRJP1 could significantly stimulate growth of Tn-5B-4 cell from Trichoplusia ni,and affect cell shape and adhesion to the substrate.Similar activities were also found in normal human neonatal skin fibroblasts NB1 RGB cell,MC3T3-E1 cell and Jurkat cell,etc.All these findings showed the potential of MRJPs in cell culture as cell growth factor.Besides,it seemed that MRJP1 could promote liver regeneration and might have a cytoprotective action on hepatocytes.Here,we used Chang’s liver cell from human as object to observe the proliferation action of MRJPs on the cell line,and to explore the potential of MRJPs substituting fetal bovine serum(FBS)as growth factor;the active mechanism of MRJPs was also investigated.
MRJPs were extracted by centrifuging from fresh royal jelly.The optimum concentration was selected by MTT assaying of the proliferation rates of the cell(PRC)cultured with serum-free mediums containing 0.05,0.1,0.2,0.3 and 0.5 mg/m L of MRJPs at 5th day.The possibility of MRJPs with optimum concentration(0.5 mg/m L)to replace FBS was investigated via comparison of the effects on PRC at the 2nd day,5th day and 8th day in both serum-free and serum-existing mediums with a positive control containing 10%FBS and a negative control containing 10%phosphate buffer solution(PBS).It was showed that MRJPs could only be used to culture the cell line when it was mixed with FBS.Then,the effects of different MRJPs/FBS(M/F)ratios(0/100,30/70,60/40,90/10,100/0)on PRC at the 2nd day,5th day and 8th day were investigated,respectively.It was shown that the M/F ratios with 30/70 and 60/40 were better for PRC of the cell line in relative to the complete medium with 10% FBS(M/F=0/100).The cell cycle distributions of the cell line cultured with different M/F ratios(0/100,30/70,60/40,100/0)were detected by flow cytometry.It was shown that the proliferation index(PI),S phase and G0/G1phase of the cell in the medium with M/F of 60/40 at the 5th day were not significantly different from the cell in complete medium(P>0.05),indicating that MRJPs might promote DNA synthesizing in S phase or RNA and protein synthesizing in G0/G1phase.
In conclusion,MRJPs mixed with FBS are suggested partially to replace FBS to culture Chang’s liver cell line in practice.
王浆主蛋白(major royal jelly proteins,MRJPs)是蜂王浆中水溶性蛋白的总称,约占王浆总蛋白的82%~90%[1],包括同源性较高(氨基酸序列一致性为60%~70%以上)的10个家族成员(MRJP1~MRJP9和MRJPψ)[15],主要特征是其蛋白序列有4个保守的半胱氨酸残基、相同的N-末端疏水序列;分子进化分析显示,为具有共同祖先——果蝇黄蛋白的同一家族[3,6].MRJPs家族有多种生理功能,如相对分子质量为5.7×104的MRJP1是决定蜜蜂级型分化的关键因素[7],能促进大鼠肝实质细胞生长,增加白蛋白产生量[8];MRJP3在体内和体外都具有免疫调节功能[9];相对分子质量为3.5×104的MRJP1四聚体能促进小鼠成骨前体细胞分化为成骨细胞[10]等.MRJPs对一些动物和人体细胞具有促生长和增殖作用,如MRJP1能促进原代大鼠肝实质肝细胞DNA合成,增加细胞分裂次数,并提高白蛋白分泌量[8],激活有丝分裂原活化蛋白激酶(mitogenactivated protein,MAP)、蛋白激酶B(protein kinase B,PKB)、蛋白激酶C(protein kinase C,PKC)等胞内信号因子[11];重组MRJP1也能促进大鼠肝实质细胞DNA合成,并保护细胞免受血清缺乏导致的凋亡[12].王浆蛋白水溶液粗提物能促进鱼内皮祖细胞(endothelial progenitor cells,EPC)[13]、昆虫Tn-5B-4细胞[14]、人外周血白血病T细胞Jurkat[15]、人皮肤成纤维细胞NB1 RGB[10]生长.通过MRJP1对人表皮角质细胞的促增殖作用研究发现,其主要机制是促进了肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)、白细胞介素-β(interleukin-β,IL-β)、转化生长因子-β(transforming growth factor-β,TGF-β)等细胞因子和基质金属蛋白酶-9(matrix metalloproteinase-9,MMP-9)的表达[16].
对MRJPs能否作为细胞生长因子、作为细胞生长因子的使用方法和浓度以及细胞对象等问题,目前尚缺乏系统研究,国内尚未见MRJPs应用于细胞培养的相关研究报道.我国是世界第一养蜂大国,目前蜂王浆产量和出口量占全球的90%[17],利用MRJPs研究开发细胞生长因子新产品,将有助于蜂王浆深加工和养蜂产业的可持续发展.为此,我们进行了MRJPs对人体正常细胞——张氏肝细胞(Chang’s liver)的促增殖活性及对细胞周期影响的研究,为MRJPs作为细胞生长因子的应用提供科学依据.
1 材料与方法
1.1 材料
1.1.1 细胞和蜂王浆 张氏肝细胞,由厦门华侨亚热带植物引种园馈赠[培养条件:含10%胎牛血清(fetal bovine serum,FBS)的RPMI-1640完全培养基,37℃,5%CO2];鲜王浆由杭州碧于天保健品有限公司提供.
1.1.2 试剂 Hyclone胎牛血清(南美洲)购自赛默飞世尔生物化学制品(北京)有限公司;RPMI-1640培养基、0.25%胰蛋白酶[含0.02%乙二胺四乙酸(ethylene diamine tetraacetic acid,EDTA)]购自吉诺生物医药技术有限公司;RNase A(Sigma)、碘化乙锭(PI)(Sigma)购自上海泽衡生物技术有限公司;MTT细胞增殖/毒性检测试剂盒购自南京凯基生物科技发展有限公司;牛血清白蛋白(bovine serum albumin,BSA)购自生工生物工程(上海)股份有限公司;二甲基亚砜(dimethyl sulfoxide,DMSO)和常规化学试剂(均为分析纯)购自国药集团化学试剂有限公司.
1.1.3 仪器与设备 FD-1-50真空冷冻干燥机(南京以马内利仪器设备有限公司);2300自动凯氏定氮仪[福斯赛诺分析仪器(苏州)有限公司];IL-161/169型气套式CO2培养箱[施都凯仪器设备(上海)有限公司];SW-CJ-1FD型超净工作台(苏州安泰空气技术有限公司);DSZ5000X型倒置显微镜(重庆澳浦光电技术有限公司);Costar 3599型96孔板(美国康宁公司);Multiskan MK型酶标仪[赛默飞世尔(上海)仪器有限公司];FACSCalibur型流式细胞仪(美国BD公司).
1.2 方法
1.2.1 MRJPs制备及浓度测定 取50 g鲜王浆溶于350 m L磷酸盐缓冲液(phosphate buffer solution,PBS)中,p H 7.4,4℃搅拌24 h,12 000 r/ min离心30 min,取上清液于透析袋(截留相对分子质量为8×103~1.4×104)中,4℃下用双蒸水(double distilled H2O,dd H2O)透析24 h,透析液即为MRJPs提取液,-20℃保存备用[18].参照GB 9697—2008[19],用凯氏定氮法测定总蛋白含量.
1.2.2 MRJPs有效浓度的筛选 取对数生长期的张氏肝细胞,以1×104/m L的密度接种到96孔板中,每孔100μL.用含10%FBS的RPMI-1640完全培养基培养24 h后换液,再在无血清的RPMI-1640中加入MRJPs,使其终质量浓度分别为0.05,0.1,0.2,0.3,0.5 mg/m L,继续培养5 d后,拍照并用MTT法检测各孔相对活细胞数.
1.2.3 MRJPs与BSA对细胞增殖影响的比较按1.2.2节接种和培养张氏肝细胞,培养24 h后换液,再在无血清RPMI-1640中分别加入MRJPs和阳性对照BSA溶液(终质量浓度均为0.5 mg/ m L),同时设添加10%PBS的无血清RPMI-1640作为阴性对照组.继续培养5 d后,拍照并用MTT法检测各处理的相对活细胞数.
1.2.4 MRJPs对细胞增殖的影响测定 1)MRJPs完全替代FBS的效果测定.按1.2.2节将张氏肝细胞接种到96孔板中(接种时不含血清),每孔100 μL.按1.2.2节确定的最佳MRJPs质量浓度(0.5 mg/m L)配制的无血清培养基培养细胞,同时分别以添加10%PBS和10%FBS的培养基作为阴性对照和阳性对照,在第2、第5和第8天用MTT法分别检测各孔相对活细胞数.2)MRJPs添加于完全培养基的效果测定.用按1.2.2节确定的最佳MRJPs浓度配制的完全培养基培养细胞,设上述相同的阴性对照,在第2天、第5天和第8天用MTT法分别检测各孔相对活细胞数.
1.2.2 不同MRJPs/FBS比例对细胞增殖的影响 根据1.2.2节实验结果配制MRJPs质量浓度为5 mg/m L的母液.按1.2.2节将张氏肝细胞接种到96孔板中,用无血清培养基中分别添加10%纯FBS、纯MRJPs(终质量浓度0.5 mg/m L),含MRJPs 30%(终质量浓度0.15 mg/m L)、60%(终质量浓度0.3 mg/m L)和90%(终质量浓度0.45 mg/m L)的FBS溶液培养细胞,在第2、第5和第8天后用MTT法检测各孔相对活细胞数,按下式计算不同培养基中细胞的相对增殖率.
相对增殖率/%=替代组MTT吸光度/纯FBS组MTT吸光度×100.
1.2.6 不同MRJPs/FBS比例对细胞周期的影响 采用MRJPs质量浓度为5 mg/m L的母液,按2×105/mL密度,将张氏肝细胞接种于25 mL培养瓶中,用无血清培养基中分别添加10%纯FBS、纯MRJPs、含MRJPs 30%与60%的FBS溶液培养细胞,5 d后收集细胞,用美国BD公司FACSCalibur型流式细胞仪检测细胞周期.上样前处理方法如下:用胰蛋白酶消化细胞后将酶除去,加入PBS制成细胞悬液,1 200 r/min离心8 min,弃上清液;用100μL PBS重悬沉淀,用1 m L注射器将细胞悬液慢慢注入1 m L、-20℃预冷的75%乙醇中,4℃过夜;第2天1 200 r/min离心8 min,弃上清液,用1 m L PBS重悬细胞;将细胞悬液转移到流式管中,1 200 r/min离心8 min,弃上清液,加入200μL RNase A(50μg/m L),37℃避光放置30 min;之后加入200μL PI(100μg/ m L),4℃放置15 min,上机检测.最后分析细胞周期各时相百分比,计算细胞增殖指数(PI).PI/%=(S+ G2/M)/(G0/G1+S+G2/M)×100.
1.3 数据分析
所有试验样品均作3组平行测试,所得数据用DPS 7.05[20]软件进行统计分析(单因素试验统计分析用邓肯新复极差法),结果用平均值±标准差表示.
2 结果与分析
2.1 促进细胞增殖的MRJPs浓度筛选
经凯氏定氮法测定,MRJPs提取液蛋白质量浓度为9.04 mg/mL.在一定范围内,由于MTT吸光度值D(490 nm)与活细胞数成正比,因此可根据吸光度大小判断相对活细胞数.由图1可见,在完全培养基中添加0.2%~0.5%的MRJPs对张氏肝细胞具有明显的促增殖作用,其作用与MRJPs质量浓度成正比(P<0.01).当培养基中MRJPs终质量浓度为0.5 mg/m L时,MTT吸光度达到最大(0.595±0.057). MRJPs质量浓度越高,贴壁存活的细胞越多(图2A~ F).当大于5 mg/mL(即终质量浓度大于0.5 mg/ m L)时MRJPs不能完全溶解,同时考虑到终质量浓度小于0.5 mg/mL的MRJPs对细胞的作用已经很显著,以及MRJPs制备效率等因素,我们选用0.5 mg/ mL作为MRJPs终质量浓度进行了深入研究.

图1 不同质量浓度MRJPs对细胞增殖的影响Fig.1 Effects of MRJPs of different concentrations on the proliferation of cells
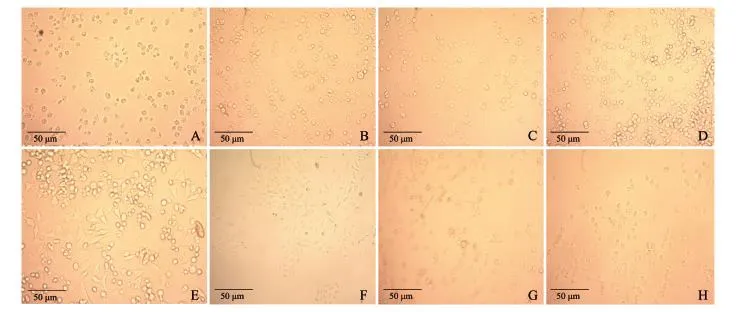
图2 第2天时不同质量浓度MRJPs处理的细胞与对照细胞Fig.2 Cells treated by MRJPs of different concentrations and controls on the 5th day
2.2 MRJPs与BSA对细胞增殖影响的比较
用同样质量浓度(5 mg/m L)的MRJPs、BSA和PBS溶液培养的细胞第5天时的生长状况见图2E,G,H.各处理的细胞相对增殖率统计结果(图3)显示,MRJPs组的细胞相对增殖率分别比阳性对照BSA和阴性对照PBS高20%以上(P<0.000 1),而阳性对照BSA的细胞相对增殖率与阴性对照PBS相比差异无统计学意义(P>0.05).说明MRJPs对张氏肝细胞的促增殖作用不同于一般蛋白质,具有明显的细胞生长因子特征.
2.3 MRJPs对细胞增殖的影响
表1为无血清条件下,用MRJPs(0.5 mg/m L)和对照培养基培养的细胞在第2、第5和第8天时MTT吸光度的差异.统计分析结果显示,用MRJPs完全替代10%FBS时,张氏肝细胞的相对活细胞数显著低于阳性对照(P<0.05).说明在无血清条件下MRJPs不能有效促进张氏肝细胞增殖,即需要与FBS适当配合使用.
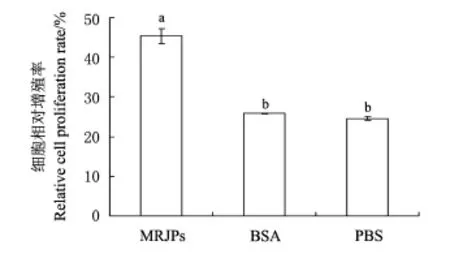
图3 MRJPs和BSA对细胞相对增殖率的影响比较Fig.3 Effects of MRJPs and BSA on relative cell proliferation rate

表1 MRJPs与FBS对细胞增殖的影响比较Table 1 Comparison of the effects of MRJPs and FBS on cell proliferation
表2反映了在完全培养基中添加MRJPs(终质量浓度为0.5 mg/m L)对张氏肝细胞增殖状况的影响.第2、第5和第8天后对培养细胞的测定结果显示,添加MRJPs处理组的相对活细胞数显著高于对照组差异极显著(P<0.01).由此可见,在完全培养基中添加MRJPs能显著促进张氏肝细胞增殖,并且表现出明显的抗衰老作用.
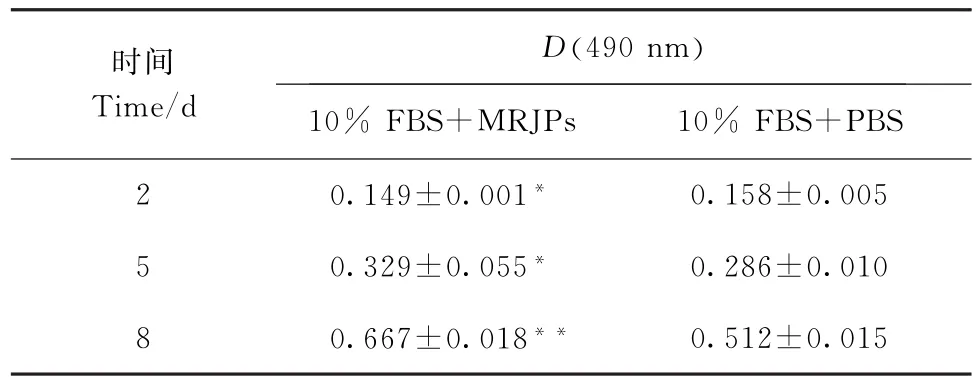
表2 FBS与MRJPs混用对细胞增殖的影响Table 2 Effects of the mixture of FBS and MRJPs on cell proliferation
2.4 不同MRJPs/FBS比例混合液对细胞增殖的影响
基于上述结果,我们进一步开展了不同MRJPs/FBS(M/F)比例(30/70,60/40,90/10,100/0)对细胞增殖的影响研究.图4为用上述配比培养基培养的细胞在第2天、第5天和第8天时的相对增殖率测定结果.经统计分析,相对于完全培养基,以用MRJPs替代30%和60%FBS的培养基细胞的增殖状况较好,两者在第2天时的相对增殖率分别达到完全培养基的87.1%和76.8%,第5天时分别达到完全培养基的91.8%和92.4%,第8天时两者甚至优于完全培养基.由于第5天和第8天时两者的细胞增殖率无显著差异(图4),宜采用MRJPs替代60%的FBS,以减少FBS用量并节约细胞培养成本.
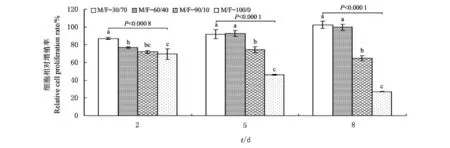
图4 不同MRJPs/FBS比例下细胞的相对增殖率比较Fig.4 Comparison of the relative cell proliferation rates under different MRJPs/FBS ratios
2.2 不同MRJPs/FBS比例混合液对细胞周期的影响
为进一步研究MRJPs对细胞的作用机制,采用流式细胞仪测定了不同M/F比例(0/100,30/70,60/40,100/0)混合液对细胞周期的影响(图5).统计分析结果(表3)显示,与完全培养基相比,用M/F=60/40培养的细胞在第5天时的增殖指数和G0/G1期细胞数比例差异均无统计学意义(P> 0.05),S期比例显著提高(P<0.05);与添加纯MRJPs的无血清培养基相比,用M/F=60/40培养的细胞显示出G0/G1期比例下降(P>0.05),细胞增殖指数、S期和G2/M细胞比例提高的趋势(P<0.05).表明用MRJPs替代60%FBS可以达到用完全培养基类似的促进细胞增殖效果.由于S期为细胞DNA合成期,DNA复制所需要的酶及组蛋白也在这一时期完成,据此推测,MRJPs所具有的促进张氏肝细胞增殖作用与促进DNA合成或与此过程相关酶的分泌有关.而完全用MRJPs替代FBS会导致细胞S期及G2/M期细胞比例降低,细胞DNA合成及有丝分裂前RNA和蛋白质(微管蛋白、促成熟因子等)合成受阻,细胞增殖指数极显著降低.
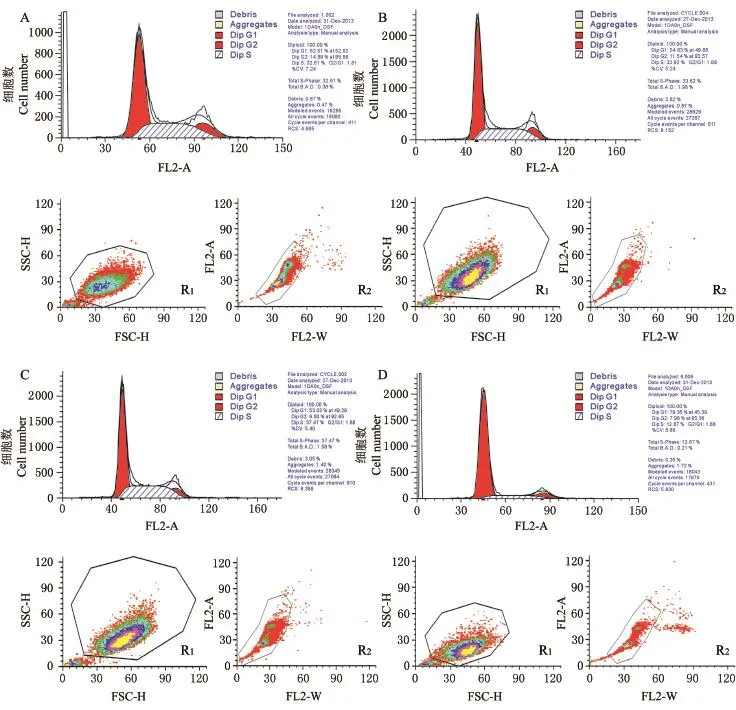
图5 不同MRJPs/FBS比例下的细胞周期Fig.5 Cell cycles under different MRJPs/FBS ratios

表3 不同MRJPs/FBS比例混合液对细胞周期的影响Table 3 Effects of different MRJPs/FBS ratios on cell cycle %
3 讨论
在细胞体外培养中,通常需要添加10%的FBS,因为FBS能提供细胞生长分裂所需的生长因子、贴壁因子等各种生理活性物质.目前虽然已有一些替代FBS的人工生长因子问世,但只对极少数细胞株有效,绝大多数细胞在培养时缺乏FBS往往难以维持正常生长.然而,FBS不仅价格昂贵,且质量受供体影响很大,不同批次FBS间经常存在生物活性不一致的情况,以致影响实验结果的重复性.此外,FBS在生产制备过程中易受支原体等外源因子污染,一旦使用了受污染的FBS,将造成实验结果报废,并影响终端产品使用安全.文献记载[2123]表明,MRJPs蛋白对大鼠肝细胞、昆虫Tn-5B-4细胞、鱼EPC细胞等多种细胞具有促进增殖和生长的作用.为此,我们进行了MRJPs对多种人体细胞的增殖作用比较,结果发现MRJPs对肝脏细胞,如永生化肝细胞Hepli4、正常肝细胞张氏肝特别有效.同时,MRJPs对张氏肝细胞的促增殖效果极显著地高于BSA,显示出不同于一般蛋白的细胞生长因子活性.因此,用MRJPs替代部分FBS培养张氏肝细胞具有重要的理论价值和应用价值.
但之前相关研究的主要目的是用MRJPs完全替代FBS,其中有培养过程中完全不添加FBS的,也有先用完全培养基短期培养到细胞贴壁再换成添加MRJPs的无血清培养基.但我们实验发现,在完全无血清条件下MRJPs难以维持张氏肝细胞增殖,而采用MRJPs替代60%以内的FBS培养细胞较为有效.这说明MRJPs中缺乏FBS的某些细胞活性因子.因此,进一步筛选有助于补充MRJPs缺少的活性成分将是今后其作为细胞生长因子研究开发的重点,同时,也需要对可采用MRJPs培养的细胞种类和范围作进一步筛选和研究.
4 结论
将MRJPs添加到完全培养基中具有促进张氏肝细胞增殖的作用,MRJPs的适宜添加量为0.5 mg/m L.用MRJPs(5 mg/m L)替代60%FBS的培养基培养张氏肝细胞,其增殖效果与完全培养基的效果接近.MRJPs与FBS配合使用可增大细胞S期及G0/G1期比例,推测MRJPs可能通过促进DNA合成、前期RNA和核糖体合成发挥其活性.
致谢 浙江大学农生环测试中心李金辉老师协助用流式细胞仪检测细胞周期,谨致谢意.
(References):
[1] Schmitzova J,Klaudiny J,Alvert S,et al.A family of major royal jelly proteins of the honeybee Apis mellifera L. Cellular and Molecular Life Sciences,1998,54(9):1020-1030.
[2] Klaudiny J,Kulifajova J,Crailsheim K,et al.New approach to the study of division of labor in the honeybee colony. Apidologie,1994,25:596-600.
[3] Albert S,Bhattacharya D,Klaudiny J,et al.The family of major royal jelly proteins and its evolution.Journal of Molecular Evolution,1999,49(2):290-297.
[4] Albert S,Klaudiny J.The MRJP/YELLOW protein family of Apis mellifera:Identification of new members in the EST library.Journal of Insect Physiology,2004,50(1):51-59.
[5] 赵亚周,田文礼,胡熠凡,等.蜜蜂蜂王浆主蛋白(MRJPs)的研究进展.应用昆虫学报,2012,49(5):1345-1353. Zhao Y Z,Tian W L,Hu Y F,et al.Research advances in major royal jelly proteins of honeybee.Chinese Journal of Applied Entomology,2012,49(5):1345-1353.(in Chinese with English abstract)
[6] Albert S,Klaudiny J,Simuth J.Newly discovered features of the updated sequence of royal jelly protein RJP57-1:Longer repetitive region on C-terminus and homology to Drosophila melanogaster yellow protein.Journal of Apicultural Research,1996,35:63-68.
[7] Kamakura M.Royalactin induces queen differentiation in honeybees.Nature,2011,473:478-483.
[8] Kamarula M,Suenobu N,Fukushima M.Fifty-seven-ku protein in royal jelly enhances proliferation of primary cultured rat hepatocytes and increases albumin production in the absence of serum.Biochemical and Biophysical Research Communications,2001,282(4):865-874.
[9] Okamoto I,Taniguchi Y,Kunikata T,et al.Major royal jelly protein 3 modulates immune responses in vitro and in vivo.Life Sciences,2003,73(16):2029-2045.
[10] Tsuruma Y,Maruyama H,Araki Y.Effect of a glycoprotein(apisin)in royal jelly on proliferation and differentiation in skin fibroblast and osteoblastic cells.Journal of the Japanese Society for Food Science and Technology,2011,58(3):121-126.
[11] Kamakura M.Signal transduction mechanism leading to enhanced proliferation of primary cultured adult rat hepatocytes treated with royal jelly 57 ku protein.Journal of Biochemistry,2002,132(6):911-919.
[12] Kamakura M,Sakaki T.A hypopharyngeal gland protein of the worker honeybee Apis mellifera L.enhances proliferation of primary-cultured rat hepatocytes and suppresses apoptosis in the absence of serum.Protein Expression and Purification,2006,45(2):307-314.
[13] Matasin Z,Gajger I T,Bedrica L.Cultivation of the EPC fish cell line in serum free media supplemented with honeybee royal jelly:Preliminary paper.Tieraerztliche Umschau,2008,63(8):446-448.
[14] Shen L R,Zhang W G,Jin F,et al.Expression of recombinant Acc MRJP1 protein from royal jelly of Chinese honeybee in Pichia pastoris and its proliferation activity in an insect cell line.Journal of Agricultural and Food Chemistry,2010,58(16):9190-9197.
[15] Shougo T,Shizuka A,Toru K,et al.Molecular characteristics and physiological functions of major royal jelly protein 1 oligomer.Proteomics,2009,9(24):5534-5543.
[16] Majtan J,Kumar P,Majtan T,et al.Effect of honey and its major royal jelly protein 1 on cytokine and MMP-9 m RNA transcripts in human keratinocytes.Experimental Dermatology,2010,19(8):73-79.
[17] 沈立荣,张瓅文,丁美会,等.蜂王浆的营养保健功能及分子机理研究进展.中国农业科技导报,2009,11(4):41-47. Shen L R,Zhang L W,Ding M H,et al.Research progress on nutritional and healthcare functions and molecular mechanism of royal jelly.Journal of Agricultural Science and Technology,2009,11(4):41-47.(in Chinese with English abstract)
[18] Salazar-Olivo L A,Paz-Gonzalez V.Screening of biological activities present in honeybee(Apis mellifera)royal jelly. Toxicology in Vitro,2005,19(5):645-651.
[19] 中华人民共和国国家质量监督检验检疫总局.GB 9697—2008,蜂王浆.北京:中国标准出版社,2008:4-5. General Administration of Quality Supervision,Inspection and Quarantine of the People’s Republic of China.GB 9697—2008,Royal jelly.Beijing:China Standards Press,2008:4-5.(in Chinese)
[20] 唐启义.DPS数据处理系统.北京:科学出版社,2010. Tang Q Y.DPS Data Processing System.Beijing:Science Press,2010.(in Chinese)
[21] Shah G.Why do we still use serum in the production of biopharmaceuticals?Developments in Biological Standardization,1999,99:17-22.
[22] Nairn C,Lovatt A,Galbraith D N.Detection of infectious bovine polyomavirus.Biologicals,2003,31(4):303-306.
[23] Greiser-Wilke I,Grummer B,Moennig V.Bovine viral diarrhoea eradication and control programmes in Europe. Biologicals,2003,31(2):113-118.
Effect of major royal jelly proteins(MRJPs)on proliferation activity of Chang's liver cell line and their mechanism of action.Journal of Zhejiang University(Agric.&Life Sci.),2015,41(1):7 14
major royal jelly proteins(MRJPs);Chang’s liver cell line;fetal bovine serum(FBS);proliferation;substitution;cell cycle
Q 253;S 896.3
A
10.3785/j.issn.1008-9209.2014.03.231
国家自然科学基金资助项目(31271848);浙江省公益性科技计划资助项目(2011C22039);杭州市重大科技创新项目(20131812A25).
沈立荣,Tel:+86 571 88982167;E-mail:shenlirong@zju.edu.cn
联系方式:于张颖,E-mail:yzy5901890@sina.com
2014 03 23;接受日期(Accepted):2014 06 05;
日期(Published online):2014 09 30
URL:http://www.cnki.net/kcms/doi/10.3785/j.issn.1008-9209.2014.03.231.html
