辣椒胞质不育系与保持系花药的细胞学和蛋白质组学差异分析
2015-06-05吕晓菡方献平柴伟国马俊平周毅飞
吕晓菡,方献平,柴伟国*,马俊平,周毅飞
(1.杭州市农业科学研究院蔬菜研究所,杭州310024;2.杭州市农业科学研究院生物技术研究所,杭州310024;3.浙江大学农业与生物技术学院,杭州310058)
辣椒胞质不育系与保持系花药的细胞学和蛋白质组学差异分析
吕晓菡1,方献平2,柴伟国1*,马俊平3,周毅飞1
(1.杭州市农业科学研究院蔬菜研究所,杭州310024;2.杭州市农业科学研究院生物技术研究所,杭州310024;3.浙江大学农业与生物技术学院,杭州310058)
为了解辣椒胞质雄性不育系与保持系花药的细胞学和蛋白质组学差异,以辣椒胞质不育系和保持系花蕾发育的6个不同时期的花药为材料,以石蜡切片于Leica DM1000显微镜下观察其发育过程的差异.然后利用SDSPAGE进行花药蛋白分离,经LC-MS/MS质谱技术和蛋白质组学分析对差异蛋白质条带进行鉴定.结果显示:花粉败育发生在四分体形成以后,与绒毡层细胞的过度液泡化及四分体周围的胼胝质不能解体有关.另外,与辣椒保持系相比,在不育系花药蛋白中有4条差异蛋白质条带(条带1~4)表达量下调,质谱分析后共鉴定出蛋白质68个,其中不重复蛋白质64个.对质谱数据利用UniProt数据库进行搜索鉴定,鉴定出的蛋白质按分子功能归类,涉及催化作用的蛋白质最多有24个;按细胞组分归类,涉及细胞内膜组织的最多有20个.按生物学过程归类,参与代谢过程的最多有24个.这说明在四分体形成以后,胞质雄性不育系花药的物质和能量代谢出现异常,导致绒毡层异常膨大,四分体不能获得物质和能量从而败育.另外,这也充分说明本研究通过蛋白质组学分析鉴定出的差异蛋白质可初步解释胞质雄性不育系与保持系在细胞学上的差异表现.
辣椒;细胞质雄性不育;花药;细胞学;蛋白质组学
SummaryCytoplasmic male sterility(CMS)lines are the excellent materials for studying the cytoplasmic and nuclear cytoplasmic interaction and have important application and theoretical value for CMS research.Three-line hybrid seed production could not only reduce the trouble of manual emasculation and seed costs,but also protect the purity of hybrid seeds.Therefore,the production of hybrid seed using CMS lines has a great potential and market demand in the pepper production.With the continuous development of science and technology,proteomics has become an important tool to decipher the biological gene function and contribute to the plant breeding.Proteome analyses helped to explore the important plant genes,thus speeding up the process of plant breeding and geneticsresearch.However,the current researchs on male sterile pepper were mostly concentrated in the physiological and biochemical indexes and the male sterile gene.The relevant report on CMS pepper protein was relatively less,and the research of sterile anther protein was even more rarely reported.Therefore,the aim of this study was to preliminarily identify the differences of cytology and proteomics involved in the peppers’anther of CMS line and its maintainer line.
The anthers at six different stages were used in the experiment.At first,the paraffin sections of anthers were observed and used to study the differences between CMS line and its maintainer line from the apparent characteristics,and then SDS-PAGE was used for anther protein separation and identification.Differential protein bands separated by SDS-PAGE,and the proteome profiles of the differential protein bands were further analyzed and identified by LC-MS/MS and proteomics analysis.
The results showed that pollen abortion occurred after metaphase.The main reason was that the callose surrounding the tetrad didn’t disintegrate normally and the tapetum cells were excessive vacuolation.Onedimensional SDS-PAGE analysis revealed that,compared with its maintainer line,there were four fewer protein bands in CMS line.A total of 64 non-redundant proteins were identified by LC-MS/MS,including 12 unique proteins from band 1,9 unique proteins from band 2,21 unique proteins from band 3,18 unique proteins from band 4,and 2 proteins from both band 1 and band 3,1 protein from both band 2 and band 3,1 protein from both band 3 and band 4.Among them,24 proteins were involved in catalysis according to molecular function;20 proteins were involved in cell endometrial tissue according to cellular components;and 24 proteins were involved in the metabolic process according to biological processes.
In conclusion,pepper CMSis mostly caused by pollen abortion.Pollen sterility is directly or indirectly related to abnormal tapetum and the lack of callose disintegration.After the differential proteome analysis between pepper CMS line and its maintainer line,we find that after metaphase,due to the decreased expression involving substance and energy metabolism in a part of the protein in the anther of male sterile line,the tapetum cells develop abnormally and the four separate microspores in development can not obtain matter and energy.Then pollen might be abortion.Therefore,in this study,the differential proteins identified by proteomics analysis can preliminarily explain the differences in cytology.
胞质雄性不育(cytoplasmic male sterility,CMS)是一种广泛存在于高等植物中,由核基因和细胞质基因共同控制的生物学现象.细胞质雄性不育系是研究细胞质遗传和核质互作的极好材料,对CMS的研究具有重要的应用和理论价值[1].辣椒杂种优势显著,但母本去雄费时费工,且杂交种子纯度难以保证.而利用CMS三系配套法生产杂交种子,不但可减少人工去雄的麻烦,降低制种成本,而且能保障杂交种子的纯度[2].目前,国内外已选育出多个辣椒胞质雄性不育系,并逐步应用于辣椒杂交种子的生产[3].因此,利用CMS三系配套法生产杂交种子在辣椒生产上具有极大的潜力和市场需求.
随着研究的不断深入及其相关技术的不断完善,蛋白质组学技术作为当今生命科学的研究热点,已经成为生物学中破译基因功能和贡献于育种程序的重要途径,建立植物的蛋白质数据库对于研究植物遗传育种有重要意义;通过蛋白质组学分析也有助于确定植物优良性状基因,从而加快植物遗传育种的进程.如今,蛋白质组学技术已经成为研究大田作物内在生理生化机制的强有力工具[4-5];它已经被广泛用于水稻[6-8]、小麦[9]、碱蓬[10]、油菜[11]和大豆[12]等作物的分子机制研究中.
1 材料与方法
1.1 供试材料
较稳定的1对辣椒细胞质不育系(DH-01-1-1A)和保持系(DH-01-1-1B),由杭州市农业科学研究院蔬菜研究所提供.
1.2 供试样品的制备
供试的辣椒材料于2012年12月上旬开始播种,2013年3月下旬移栽入大棚,至2013年6月上旬辣椒材料进入盛花期,在上午8:00—9:00将花蕾按从小到大分为6个不同时期采摘(图1).然后,用镊子将花蕾中的花药剥离出备用(图2).

图1 辣椒6个不同发育时期的花蕾Fig.1 Six different periods’buds of peppers

图2 辣椒6个不同发育时期的花药Fig.2 Six different periods’anthers of peppers
1.3 花药的石蜡切片
所有患者均在全麻下行后路三维矫形植骨融合内固定术,内固定器械为山东威高Upass系统或美国强生Expedium系统。椎弓根螺钉均为徒手置入,术中采用旋棒、平移、去旋转及原位弯棒等矫形技术,同种异体骨和咬碎自体骨混合后作为植骨材料。手术全程在体感诱发电位(sSEP)和运动诱发电位(MEP)监护下完成。
于2013年6月初辣椒细胞不育系和保持系植株进入盛花期,在晴天上午取不育系及其保持系处于生长6个不同发育时期的花蕾(图1),立即浸没于卡诺固定液中,24 h后用各级乙醇脱水,二甲苯透明,取不同大小的花蕾采用常规石蜡切片程序包埋,制成厚度为10μm的连续切片,二甲基蓝染色,中性树胶干燥封片.在Leica DM 1000显微镜下观察并拍照记录.
1.4 花药蛋白SDS-PAGE分析
利用TCA-丙酮沉淀法提取花药中的总蛋白质,得到的蛋白质样品在-80℃超低温保存.SDSPAGE电泳参照王勇等[13]的方法.采用5%浓缩胶和12%分离胶,样品与含有β-巯基乙醇的上样缓冲液以1∶1体积比混合,每孔上样量12μL,采用10 m A电流电泳,待蛋白质带进入分离胶时改用24 m A电流电泳,至距凝胶下端1 cm处停止电泳,凝胶用考马斯亮蓝R-250过夜染色,次日用清水褪色3 h,脱色后采用凝胶成像系统(Bio-Rad公司)照相.
1.2 蛋白质胶内酶解及肽段提取
选取辣椒细胞质不育系与保持系出现的差异蛋白质条带,用洁净的刀片切下,参照Shevchenko等[14]的方法将切下的蛋白质条带分别进行胶内酶解,并洗脱,得到肽段混合物.蛋白质胶内酶解:胶条首先用500μL含50%乙腈的浓度为25 mmol/L的碳酸氢铵洗脱,弃去上清液,重复2次,每次60 min;用500μL H2O洗脱1次,弃去上清液;加入500μL乙腈脱水;在56℃条件下使用10 mmol/L二硫苏糖醇处理1 h,还原打开二硫键;在暗室使用55 mmol/L碘乙酰胺处理45 min,进行半胱氨酸的皖基化封闭;用膜蛋白酶溶液(10 ng/μL溶于25 mmol/L碳酸氢铵溶液中)覆盖;冰上30 min,去除多余酶液,加入25μL 25 mmol/L碳酸氢铵,37℃消化过夜;加5%甲酸(formic acid,FA)终止反应.肽段提取:用含有0.1%甲酸的50%乙腈200μL提取1次;用含有0.1%甲酸的100%乙腈200μL提取2次;收集所有的上清液,并真空干燥,得到肽段混合物.
1.6 蛋白质条带基于LTQ Orbitrap Q-Exactive的LC-MS/MS分析
将真空抽干的每个组分分别用缓冲液A(2%乙腈,0.1%叶酸)复溶至约0.5μg/μL,15 000 g离心10 min,除去不溶物质.每个组分上样5μL,通过赛默飞EASY nlc-1000高效液相色谱仪进行分离.分离程序:以300 n L/min的流速进行梯度洗涤90 min;洗涤梯度为前70 min缓冲液B(99.9%乙腈,0.1%叶酸)从0%上升到35%;再从35%到100%线性洗涤5 min.最后用100%的缓冲液B洗柱15 min.经过液相分离的肽段进入到Q-Exactive质谱仪.仪器的分辨率设置为70 000(质荷比/半峰宽).用碰撞能量为35%的CID(collision induced dissociation)模式对肽段进行筛选,在离子阱中检测信号.每个峰强度超过5 000的一级母离子打8个二级谱图.扫描的质荷比范围为350~1 800.
1.7 质谱数据的蛋白质数据库搜寻鉴定与功能注释
质谱采集到的原始数据先采用Proteome Discovery软件在NCBI数据库进行搜索和匹配.同时,在搜索过程中设定参数:消化酶为胰酶、固定修饰为carbamidomethyl(C)、可变修饰为oxidation(M),每个肽允许有1个不完全裂解位点,物种来源选择绿色植物,离子选择[M+H]+和average.对鉴定蛋白通过UniProt基因本体(gene ontology,GO)数据库进行注释及分析,预测这些蛋白质可能的功能并对其进行功能分类统计.
2 结果与分析
2.1 不同发育时期辣椒花药的细胞学观察
为进一步对花粉败育的原因进行探索,我们按从小到大选取6个不同时期的辣椒不育系和保持系花蕾做石蜡切片(图3),结果发现,不育系植株的花药和保持系植株相比,明显差异从减数分裂四分体时期(图3C、I)开始.败育发生在四分体形成以后,由于四分体周围的胼胝质不能解体和绒毡层细胞的过度液泡化,径向肥大,使得四分体被挤向药室中央且原生质体浓厚,然后逐渐解体.
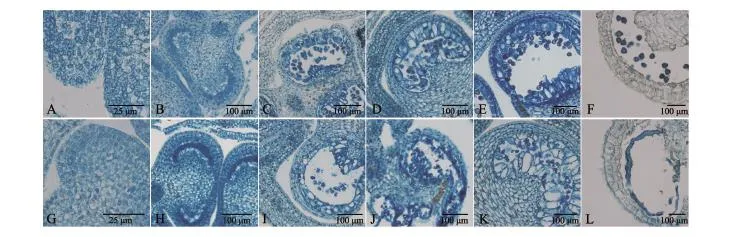
图3 保持系和不育系小孢子发育过程Fig.3 Microspore development process of cytoplasmic male sterile line and its maintainer line
2.2 辣椒细胞质不育系与保持系花药蛋白的SDSPAGE分析
对辣椒不育系和保持系花药蛋白SDS-PAGE分析(图4)表明,在保持系的6个不同发育时期均新出现了4条差异蛋白质条带,表明这4条蛋白质条带很可能是与植株可育相关的重要应答蛋白带.
2.3 蛋白质条带的质谱鉴定
用LTQ Orbitrap Q-Exactive对酶解的肽段进行分析,Sequest软件搜索NCBI数据库后,从条带1中鉴定出蛋白质14个,从条带2中鉴定出蛋白质10个,从条带3中鉴定出蛋白质25个,从条带4中鉴定出蛋白质19个(表1).
通过比较质谱鉴定的条带1、条带2、条带3和条带4蛋白质条带中的蛋白质种类,发现条带1和条带3共有蛋白质2个,条带2和条带3共有蛋白质1个,条带3和条带4共有蛋白质1个,即在4个样品中共鉴定出不重复蛋白质64个.鉴定蛋白质的登录号及注释信息见表1.

图4 辣椒细胞质不育系与保持系蛋白质的SDS-PAGE图谱Fig.4 SDS-PAGE on proteins from haemolymph of cytoplasmic male sterile line and its maintainer line

表1 质谱鉴定条带1、条带2、条带3和条带4对应的蛋白质Table1 Non-redundant list of proteins identified by LC-MS/MS from band1,band2,band3 and band4
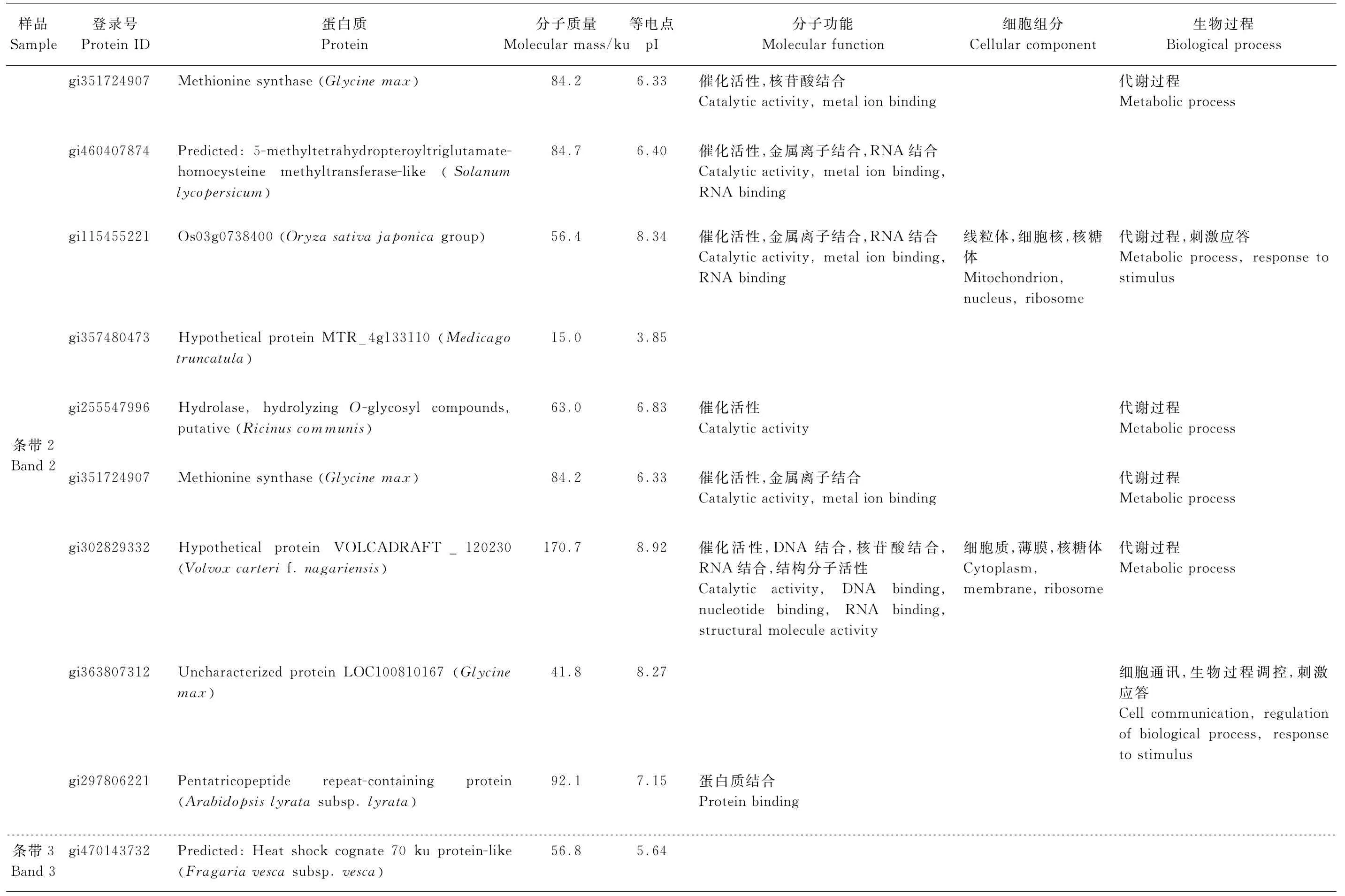
续表1 质谱鉴定条带1、条带2、条带3和条带4对应的蛋白质Continuation of Table1 Non-redundant list of proteins identified by LC-MS/MS from band1,band2,band3 and band4

续表1 质谱鉴定条带1、条带2、条带3和条带4对应的蛋白质Continuation of Table1 Non-redundant list of proteins identified by LC-MS/MS from band1,band2,band3 and band4

续表1 质谱鉴定条带1、条带2、条带3和条带4对应的蛋白质Continuation of Table1 Non-redundant list of proteins identified by LC-MS/MS from band1,band2,band3 and band4

续表1 质谱鉴定条带1、条带2、条带3和条带4对应的蛋白质Continuation of Table1 Non-redundant list of proteins identified by LC-MS/MS from band1,band2,band3 and band4
从图5中可以看出,在这4个样品中鉴定出的蛋白质分子质量大多集中在30~100 ku区间内,等电点大多集中在5.5~8.5区间内.

图5 质谱差异蛋白质条带的蛋白质物理性质分布图Fig.5 Distribution of physical properties for proteins identified by LC-MS/MS for the differential protein bands
2.4 质谱鉴定蛋白质条带的蛋白质GO分类
对条带1、条带2、条带3和条带4鉴定蛋白质的GO分析结果(图6~8)表明,鉴定蛋白质被注释到3个本体的23个GO条目(term)中,其中,在细胞组分本体中包括液泡、细胞骨架、细胞溶质、细胞核、线粒体、细胞外物质、核糖体、细胞质、细胞内薄膜组织.涉及7种分子功能,包括结构分子活性、RNA结合、蛋白质结合、DNA结合、金属离子结合、核苷酸结合、催化活性.参与7个生物学过程,包括细胞通讯、细胞发育、细胞分化、生化过程调控、细胞组织进程、刺激应答、代谢过程.

图6 条带1、条带2、条带3和条带4鉴定蛋白质在分子功能本体上的注释Fig.6 Molecular function ontology annotation of proteins identified from band 1,band 2,band 3 and band 4

图7 条带1、条带2、条带3和条带4鉴定蛋白质在生物过程本体上的注释Fig.7 Biological process ontology annotation of proteinsidentified from band 1,band 2,band 3 and band 4
图8 条带1、条带2、条带3和条带4鉴定蛋白质在细胞组分本体上的注释Fig.8 Cellular component ontology annotation of proteins identified from band 1,band 2,band 3 and band 4
经统计,这4个样品中鉴定到的蛋白质按分子功能归类时,涉及催化作用的蛋白质最多有24个;按细胞组分归类时,涉及细胞内膜组织的最多有20个;按生物学过程归类时,参与代谢过程的最多有24个.
3 讨论
植物雄性不育是一种遗传现象,具体表现为在有性繁殖过程中花药、花粉或雄配子功能丧失或产生的合子在正常环境下无法存活,在辣椒上不育的表现通常是花粉败育[15].在本研究中发现辣椒不育系花粉败育的细胞学机制可能是由于小孢子母细胞在减数分裂形成四分体后至单核花粉粒时期,四分体周围的胼胝体不能及时降解所致.在造孢细胞期,不育系和保持系特征相似,无明显差异,不育系小孢子败育发生在四分体小孢子形成后,由于绒毡层细胞发育异常,高度液泡化,径向异常膨大,四分体受到挤压后破裂并降解,无法形成正常的花粉粒而导致败育.这一发现与Horner[16]、逯红栋等[17]的观点基本一致.国内外有关辣椒胞质雄性不育系的细胞学比较研究结果不尽一致,这种不一致可能来自于CMS类型、核背景以及植株生长发育条件的差异[18].但无论雄性不育发生在小孢子发育的哪个阶段,都是由于绒毡层细胞的异常发育导致了小孢子的败育,均直接或间接与绒毡层的发育相关.
在辣椒雄性不育系中,可溶性蛋白质含量从蕾期开始逐渐降低,在其保持系中,蛋白质含量则呈逐渐升高的趋势,远高于不育系,推断不育系花药中可溶性蛋白的缺乏是由于来自绒毡层的物质运输被阻断而成为花粉发育的限制因素[19].本研究发现,与保持系相比,在不育系的6个不同发育时期均少了4条差异蛋白质条带.本研究对这4条蛋白质带进行了质谱鉴定,鉴定出不重复蛋白质64个.对这些蛋白质进行GO分类后发现,这些蛋白质中有相当一部分涉及花药的物质和能量代谢过程.这说明与保持系相比,不育系的花药中与物质和能量代谢相关的蛋白质表达量明显下降.这些蛋白质的表达量降低或不表达可能使得细胞供能不足、淀粉不能正常合成,导致花粉败育,也可能是由于不育基因导致过氧化物酶同工酶基因表达异常,扰乱了花药中物质和能量代谢平衡,最终导致雄性不育.
本次研究鉴定出的差异蛋白多为酶类和修饰蛋白(表1).其中,发现在不育系中磷酸烯醇式丙酮酸羧化酶(phosphoenolpyruvate carboxylase,PEPC,EC 4.1.1.31)的表达量明显下降.PEPC是一种广泛存在的细胞质酶,催化磷酸烯醇式丙酮酸(PEP)和HCO3-生成草酰乙酸(OAA),后者可转化生成三羧酸循环的多种中间产物[20-22].PEPC表达量下降或不表达可能使植株体内的三羧酸循环受阻,导致营养物质合成与代谢过程的紊乱,而使胼胝质不能正常解体,液泡过度膨大而导致花粉败育.另外,研究还发现,由于催化葡糖-1-磷酸(G-1-P)与葡糖-6-磷酸(G-6-P)之间可逆性转化的酶类[23]即葡糖磷酸变位酶(phosphoglucomutase,PGM,EC 2.7.5.1)的表达量下调,使得不育系细胞功能不足、淀粉和蔗糖不能正常合成.这也是导致花粉败育的可能原因之一.
4 结论
辣椒细胞质不育大多是花粉败育引起的,而花粉败育的细胞学表现为四分体形成以后,其周围的胼胝质不能解体以及绒毡层发育异常.通过对辣椒胞质雄性不育系和保持系的蛋白质组学差异分析发现,在四分体时期由于不育系花药中涉及物质和能量代谢的一部分蛋白表达量下降或许不表达,使绒毡层细胞发育异常,导致四分体小孢子在发育中不能获得物质和能量而败育.另外,在本次研究中蛋白质组学鉴定出的差异蛋白可初步解释细胞学的差异表现.这说明利用细胞学和蛋白质组学相结合来研究辣椒不育,特别是花粉败育导致辣椒不育,可以初步探讨与辣椒花粉败育相关的蛋白质,有利于今后深入研究这些相关蛋白在辣椒花粉败育中的功能,对揭示辣椒不育系中花粉败育的具体分子机制也有重要意义.
(References):
[1] 文李,刘盖,张再君,等.红莲型水稻细胞质雄性不育花药蛋白质组学初步分析.遗传,2006,28(3):311-316. Wen L,Liu G,Zhang Z J,et al.Preliminary proteomics analysis of the total proteins of HL type cytoplasmic male sterility rice anther.Hereditas,2006,28(3):311-316.(in Chinese with English abstract)
[2] 郭爽,常绍东,黄贞,等.辣椒雄性不育研究进展.辣椒杂志,2012(2):1-5. Guo S,Chang S D,Huang Z,et al.Progress in hot pepper male sterility research.Journal of China Capsicum,2012(2):1-5.(in Chinese with English abstract)
[3] 魏兵强,王兰兰,陈灵芝.辣椒胞质雄性不育转育模式的探讨.种子,2008,27(9):96-97. Wei B Q,Wang L L,Chen L Z.The study on transformation pattern of cytoplasmic male sterility in pepper.Seed,2008,27(9):96-97.(in Chinese with English abstract)
[4] Komatsu S,Konishi H,Shen S,et al.Rice proteomics:Astep toward functional analysis of the rice genome. Molecular&Cellular Proteomics,2003,2:2-10.
[5] Salekdeh G H,Komatsu S.Crop proteomics:Aim at sustainable agriculture of tomorrow.Proteomics,2007,7:2976-2996.
[6] Abbasi F M,Komatsu S.A proteomic approach to analyze salt responsive proteins in rice leaf sheath.Proteomics,2004,4:2072-2081.
[7] Kim D W,Rakwal R,Agrawal G K,et al.A hydroponic rice seedling culture model system for investigating proteome of salt stress in rice leaf.Electrophoresis,2005,26:4521-4539.
[8] Parker R,Flowers T J,Moore A L,et al.An accurate and reproducible method for proteome profiling of the effects of salt stress in the rice leaf lamina.Journal of Experimental Botany,2006,57:1109-1118.
[9] Caruso G,Cavaliere C,Guarino C,et al.Identification of changes in Triticum durum L.leaf proteome in response to salt stress by two-dimensional electrophoresis and MALDITOF mass spectrometry.Analytical and Bioanalytical Chemistry,2008,391:381-390.
[10] Askari H,Edqvist J,Hajheidari M,et al.Effects of salinity levels on proteome of Suaeda aegyptiaca leaves. Proteomics,2006,6:2542-2554.
[11] Srivastava S,Fristensky B,Kav N V.Constitutive expression of a PR10 protein enhances the germination of Brasica napus under saline conditions.Plant and Cell Physiology,2004,45:1320-1324.
[12] Aghaei K,Ehsanpour A A,Komatsu S.Proteome analysis of soybean hypocotyl and root under salt stress.Amino Acids,2009,36:91-98.
[13] 王勇,李彦卓,王伯阳,等.柞蚕胚胎发育期蛋白质的2DPAGE图谱分析.蚕业科学,2011,37(4):658-665. Wang Y,Li Y Z,Wang B Y,et al.2D-PAGE image analysis of proteins from developing embryos of antheraea pernyi,Science of Sericulture,2011,37(4):658-665.(in Chinese with English abstract)
[14] Shevchenko A,Wilm M,Vorm O,et al.Mass spectrometric sequencing of protein from sliver-stained polyacrylamide gels.Analytical Chemistry,1996,68(5):850-858.
[15] 韩璐,王晶,郑蕊.蛋白质组学在植物雄性不育研究中的应用.北方园艺,2011(18):206-208. Han L,Wang J,Zheng R.Application of proteomics to the plant male steriliy.Northern Horticulture,2011(18):206-208.(in Chinese with English abstract)
[16] Horner H.Microsporogenesis in normal and cytoplasmic male sterile pepper.American Jounal of Botany,1973,Suppl:60-64.
[17] 逯红栋,巩振辉,王晓敏,等.辣椒雄性不育材料小孢子发生的细胞形态学观察.西北植物学报,2006,26(9):1842-1845. Lu H D,Gong Z H,Wang X M,et al.Cytological studies on microsporogenesis of the male sterile lines of pepper.Acta Botanica Boreali-Occidentalia Sinica,2006,26(9):1842-1845.(in Chinese with English abstract)
[18] 李莹莹,魏佑营,张瑞华,等.辣椒雄性不育系与可育系小孢子发生的细胞学观察.植物研究,2006,26(4):411-415. Li Y Y,Wei Y Y,Zhang R H,et al.Cytological investigation on microsporogenesis of male sterility of pepper.Bulletin of Botanical Research,2006,26(4):411-415.(in Chinese with English abstract)
[19] 邓明华,文锦芬,邹学校,等.辣椒细胞质雄性不育系的物质代谢和过氧化物酶分析.云南农业大学学报,2007,22(6):791-794. Deng M H,Wen J F,Zou X X,et al.A study on material metabolism and peroxidase in cytoplasmic male-sterile lines and their maintainer lines of hot pepper.Journal of Yunnan Agricultural University,2007,22(6):791-794.(in Chinese with English abstract)
[20] Izui K,Matsumura H,Furumoto T,et al. Phosphoenolpyruvate carboxylase:A new era of structural biology.Annual Review of Plant Biology,2004,55:69-84.
[21] Gowik U,Westhoff P.C4-phosphoenolpyruvate carboxylase// Raghavendra A S,Sage R F.C4Photosynthesis and Related CO2Concentrating Mechanisms.Advances in Photosynthesis and Respiration.Dordrecht:Springer,2010:257-275.
[22] 魏绍巍,黎茵.植物磷酸烯醇式丙酮酸羧化酶的功能及其在基因工程中的应用.生物工程学报,2011,27(12):1702-1710. Wei S W,LI Y.Functions of plant phosphoenolpyruvate carboxylase and its applications for genetic engineering. Chinese Journal of Biotechnology,2011,27(12):1702-1710.(in Chinese with English abstract)
[23] 曹宛虹,王永健.植物中的葡糖磷酸变位酶.生物工程进展,2000,20(5):32-35. Cao W H,Wang Y J.Phosphoglucomutase in plants.China Biotechnology,2000,20(5):32-35.(in Chinese with English abstract)
Comparative analysis of cytology and proteomics in anthers between cytoplasmic male sterile line and its maintainer line of peppers.Journal of Zhejiang University(Agric.&Life Sci.),2015,41(1):44-55
LüXiaohan1,Fang Xianping2,Chai Weiguo1*,Ma Junping3,Zhou Yifei1(1.Institute of Vegetables,Hangzhou Academy of Agricultural Sciences,Hangzhou 310024,China;2.Institute of Biology,Hangzhou Academy of Agricultural Sciences,Hangzhou 310024,China;3.College of Agriculture and Biotechnology,Zhejiang University,Hangzhou 310058,China)
pepper;cytoplasmic male sterile;anther;cytology;proteomics
Q 246;Q 51;S 641.3
A
10.3785/j.issn.1008-9209.2014.04.242
浙江省重大科技专项(2011C02001);浙江省农业新品种选育重大科技专项(2012C12903).
柴伟国,E-mail:kuni@21.com
联系方式:吕晓菡,E-mail:13758280949@163.com
2014 04 24;接受日期(Accepted):2014 11 12;
日期(Published online):2015 01 19 URL:http://www.cnki.net/kcms/detail/33.1247.S.20150119.1653.004.html
