Abundance and Distribution of Fatty Acids in Sediments of the South Mid-Atlantic Ridge
2015-04-05HUANGXinZENGZhigangCHENShuaiYINXueboWANGXiaoyuanMAYaoYANGBaojuRONGKunboSHUYunchaoandJIANGTao
HUANG Xin, ZENG Zhigang, CHEN Shuai YIN Xuebo WANG Xiaoyuan MA Yao, YANG Baoju, RONG Kunbo, SHU Yunchao, and JIANG Tao
1)Key Laboratory of Marine Geology and Environment,Institute of Oceanology,Chinese Academy of Sciences,Qingdao266071,P.R.China
2)University of Chinese Academy of Sciences,Beijing100049,P.R.China
Abundance and Distribution of Fatty Acids in Sediments of the South Mid-Atlantic Ridge
HUANG Xin1),2), ZENG Zhigang1),*, CHEN Shuai1), YIN Xuebo1), WANG Xiaoyuan1), MA Yao1),2), YANG Baoju1),2), RONG Kunbo1),2), SHU Yunchao1),2), and JIANG Tao1),2)
1)Key Laboratory of Marine Geology and Environment,Institute of Oceanology,Chinese Academy of Sciences,Qingdao266071,P.R.China
2)University of Chinese Academy of Sciences,Beijing100049,P.R.China
Sediment samples obtained from the South Mid-Atlantic Ridge were studies by gas chromatography-mass spectrometer (GC-MS) for the abundance and distributions of total fatty acids (TFAs). Approximately 34 fatty acids were identified, with the chain-lengths ranging from C12to C30. The total concentrations of TFAs (∑TFA) ranged from 7.15 to 30.09 μg g-1dry sediment, and∑TFA was weakly correlated with bitumen content (R2= 0.69). The ∑TFA of samples around hydrothermal areas were significantly higher than that of samples away from hydrothermal areas, indicating intense primary production and large biomass in the hydrothermal areas, and suggesting a close relationship between hydrothermal activity and ∑TFA of samples. The characteristics of the TFA composition in the present study are rich in monounsaturated fatty acids and lacking in polyunsaturated fatty acids, and the ratios between the concentrations of monounsaturated fatty acids and ΣTFAs in samples close to the hydrothermal areas, are about 0.8, but for samples far from the hydrothermal areas, they are only about 0.5. Several fatty acids (e.g., a/iC15:0 and C16:1ω7), which are signature biomarkers for sulfur-metabolizing bacteria, show the same distribution trend as ∑TFA of samples, further highlighting the close relationship between fatty acid content and hydrothermal activity and/or hydrothermal communities. The metabolic activities of hydrothermal communities, especially those of microorganisms, are likely the main source of fatty acids in samples.
South Mid-Atlantic Ridge; sediment; fatty acids; hydrothermal activity; microorganism
1 Introduction
Chemosynthetic bacteria are major players in seafloor hydrothermal ecosystem, and are also the dominant source of organic matter around the seafloor hydrothermal vents (Yamanaka and Sakata, 2004). As the major constituents of cell membranes, fatty acids are closely associated with biological activity. The distinct structures of fatty acids make them useful as biomarkers of bacteria and other microorganisms (Bobbie and White, 1980; van Vleet and Quinn, 1979). Fatty acid analysis provides information about the surrounding ecological and environmental conditions.
Many researchers have investigated the fatty acids in sulfides (Leinet al., 2003; Simoneitet al., 2004; Liet al., 2011), rocks (Bassezet al., 2009), and sediments (Ohkouchi, 1995; Venkatesanet al., 2003; Yamanaka and Sakata, 2004; Morgunovaet al., 2012; Shulgaet al., 2012). Extensively investigated are also fatty acids of submarine hydrothermal organisms: tube worms (Pondet al., 2002), bivalves (Fouadet al., 1992; Pranalet al.,1997; Colacoet al., 2009), barnacles (Huanget al., 2013), gastropods (Saito and Hashimoto, 2010), shrimp (Pondet al., 1997, 2000; Saito, 2011), crabs (Saito, 2011), and fish (Guerreiroet al., 2004; Pondet al., 2008). Ohkouchi (1995) analyzed the concentration of fatty acids of sediments in the Central Pacific along 175°E from 48°N to 15°S, and discovered that the concentration of fatty acids of sediments was closely related to the latitude of sediments. The concentration of fatty acids of hydrothermal sediments and massive sulfide in Rainbow hydrothermal field were detected by Simoneitet al. (2004), and they affirmed the relationship between hydrothermal activity and TFAs. Liet al. (2011) detected the concentration of fatty acids of sulfide chimneys in the Main Endeavour segment of Juan de Fuca Ridge, and showed the distribution of fatty acid in the sulfide chimneys. However, there were few researches about organic matter in the South Mid-Atlantic Ridge, and we have not found the study of fatty acids in sediments affected by hydrothermal activity in this area.
In 2010-2012, hydrothermal sediments were obtained during the DY115-22 and DY115-26 cruises organized by the China Ocean Mineral Resources R&D Association (COMRA) in the South Mid-Atlantic Ridge. We meas-ured the abundance and distribution of total fatty acids of 10 sediment samples to understand the ecological and environmental conditions of this hydrothermal system, and obtain the influence of seafloor hydrothermal activities on the fatty acid in sediments.
2 Methods
2.1 Geologic Setting
The slow-spreading Atlantic Ridge, which accounts for about 40% of the total length of global mid-ocean ridge, stretches from 87°N (only about 330 km from the North Pole) to 54°S. By the Romanche trench near the equator, the Mid-Atlantic Ridge is divided into the North Mid-Atlantic Ridge and the South Mid-Atlantic Ridge. The South Mid-Atlantic Ridge turns to the Atlantic-Indian Ridge near 54°S, crosses the Crozet plateau, and continues eastwards to the Southwest Indian Ridge and the west to the Scotia Ridge.
In recent years, scientists have discovered several hydrothermal areas in the South Mid-Atlantic Ridge. In 2009, the DY115-21 cruise found two new hydrothermal areas between the 13°-14°S segments of the South Mid-Atlantic Ridge, and obtained hydrothermal sulfide chimney samples (Taoet al., 2011). In 2010-2012, DY115-22 and DY115-26 cruises continued to investigate the South Mid-Atlantic Ridge, and collected a variety of hydrothermal sulfides, sediment, and rocks. In this study, the sampling location (except 22V-TVG14) are centered in the mid-ocean ridge between 12° to 15°S of the South Mid-Atlantic Ridge (Fig.1), where the spreading rate is about 3.4 cm year-1(DeMetset al., 1994).
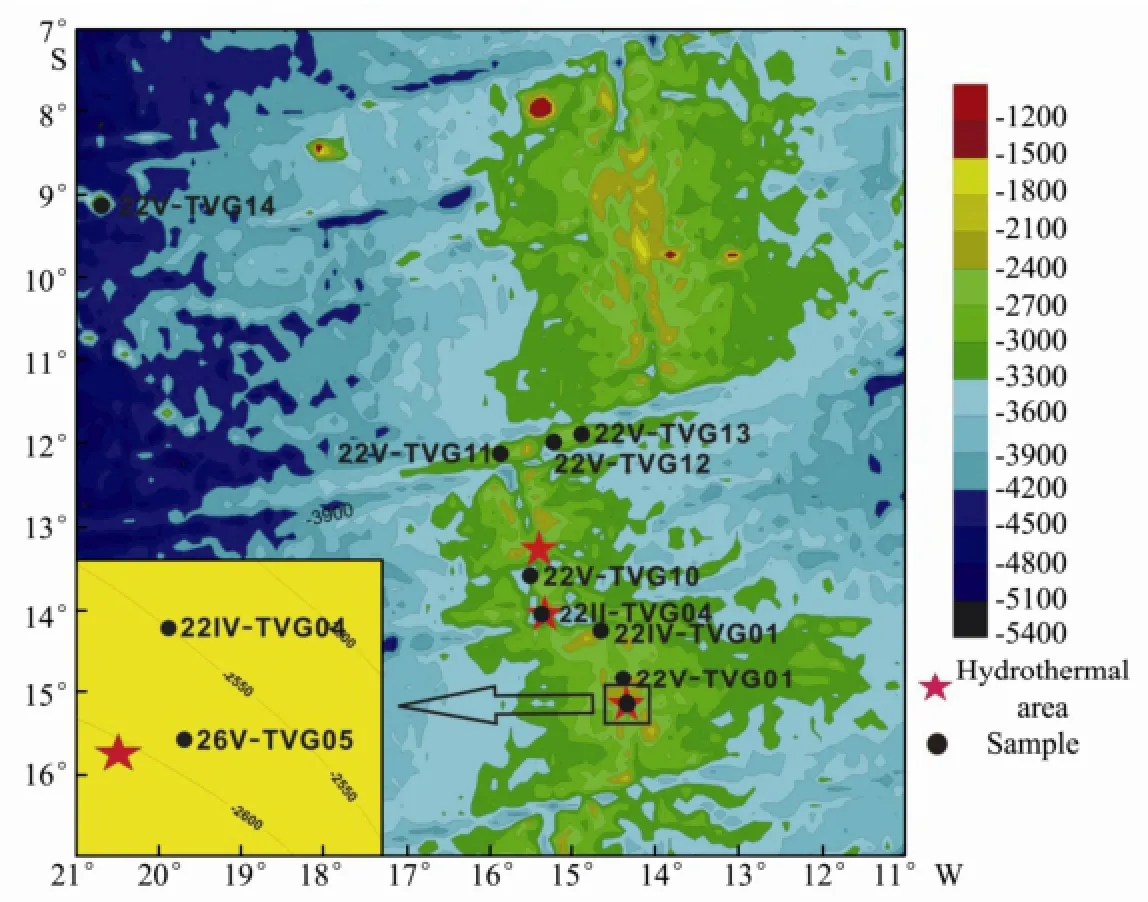
Fig.1 Sample collection site in the South Mid-Atlantic Ridge.
2.2 Sampling and Analyses
In 2010-2012, samples were collected by a TV-grab on the DY115-22 and DY115-26 cruises of R/VDa Yang Yi Haoconducted by COMRA in the South Mid-Atlantic Ridge. Sites, water depths and descriptions of samples are summarized in Table 1.
After collection, the samples were placed in bags and stored at -20℃ until analysis. About 200 g of sediment from each sample was placed into dry acid-clean glass beakers, and dried at for 48 h. The dried sediment was powdered in an agate mortar to 100 meshes and dried for 24 h.
The extraction and analysis of fatty acids was performed at the Lanzhou Center for Oil and Gas Resources, Institute of Geology and Geophysics, Chinese Academy of Sciences. The extraction process was as follows: bitumen were extracted by soxhlet extractor with chloroformfor 72 h. N-hexane was used to remove asphaltene and solubilize organic matter. Soluble organic matter was separated by column chromatography (silica-gel 60; i.d.:15 mm; length: 35 mm), and the acid fraction was eluted by methanol. After methyl esterification by BF3-MeOH, the acid fraction was analyzed by GC-MS.
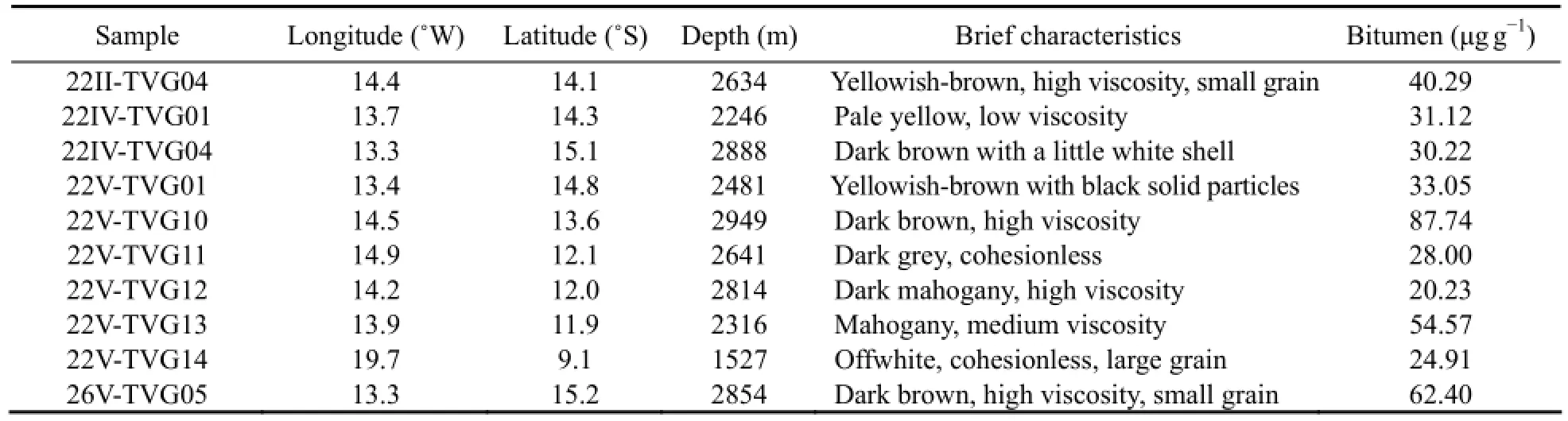
Table 1 Sample locations and principal characteristics
The gas chromatograph was a 6890N gas chromatography analyzer with a 30-m DB-5MS fused silica capillary column (i.d.: 0.2 mm; film thickness: 0.2 μm). The carrier gas was He. The GC temperature program used was as follows: injection at 80℃, 2 min isothermal; from 80 to 290℃ at 4℃ min-1; 20 min isothermal. The mass spectrometer (5973N) was operated in EI model at 70 eV.
3 Results
3.1 Total Concentrations of TFAs
Total concentrations of total fatty acids are given in Table 2. The value of ΣTFAs ranged from 7.15 to 30.09 μg g-1dry weight; sample 22V-TVG10 contained the highest fatty acid concentrations. The value of ΣTFAs in samples was in accord with that in the surface sediments in the Central Pacific along 175°E from 48°N to 15°S (1.82-23.8 μg g-1) (Ohkouchi, 1995). The concentration of bitumen and ΣTFA in samples were weakly correlated (R2= 0.69) (Fig.2).
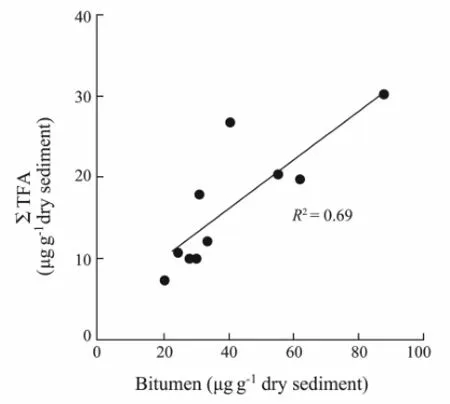
Fig.2 Relationship between the total concentrations of TFA (∑TFA) and bitumen concentration.
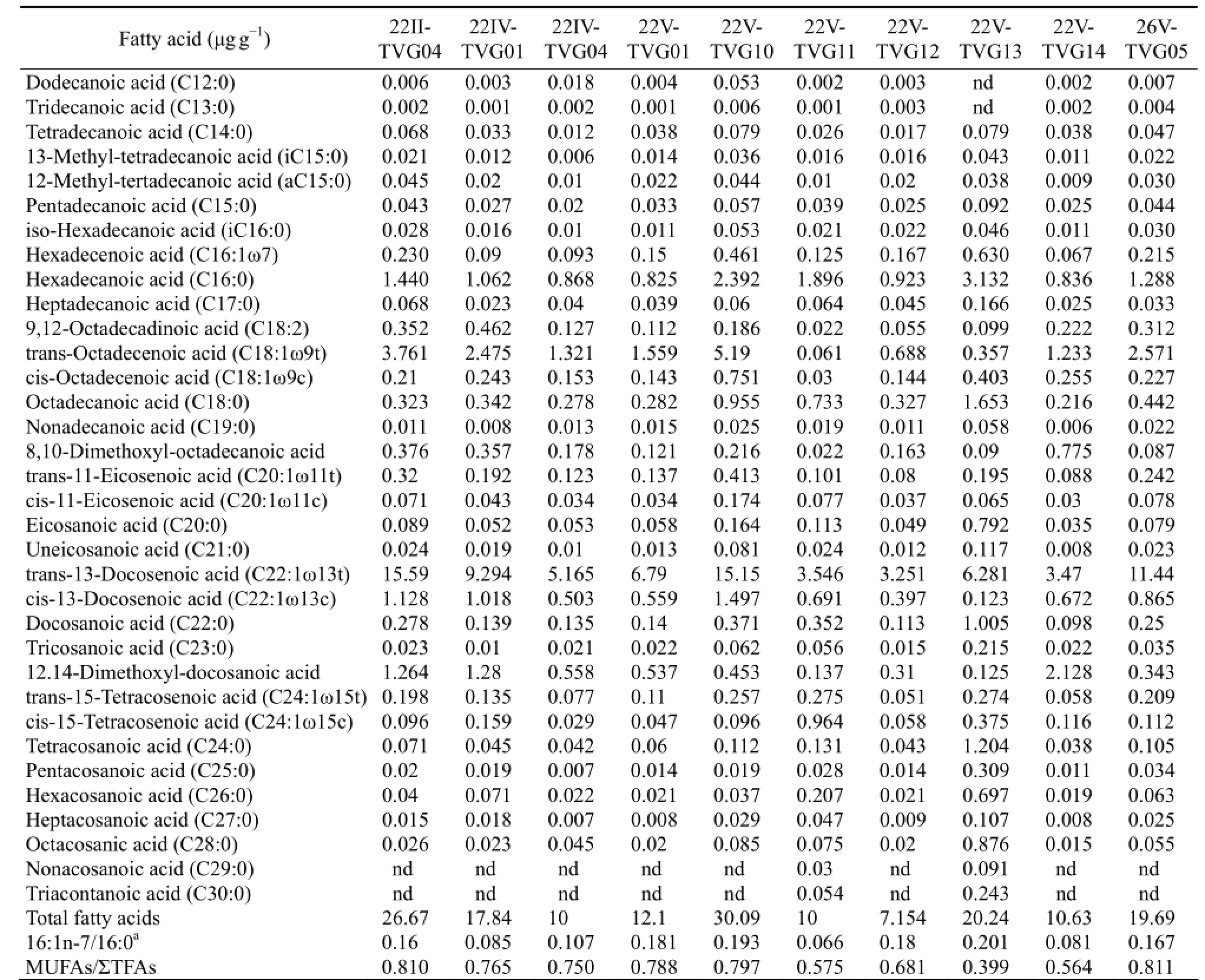
Table 2 Content of the total fatty acids in the sediments
3.2 Individual TFA Compositions
The composition and abundance of TFAs are given in Table 2. Approximately 34 fatty acids were identified, with chain-lengths ranging from C12to C30. The composition of TFAs are in accord with that of hydrothermal sediments of the western Pacific Ocean (Yamanaka and Sakata, 2004). The ratios between the sum of saturated high-molecular-weight fatty acids and the sum of saturated low-molecular-weight fatty acids [∑(〈C20)/ ∑(≥C20)] ranged from 0.34 to 0.54.
3.2.1 Saturated fatty acid
Saturated fatty acids were the most plentiful among fatty acids detected in the samples. Unbranched saturated fatty acids were the major component of saturated fatty acids in the samples, with the chain-lengths ranging from C12to C30. The dominant acid was hexadecanoic acid (C16:0), which constituted about 10%-30% of ΣTFA, followed by octadecanoic acid (C18:0). The majority of the saturated fatty acids were even in carbon chain length.
12-Methyl-tertadecanoic acid (aC15:0), 13-methyltetradecanoic acid (iC15:0) and iso-hexadecanoic acid (iC16:0) were all detected. 8,10-Dimethoxyl-octadecanoic acid and 12,14-dimethoxyl-docosanoic acid were also discovered in the samples with concentrations ranging from 1% to 10% of ΣTFA. Other branched fatty acid and cyclic fatty acid were not detected.
3.2.2 Unsaturated fatty acid
Unsaturated fatty acids detected in the samples were all even-carbon (16, 18, 20, 22 and 24) fatty acids without branched chains. Except 9,12-octadecadinoic acid (C18:2), unsaturated fatty acids were all monounsaturated fatty acids. The concentration of trans-13-docosenoic acid (C22:1ω13t), which was the highest among unsaturated fatty acids, was between 3.25 and 15.29 μg g-1dry weight, and about 50% of ΣTFA. The concentration of trans-octadecenoic acid (C18:1ω9t) was also high, and about 20-30% of ΣTFA. In addition, hexadecenoic acid (C16:1ω7), cis-octadecenoic acid (C18:1ω9c) and 9,12-octadecadinoic acid (C18:2) were all discovered in samples.
4 Discussion
ΣTFA is related to ecosystem productivity, and may be used as an indicator of biomass (Morris and Culkin, 1976). Samples 22V-TVG10, 26V-TVG05, 22V-TVG13 and 22II-TVG04 all contained high ΣTFA and high bitumen, indicating high biomass (Fig.3). The remaining samples (e.g. 22V-TVG14) were lower in ΣTFA and bitumen content, indicating lower biomass. Samples 22V-TVG10, 26V-TVG05 and 22II-TVG04 were located near the hydrothermal areas, suggesting a close relationship between high ΣTFA and hydrothermal activity (Fig.1). Moreover, high ΣTFA in 22V-TVG13 indicates that there may be an unknown hydrothermal source near this sampling location. However, several samples (e.g. 22IVTVG04) near the hydrothermal area have low ΣTFA, which may be related to the sedimentation rate, hydrothermal community size, hydrothermal plume altitude and sea water current direction (Braultet al., 1984). Braultet al. (1984) detected similar sample content in hydrothermal settings in EPR13°N.
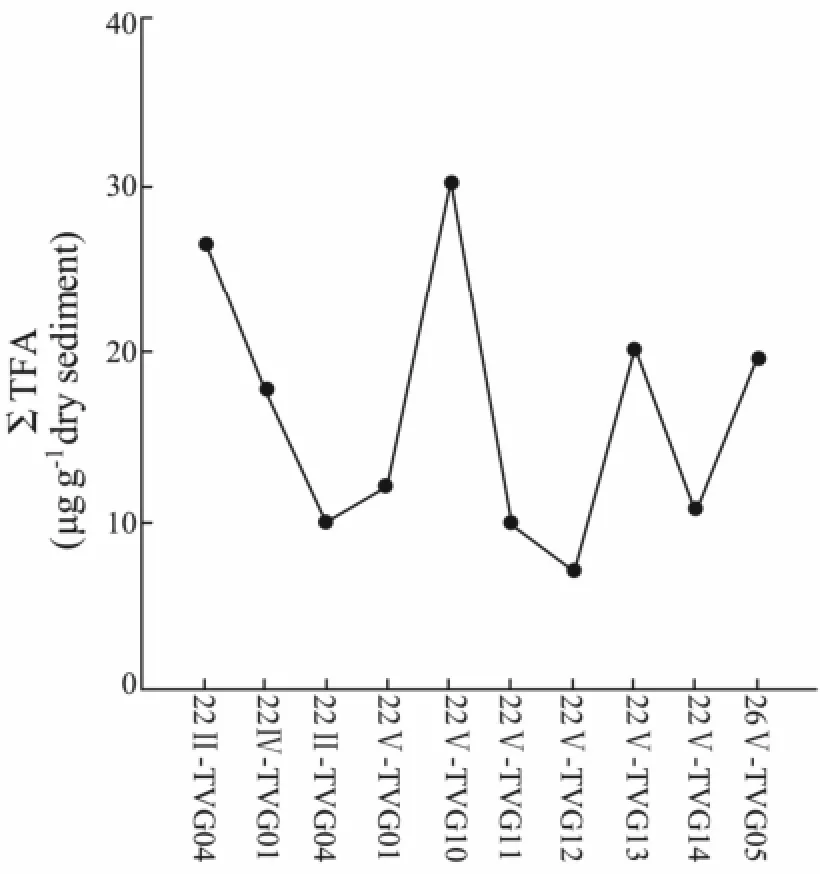
Fig.3 The abundance of ΣTFA in samples.
We did not detect polyunsaturated fatty acids, suggesting that the fatty acids in our samples were not derived from marine invertebrates or algae (Farringtonet al., 1973; Joseph, 1982; Simoneit, 1977). The characteristics of the TFAs composition in the present study, rich in monounsaturated fatty acids and lacking in polyunsaturated fatty acids, are common in the vent sediments and animal samples (Yamanaka and Sakata, 2004). The concentrations of monounsaturated fatty acids are high in samples, and the ratios between the concentrations of monounsaturated fatty acids and ΣTFAs in samples 22VTVG10, 26V-TVG05 and 22II-TVG04 collected near the hydrothermal areas, are about 0.8 (Table 2), which is in accord with the characteristics of the TFAs composition in the vent sediments and animal samples, indicating more influence of hydrothermal activity on these samples. However, the ratio from sample 22V-TVG14, which is far from the hydrothermal areas, is only about 0.5 (Table 2), indicating less influence of hydrothermal activity and being in accordance with above conclusion about ΣTFA.
Sulfur-based metabolic processes are important in the hydrothermal environment (Simoneit, 1977; Correet al., 2001; Brazeltonet al., 2006). High-temperature hydrothermal fluids contain hydrogen sulfide in concentrations several orders of magnitude higher than that of surrounding ambient seawater (Dinget al., 2001) and supply a critical substrate for chemosynthesis bacteria (McCollom and Shock, 1997; Van Dover, 2002). Sulfur metabolism supplies electron donors and acceptors for energy metabolism to the dense chemosynthetic-based vent ecosystems (McCollom and Shock, 1997). Some previous studies have shown a predominance of sulfate-reducing bacteria (SRB) and sulfur-oxidizing bacteria (SOB) in thehydrothermal systems (McCaffreyet al., 1989; Guezennec and Fiala-Medioni, 1996; Zhanget al., 2005).
Some individual fatty acids including iC16:0, a/iC15:0, and C18:1ω9c/t (Liet al., 2011; Edlundet al., 1985; Doωlinget al., 1986; Kohringet al., 1994) are summed as the biomarker of SRB in the present study (Table 2). From Fig.4, the concentrations of these fatty acids in samples 22V-TVG10, 26V-TVG05, 22II-TVG04 and 22V-TVG13 are higher than those in other samples, indicating more influence of SRB on fatty acids, and also suggesting more influence of hydrothermal activity on these samples.
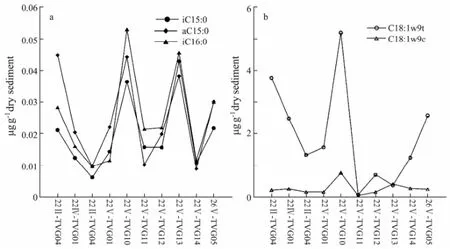
Fig.4 The abundance of several selected fatty acids.
Many previous studies have considered C16:1ω7 as the signature biomarker for SOB (Yamanaka and Sakata, 2004; Liet al., 2011; McCaffreyet al., 1989; Guezennec and Fiala-Medioni, 1996; Zhanget al., 2005; Liet al., 2007). The fatty acid C16:1ω7 was detected in all samples, indicating some contribution of SOB to fatty acids in samples. Yamanaka and Sakata (2004) reported the ratio of 16:1n-7/16:0 could be used to indicate the relative contribution of bacterial input and hydrothermal influence. The ratios of 16:1n-7/16:0 were larger in the samples of 22II-TVG04, 22V-TVG10, 22V-TVG13 and 26V-TVG05 than in other samples (Table 2), indicating a higher contribution of bacteria and more influence of hydrothermal activity, which is also in accord with the above conclusion.
In samples, we did not detect C18:1ω7, a common biomarker for SOB (Yamanaka and Sakata, 2004; Liet al., 2011; McCaffreyet al., 1989; Guezennec and Fiala-Medioni, 1996; Zhanget al., 2005; Liet al., 2007), and iC17:0, a common biomarker for SRB (Liet al., 2011; Edlundet al., 1985; Doωlinget al., 1986; Kohringet al., 1994) may be related to the species of microorganisms, and long time mixture of sediment and seawater. However, other biomarkers and their distribution make it difficult for us to ignore the contribution of microorganisms associated with sulfur metabolism (e.g., SRB and SOB) to the fatty acids in the samples.
5 Conclusion
The distribution of ΣTFA in these samples reflects intense biomass and biological activity. The TFAs composition in the present study, rich in monounsaturated fatty acids and lacking in polyunsaturated fatty acids, are in accord with most of the hydrothermal sediment in previous researches, indicating that the metabolic activities of hydrothermal communities (especially microbial metabolic activities) are likely to be the main source of fatty acids in samples. Microorganisms associated with sulfur metabolism play an important role in the abundance and distribution of fatty acids in these samples, which is not only reflected in the high concentration of biomarkers for microorganisms associated with the sulfur metabolism, but also reflected in the ratio of 16:1n-7/16:0 in samples. In conclusion, the influence of the hydrothermal activity and the microbial community are clear from the abundance and distribution of fatty acids in the sediment samples.
Acknowledgements
The authors would like to thank the crew of the COMRA cruises (DY115-22 and DY115-26) for their help with sampling operations, and thank Professor MENG Qianxiang for his help with sampling analysis. This work was supported by the National Key Basic Research Program of China (Grant No. 2013CB429700), National Special Fund for the 12th Five Year Plan of COMRA (Grant Nos. DY125-12-R-02, DY125-12-R-05, DY125- 11-R-05), National Natural Science Foundation of China (Grant Nos. 41325021, 40830849, 40976027), Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDA11030302), and the Shandong Province Natural Science Foundation of China for Distinguished Young Scholars (Grant No. JQ200913).
Bassez, M. P., Takano, Y., and Ohkouchi, N., 2009. Organic analysis of peridotite rocks from the Ashadze and Logatchev hydrothermal sites.International Jourmal of Molecular Sciences, 10: 2986-2998.
Bobbie, R. J., and White, D. C., 1980. Characterization of benthic microbial community structure by high-resolution gas chromatography of fatty acid methyl esters.Applied and Environmental Microbiology, 39: 1212-1222.
Brault, M., Marty, J. C., and Saliot, A., 1984. Fatty acids from particulate matter and sediment in hydrothermal environments from the east Pacific rise, near 13°N.Organic Geochemistry, 6: 217-222.
Brazelton, W., Schrenk, M., Kelley, D., and Barass, J., 2006. Methane and sulfur-metabolizing microbial communities dominate the lost city hydrothermal field ecosystem.Applied and Environmental Microbiology, 72: 6257-6270.
Colaco, A., Prieto, C., Martins, A., Figueiredo, M., Lafon, V., Monteiro, M., and Banadarra, N., 2009. Seasonal variations in lipid composition of the hydrothermal vent mussel Bathymodiolus azoricus from the Menez Gwen vent field.Marine Environmental Research, 67: 146-152.
Corre, E., Reysenbach, A. L., and Prieur, D., 2001. ε-Proteobacterial diversity from a deep-sea hydrothermal vent on the Mid-Atlantic Ridge.FEMS Microbiology Letters, 205: 329-335.
DeMets, C., Gordon, R. G., Argus, D. F., and Stein, S., 1994. Effect of recent revisions to the geomagnetic reversal time scale on estimate of current plate motions.Geophysical Research Letters, 21: 2191-2194.
Ding, K., Seyfried, W. E., Tivey, M. K., and Bradley, A. M., 2001.In situmeasurement of dissolved H2and H2S in high-temperature hydrothermal vent fluids at the Main Endeavour Field, Juan de Fuca Ridge.Earth and Planetary Science Letters, 186 (3-4): 417-425.
Dowling, N. J. E., Widdel, F., and White, D. C., 1986. Phospholipid ester-linked fatty acid biomarkers of acetateoxidizing sulphate-reducers and other sulphide-forming bacteria.Journal of General Microbiology, 132: 1815-1825.
Edlund, A., Nichols, P. D., Roffey, R., and White, D. C., 1985. Extractable and lipopolysaccharide fatty acid and hydroxyl acid profiles fromDesulfovibriospecies.Journal of Lipid Research, 26: 982-988.
Farrington, J. W., Quinn, J. G., and Davis, W. R., 1973. Fatty acid composition ofNephtys incisaandYoldia eimatula.Journal of the Fisheries Research Board of Canada, 30:181-185.
Fouad, B. M., Jean, C. M., and Aline, F. M., 1992. Fatty acid composition in deep hydrothermal vent symbiotic bivalves.Journal of Lipid Research, 33: 1797-1806.
Guerreiro, V., Narciso, L., Almeida, A. J., and Biscoito, M., 2004. Fatty acid profiles of deep-sea fishes from the Lucky Strike and Menez Gwen hydrothermal vent fields (Mid-Atlantic Ridge).Cybium, 28 (1): 33-44.
Guezennec, J., and Fiala-Medioni, A., 1996. Bacterial abundance and diversity in the Barbados Trench determined by phospholipids analysis.FEMS Microbiology Ecology, 19:83-93.
Huang, X., Zeng, Z., Chen, S., Yin, X., Wang, X., Zhao, H., Yang, B., Rong, K., and Ma, Y., 2013. Component characteristics of organic matter in hydrothermal barnacle shells from Southwest Indian Ridge.Acta OceanologicaSinica, 12: 60-67.
Joseph, J. D., 1982. Lipid composition of marine and estuarine invertebrates. Part II. Mollusca.Progress in Lipid Research, 21: 109-153.
Kohring, L. L., Ringelberg, D. B., Devereux, R., Stahl, D. A., Mittelman, M. W., and White, D. C., 1994. Comparison of phylogenetic relationship based on phospholipid fatty acid profiles and ribosomal RNA sequence similarities among dissimilatory sulfatereducing bacteria.FEMS Microbiology Letters, 119: 303-308.
Lein, A. Y., Peresypkin, V. I., and Simoneit, B. R. T., 2003. Origin of hydrocarbons in hydrothermal sulfide ores in the Mid-Atlantic Ridge.Lithology and Mineral Resources, 38 (5):383-393.
以关系到千家万户的粮食加工行业为例,在解决粮食供应问题后,人们的生活开始由温饱型向小康型逐步过渡。如小型砻谷机,碾米机和200型榨油机、“东方红”牌粮机和“双狮”牌砻谷胶辊等具有代表性的机械,开始被运用到米、面、油的深加工、精加工上来,以提高原料的利用率。
Li, J., Zhou, H., Peng, X., Fu, M., Chen, Z., and Yao, H., 2011. Abundance and distribution of fatty acids within the walls of an active deep-sea sulfide chimney.Journal of Sea Research, 65: 333-339.
Li, Y. L., Peacock, A. D., White, D. C., Geyer, R., and Zhang, C., 2007. Spatial patterns of bacterial signature biomarkers in marine sediments of the Gulf of Mexico.Chemical Geology, 238: 168-179.
McCaffrey, M. A., Farrington, J. W., and Repeta, D. J., 1989. Geochemical implications of the lipid composition ofThioplocaspp. from the Peru upwelling regions 15°S.Organic Geochemistry, 14: 61-68.
McCollom, T., and Shock, E. L., 1997. Geochemical constraints on chemolithoautotr-ophic metabolism by microorganisms in seafloor hydrothermal systems.Geochimica et Cosmochimica Acta, 61: 4375-4391.
Morgunova, I. P., Ivanov, V. N., Litvinenko, I. V., Petrova, V. I., Stepanova, T. V., and Cherkashev, G. A., 2012. Geochemistry of organic matter in bottom sediments of the ashadze hydrothermal field.Oceanology, 52 (3): 345-353.
Morris, R. J., and Culkin, F., 1976. Marine lipids: Analytical techniques and fatty acid ester analyses.Oceanography and Marine Biology, 14: 391-433.
Pond, D. W., Allen, C. E., Bell, M. V., Dover, C. L., Fallick, A. E., Dixon, D. R., and Sargent, J. R., 2002. Origins of long-chain polyunsaturated fatty acids in the hydrothermal vent wormsRidgea piscesaeandProtis hydrothermica.Marine Ecology Progress Series, 225: 219-226.
Pond, D. W., Dixon, D. R., Bell, M. V., Fallick, A. E., and Sargent, J. R., 1997. Occurrence of 16:2(n-4) and 18:2(n-4) fatty acids in the lipids of the hydrothermal vent shrimpsRimicaris exoculataandAlvinocaris markensis: Nutritional and trophic implications.Marine Ecology Progress Series, 156: 167-174.
Pond, D. W., Fallick, A. E., Stevens, C. J., Morridon, D. J., and Dixon, D. R., 2008. Vertebrate nutrition in a deep-sea hydrothermal vent ecosystem: Fatty acid and stable isotope evidence.Deep-Sea ResearchI, 55: 1718-1726.
Pond, D. W., Gebruk, A., Southward, E. C., Southward, A. J., Fallick, A. E., Michael, V. B., and Sargent, J. R., 2000. Unusual fatty acid composition of storage lipids in the bresilioid shrimpRimicaris exoculatacouples the photic zone with MAR hydrothermal vent sites.Marine Ecology Progress Series, 198: 171-173.
Pranal, V., Medioni, A. F., and Guezennec, J., 1997. Fatty acid characteristics in two symbiont-bearing mussels from deepsea hydrothermal vents of the south-western Pacific.Marine Biological Association of the UK, 77: 473-492.
Pranal, V., Medioni, A. F., and Guezennec, J., 1996. Fatty acid characteristics in two symbiotic gastropods from a deep hydrothermal vent of the West Pacific.Marine Ecology Progress Series, 142: 175-184.
Saito, H., and Hashimoto, J., 2010. Characteristics of the fatty acid composition of a deep-sea vent gastropod, ifremeria nautilei.Lipids, 45: 537-548.
Saito, H., 2011. Characteristics of fatty acid composition of the deep-sea vent crab,Shinkaia crosnieriBaba and Williams.Lipids, 46: 723-740.
Shulga, N. A., and Peresypkin, V. I., 2012. New data on the composition of organic matter in the hydrothermal deposits of the Mid-Atlantic Ridge (Broken Spur, Snake Pit, TAG).Doklady Earth Sciences, 444 (2): 773-775.
Simoneit, B. R. T., 1977. Organic matter in eolian dusts over the Atlantic Ocean.Marine Chemistry, 5: 443-464.
Simoneit, B. R. T., Lein, A. Y., Peresypkin, V. I., and Osipov, G. A., 2004. Composition and origin of hydrothermal petroleum and associated lipids in the sulfide deposits of the Rainbow Field (Mid-Atlantic Ridge at 36°N).Geochimica et Cosmochimica Acta, 68 (10): 2275-2294. Tao, C., Li, H., Yang, Y., Ni, J., Cui, R., Chen, Y., He, Y., Li, J., Huang, W., Lei, J., and Wang, Y., 2011. Two hydrothermal fields found on the southern Mid-Atlantic Ridge.Science China Earth Sciences, 54: 9.
Van Dover, C. L., 2000.The Ecology of Deep-Sea Hydrothermal Vents. Princeton University Press, Princeton, 424pp.
Van Vleet, E. S., and Quinn, J. G., 1979. Early diagenesis of fatty acids and isoprenoid alcohols in estuarine and coastal sediments.Geochimica et Cosmochimica Acta, 43: 289-303.
Venkatesan, M. I., Ruth, E., Rao, P. S., Nath, B. N., and Rao, B. R., 2003. Hydrothermal petroleum in the sediments of the Andaman Backarc Basin, Indian Ocean.Applied Geochemistry, 18: 845-861.
Yamanaka, T., and Sakata, S., 2004. Abundance and distribution of fatty acids in hydrothermal vent sediments of the western Pacific Ocean.Organic Geochemistry, 35: 573-582.
Zhang, C., Huang, Z., Cantu, J., Pancost, R. D., Brigmon, R. L., Lyons, T. W., and Sassen, R., 2005. Lipid biomarkers and carbon isotope signatures of a microbial (Beggiatoa) mat associated with gas hydrates in the Gulf of Mexico.Applied and Environmental Microbiology, 71: 2106-2112.
(Edited by Ji Dechun)
(Received March 5, 2014; revised April 18, 2014; accepted May 20, 2014)
© Ocean University of China, Science Press and Springer-Verlag Berlin Heidelberg 2015
* Corresponding author. E-mail: zgzeng@ms.qdio.ac.cn
猜你喜欢
杂志排行
Journal of Ocean University of China的其它文章
- Impacts of the Two Types of El Niño on Pacific Tropical Cyclone Activity
- Numerical Simulation of Typhoon Muifa (2011) Using a Coupled Ocean-Atmosphere-Wave-Sediment Transport (COAWST) Modeling System
- Estimating the Turbulence Characteristics in the Bottom Boundary Layer of Monterey Canyon
- Composition and Origin of Ferromanganese Crusts from Equatorial Western Pacific Seamounts
- Hydroelastic Analysis of a Very Large Floating Structure Edged with a Pair of Submerged Horizontal Plates
- A Storm Surge Intensity Classification Based on Extreme Water Level and Concomitant Wave Height
