绵羊胚胎发育中后期骨骼肌BTG2/3的表达动态及其与Myostatin的关系
2015-03-22刘瑞凿吴明明王慧华朱才业赵福平魏彩虹杜立新
刘瑞凿,刘 真,吴明明,2,王慧华,朱才业,张 莉,赵福平,魏彩虹*,杜立新*
绵羊胚胎发育中后期骨骼肌BTG2/3的表达动态及其与Myostatin的关系
刘瑞凿1,刘 真1,吴明明1,2,王慧华1,朱才业1,张 莉1,赵福平1,魏彩虹1*,杜立新1*
(1.中国农业科学院北京畜牧兽医研究所,北京 100193;2.中国农业大学动物科技学院,北京 100193)
旨在探究绵羊胚胎中后期骨骼肌BTG2/3(B cell translocation gene 2/3)的动态表达情况,探索肌肉生长抑制素(Myostatin,MSTN)基因对其表达的影响。以特克赛尔(Texel)和乌珠穆沁(Ujimqin)绵羊85、100、120和135 d 4个不同发育阶段的胎儿背最长肌为研究对象,利用Real-time PCR技术分别检测MSTN及BTG2/3的表达差异;选取100 d各胚胎半膜肌、背最长肌、半腱肌和股四头肌检测不同组织BTG2/3的表达差异;构建稳定慢病毒干扰载体pFU-GW-myostatin,分别感染处于增殖和分化阶段的绵羊成肌细胞,检测BTG2/3表达水平变化。结果显示,随着胚胎生长,特克赛尔羊胎儿MSTN表达量持续下降,而乌珠穆沁羊胎儿先下降后上升,特克赛尔羊胎儿BTG2表达量呈先升高后降低趋势,而乌珠穆沁羊表现出先降低后升高再降低的趋势;BTG3的表达量在特克赛尔羊与BTG2表达趋势相同,而乌珠穆沁羊则与BTG2相反;100 d胎儿4种不同骨骼肌组织中,特克赛尔羊与乌珠穆沁羊相比,背最长肌和半腱肌中BTG2表达量极显著偏高(P<0.01),而半膜肌和背最长肌中BTG3表达量极显著偏低(P<0.01);慢病毒干扰载体对成肌细胞具有较高的感染和干扰效率,增殖和分化阶段的成肌细胞被MSTN干扰后,BTG2和BTG3表达量极显著降低(P<0.01)。综上表明,BTG2和BTG3对绵羊胎儿中后期骨骼肌发育有重要作用,可能参与myostatin调节通路。本研究对绵羊胚胎发育过程的分子机制研究具有重要意义。
BTG2/3;特克赛尔;乌珠穆沁;胎儿;myostatin干扰
BTG/TOB家族被称为“APRO”,首先在哺乳动物被发现,在细胞生长中起到抗增殖的作用,影响细胞分化、发育和凋亡[1]。BTG/TOB家族成员(BTG1、Tob1/2、ANA、PC3B)的异常表达,造成细胞增殖受抑制[2-6]。BTG2和BTG3是BTG/TOB家族的重要成员,他们都能与CAF1和CCR4等相关因子结合[4,7],BTG2还可与PRMT1和HOXB9相互作用[8-9],而BTG3并未发现有此功能。
BTG2在细胞分裂G0/G1期开始表达[10],之后表达量下降。BTG2基因含有p53野生型基因的反应元件[11],p73和p53可诱导与人类具有同源性的BTG2基因[12]。利用高密度芯片技术对基因的表达趋势进行研究,发现BTG2基因对细胞的分化、增殖和凋亡起调节作用[13]。更多的研究证明PC3/BTG/TOB还共同参与了胚胎干细胞、肌细胞和造血干细胞等多种细胞的分化发育[14-15]。BTG3在G1期表达量最高,G1-S期表现出峰值[16]。BTG3表达缺失与肺癌的发生有关[17],也能增强BMP诱导的骨形成[18]。肌肉生长抑制素(Myostatin,MSTN) 基因是TGF-β超家族成员,是骨骼肌生长的负调控因子,为生长发育性状的候选基因。近期多项研究结果表明,BTG2与小尾寒羊多脊柱性状相关,BTG3对猪骨骼肌生长具有重要作用[19-21],但未见BTG/TOB家族与绵羊肌肉发育相关的报道。
胚胎期是动物生长发育的关键阶段,决定了其早期甚至成年期生长发育的表型性状。特克赛尔羊为引入肉用品种绵羊,具有典型的双肌表型;乌珠穆沁羊是肉脂兼用型本地品种绵羊。两品种生长发育性状存在明显差异,是研究绵羊肌肉生长发育的理想模型。本研究以特克赛尔羊和乌珠穆沁羊为研究对象,通过绵羊不同日龄阶段胎儿BTG2/3基因的表达趋势,分析其在绵羊肌肉发育的作用;并通过慢病毒载体下调MSTN基因表达,分析基因干扰对BTG2和BTG3在成肌细胞增殖和分化中的表达影响,初步揭示MSTN与BTG/TOB家族的关系,为BTG/TOB家族在绵羊肌肉发育的功能研究提供基础资料。
1 材料与方法
1.1 材料
特克赛尔羊(Texel)为山西省右玉县宏宇种羊场从国外引进原种肉羊;乌珠穆沁羊(Ujimqin)来自内蒙古自治区锡林郭勒乌珠穆沁羊业有限责任公司。上述羊场均为种养繁育场,实施肉羊标准化饲养,饲喂标准及环境基本一致。
Trizol购自Invitrogen公司(美国);DEPC水、反转录试剂盒购自TaKaRa公司(日本); DMEM/F12培养液、胎牛血清、0.25%胰酶、双抗和PBS购自Gibco公司(美国);I 型胶原酶(Sigma,美国);CO2培养箱(Thermo,美国);离心机(Eppendorf 5430R,德国);倒置显微镜(Nikon TE2000-U,日本);实时荧光定量PCR仪(ABI7500,美国);培养瓶(Corning,美国)。
1.2 方法
1.2.1 样品采集及RNA提取 分别在特克赛尔和乌珠穆沁母羊妊娠第85、100、120和135天进行剖腹产,各阶段分别取3只胚胎。立即取背最长肌于液氮中保存,用于不同阶段背最长肌RNA分析。100日龄各胚胎另取半膜肌、半腱肌和股四头肌于液氮中保存,用于不同组织RNA分析。
采用Trizol一步法提取总RNA。肌肉组织放入盛有液氮的研钵中充分研磨,与1 mL Trizol共同加入无RNA酶的1.5 mL离心管;细胞样先用1 mL PBS清洗两次,24孔板加入200 μL Trizol,反复吹打,使细胞溶解。之后按照生产商提供的方法提取。检测总RNA的质量及完整性。
1.2.2 成肌细胞的分离及分化培养 无菌条件取出背最长肌,70%酒精处理5~10 s;用组织镊及剪刀小心去除非肌肉组织,用剪刀将剥离的肌肉剪成约1 mm3的小块,加入2 g·L-1的I型胶原酶,37 ℃消化30 min。吹打均匀后,静止沉淀5 min,吸取上层细胞悬液置于离心管中,1 000 r·min-1离心5 min,弃上清;加入含20% FBS的DMEM/F12基础培养基(生长培养基),移液器吹打成单细胞悬液,移入培养瓶中,37 ℃ 5% CO2培养箱中培养,采用差速贴壁法进行细胞纯化。细胞接种于培养瓶1 h后,将未贴壁细胞移入另一培养瓶中进行培养,重复一次,则后贴壁培养的细胞为较纯的成肌细胞,24 h后进行首次换液,换液后可去血细胞与其他肌源细胞等。
处理成单细胞后按每孔1.0×106个细胞密度将肌细胞接种于6孔板,待细胞贴壁后,将基础培养基换为含2%马血清的DMEM/F12培养基(分化培养基),置于37 ℃,5% CO2培养箱中培养。一般48 h后会看到细胞形态变化,72 h后可看到明显肌管。
1.2.3MSTN慢病毒干扰载体及其感染方法 基于本实验室的前期报道[22],选用位点322设计绵羊MSTNsiRNA,利用靶位点设计序列,Sense:5′- TaaGACGATGACTACCACGTTACTCGAGTAA-CGTGGTAGTCATCGTCttTTTTTTC-3′,Antis-ense:5′-TCGAGAAAAAAaaGACGATGACTAC-CACGTTACTCGAGTAACGTGGTAGTCATCG-TCttA-3′,并由上海吉凯基因化学技术有限公司进行pFU-GW-myostatin慢病毒包装。
感染前24 h接种1×106个细胞于6孔培养板中。感染前弃去原有培养基,更换转染试剂(1 μL浓度为5 μg·mL-1的polybrene,10 μL滴度为109TU·mL-1的慢病毒载体,900 μL的增强转染试剂)。8~12 h以后观察细胞状态。如果细胞状态与未感染组无明显差异,表明慢病毒对细胞没有明显毒性作用,除去上清,更换为新鲜培养基培养。感染72 h后,观察荧光表达情况并收集细胞。
1.2.4 实时荧光定量PCR 用PrimeScript®1st Strand cDNA Synthesis Kit(TaKaRa,日本)试剂盒合成cDNA,试验操作按产品说明书进行。以cDNA为模板,引物参照本实验室前期设计的序列(表1),β-actin作为内参基因。ABI 7500荧光定量PCR仪进行qPCR试验,利用Power SYBR®Green PCR Master Mix试剂盒(ABI,美国)进行qPCR反应,每个样品设3个重复,反应体系为20 μL:2×SYBR Premix ExTaq10 μL,上下游引物(10 μmol·L-1)各0.4 μL, cDNA模板100 ng,加入灭菌蒸馏水至20 μL。反应条件:95 ℃预变性10 min;95 ℃变性15 s,60 ℃退火延伸30 s,40个循环。
表1 Real-time PCR引物序列
Table 1 Primer sequences for Real-time PCR analysis
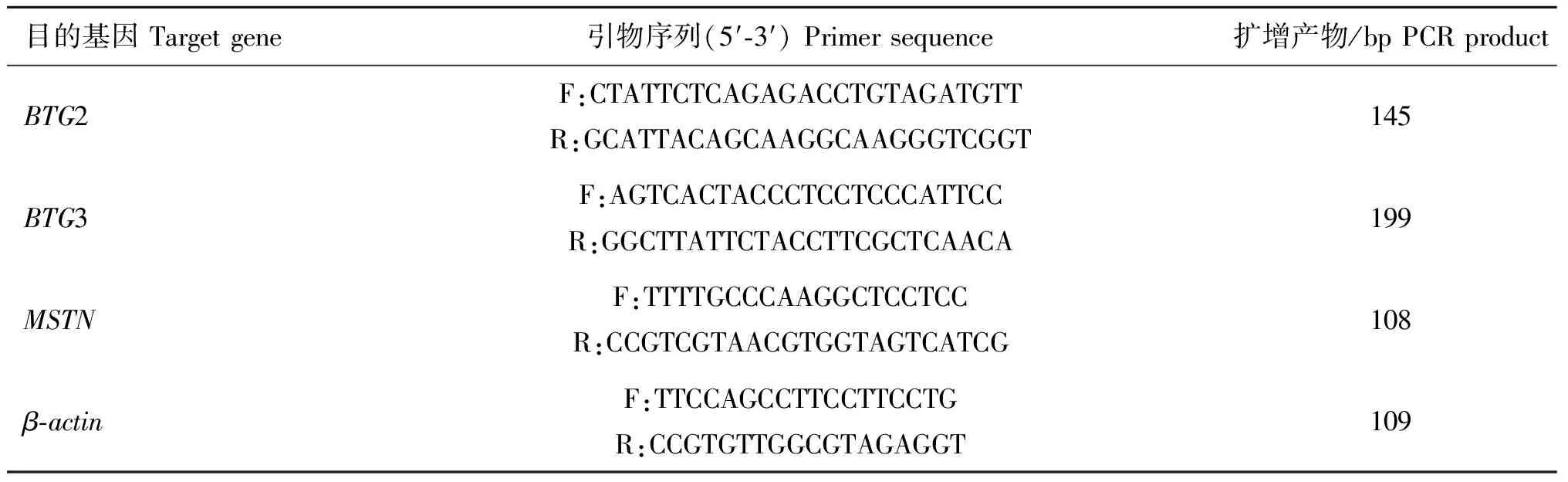
目的基因Targetgene引物序列(5′⁃3′)Primersequence扩增产物/bpPCRproductBTG2F:CTATTCTCAGAGACCTGTAGATGTTR:GCATTACAGCAAGGCAAGGGTCGGT145BTG3F:AGTCACTACCCTCCTCCCATTCCR:GGCTTATTCTACCTTCGCTCAACA199MSTNF:TTTTGCCCAAGGCTCCTCCR:CCGTCGTAACGTGGTAGTCATCG108β⁃actinF:TTCCAGCCTTCCTTCCTGR:CCGTGTTGGCGTAGAGGT109
1.2.5 数据分析 数据分析用SAS 9.2软件,单因素方差分析及t检验完成。
2 结 果
2.1 特克赛尔和乌珠穆沁羊不同日龄胎儿背最长肌MSTN、BTG2/3的表达趋势
用Trizol一步法提取肌肉组织总RNA,将提取的总RNA进行琼脂糖凝胶电泳分析,结果显示,28S、18S清晰可见,且28S条带亮度约为18S的两倍(图略)。经紫外分光光度仪测定,所提取总RNA的A260 nm/A280 nm值均为1.8~2.0,表明无蛋白和酚等污染,可用于后续试验。
乌珠穆沁和特克赛尔羊不同日龄胎儿的背最长肌MSTN和BTG2/3 的Real-time PCR检测结果显示,对于MSTN基因,特克赛尔羊胎儿MSTN表达量持续下降,而乌珠穆沁羊胎儿呈先下降后上升趋势,100 d表达量最低,且与特克赛尔羊差异显著(P<0.05)(图1A);对于BTG2基因,特克赛尔羊胎儿BTG2表达量先升高后降低,100 d表达量达到顶峰,135日龄表达最低;而乌珠穆沁羊胎儿85 d表达量最高,100 d出现第一次低谷,120 d有另一个高峰,出生前即135 d表达量最低(图1B);对于BTG3基因,特克赛尔羊胎儿表现出与BTG2相同的趋势,乌珠穆沁羊胎儿表现出与BTG2相反的趋势——100 d表达最高,85及120 d表达最低(图1C)。
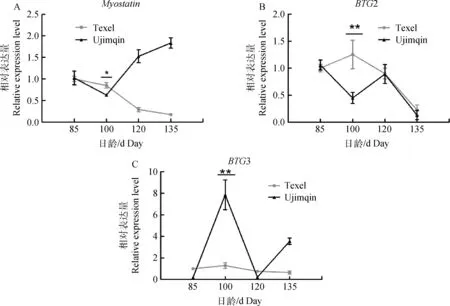
A.背最长肌MSTN表达量变化;B.背最长肌BTG2表达量变化;C.背最长肌BTG3表达量变化。*代表差异显著(P<0.05),** 代表差异极显著(P<0.01)。下同A.Changes for expression of MSTN;B.Changes for expression of BTG2;C.Changes for expression of BTG3.Analysis of variance was performed and significant variations are indicated by the *(P<0.05) and **(P<0.01).The same as below图1 特克赛尔和乌珠穆沁羊不同日龄胎儿背最长肌MSTN、BTG2和BTG3的表达趋势Fig.1 Expression of MSTN,BTG2 and BTG3 in longissimus dorsi at different fetal stages in Texel and Ujimqin
2.2 特克赛尔和乌珠穆沁羊100日龄胎儿不同骨骼肌中BTG2/3的差异表达
乌珠穆沁和特克赛尔羊胎儿100 d不同骨骼肌组织BTG2/3 Real-time PCR检测结果显示,对于BTG2基因,特克赛尔羊背最长肌和半腱肌的相对表达量极显著高于乌珠穆沁羊(P<0.01),而半膜肌荧光显微镜观察结果显示,细胞绿色荧光表达效率在95%以上,且传代后仍有高强度荧光表达,观察和股四头肌两种绵羊差异不显著(图2A);对于BTG3基因,乌珠穆沁羊背最长肌和半膜肌的相对表达量极显著高于特克赛尔羊(P<0.01),而半腱肌和股四头肌两种绵羊差异不显著(图2B)。
2.3 慢病毒载体干扰效果验证
MSTN慢病毒干扰载体转染成肌细胞72 h后,转染前后成肌细胞形态,没有发生明显变化。
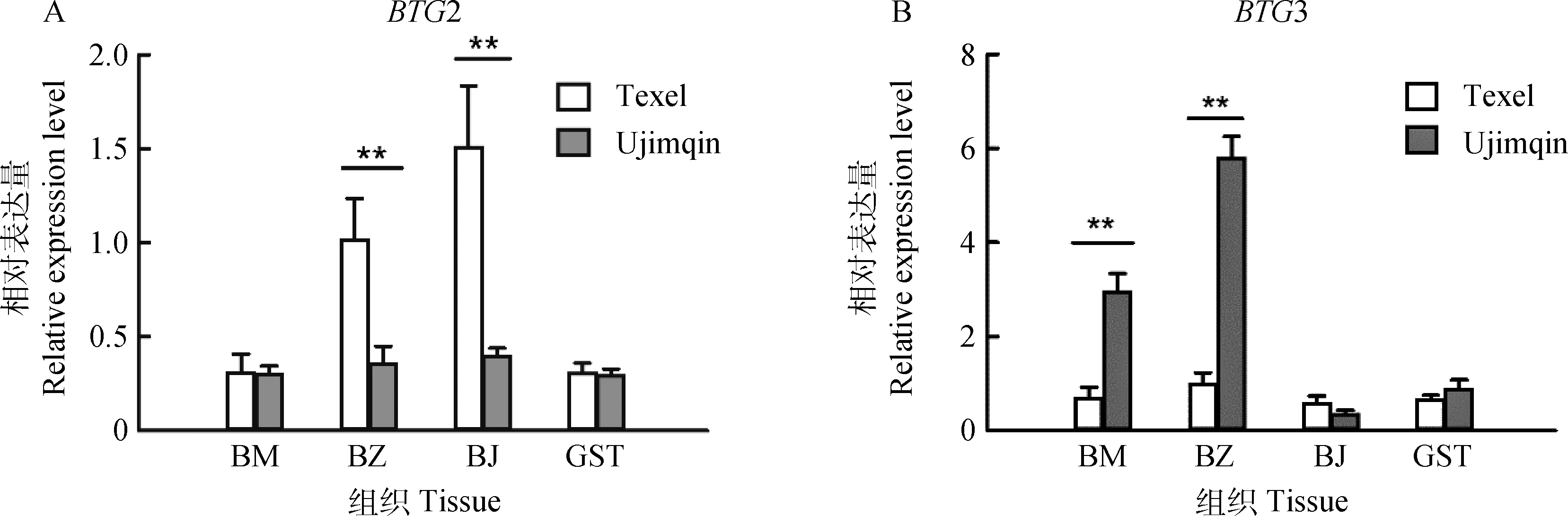
BM.半膜肌;BZ.背最长肌;BJ.半腱肌;GST.股四头肌BM.Semimembranosus;BZ.Longissimus dorsi;BJ.Semitendinosus;GST.Quadriceps femoris图2 特克赛尔和乌珠穆沁羊 100日龄胎儿不同骨骼肌BTG2、BTG3差异表达Fig.2 Expression of BTG2 and BTG3 in the skeletal muscle at fetal 100 d of age in Texel and Ujimqin
增殖和分化阶段感染成肌细胞,MSTNqPCR检测结果显示,增殖和分化阶段感染后的细胞MSTN表达量极显著低于空白对照(P<0.01),且增殖阶段MSTN表达量约4倍于分化阶段,差异极显著(P<0.01)(图3),说明成肌分化过程抑制MSTN表达,与前期研究相符[23]。
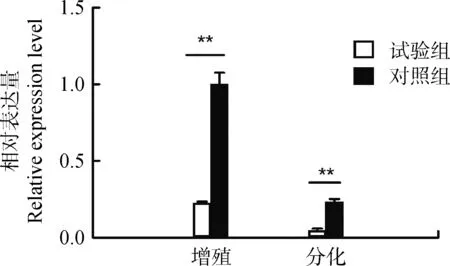
图3 慢病毒干扰MSTN后差异表达Fig.3 Expression analysis of MSTN after transfected by lentiviral vectors
2.4MSTN干扰对BTG2、BTG3表达的影响
成肌细胞增殖和分化阶段进行慢病毒感染,BTG2/3 Real-time PCR检测结果表明,与空白对照相比,增殖阶段MSTN干扰后,BTG2和BTG3表达量均下调,差异极显著(P<0.01);分化阶段MSTN干扰后,BTG2和BTG3表达量同样降低,差异极显著(P<0.01)(图4);且两基因增殖阶段表达量极显著高于分化阶段(P<0.01)。结果提示MSTN会直接或间接促进BTG2和BTG3表达。
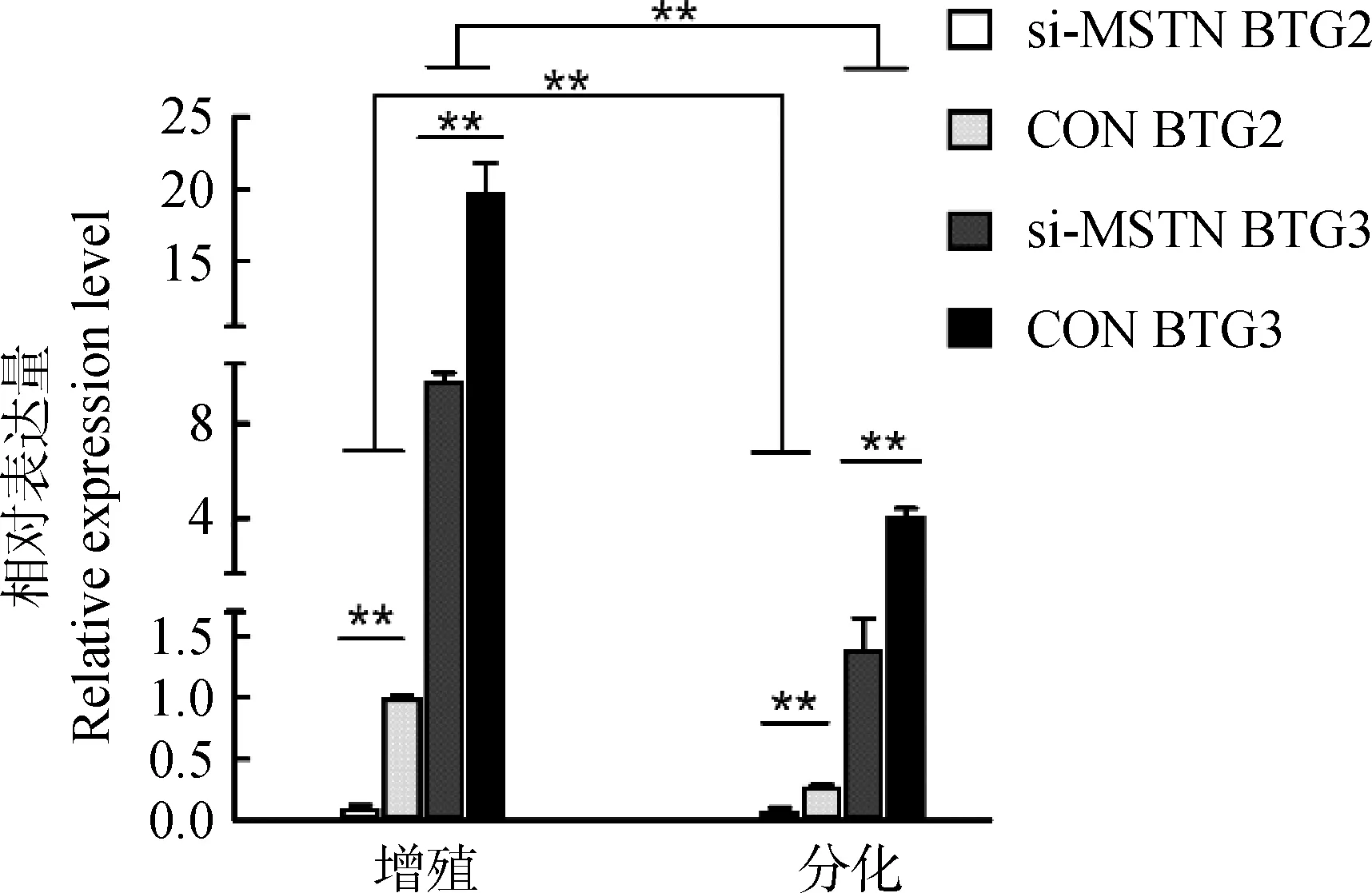
si-MSTN BTG2.MSTN干扰后BTG2表达量;CON BTG2.对照组BTG2表达量;si-MSTN BTG3.MSTN干扰后BTG3表达量;CON BTG3.对照组BTG3表达量si-MSTN BTG2.BTG2 expression level after MSTN interference;CON BTG2.BTG2 expression level after control;si-MSTN BTG3.BTG3 expression level after MSTN interference;CON BTG3.BTG3 expression level of control图4 MSTN干扰对BTG2、BTG3影响Fig.4 Impact of MSTN interference on BTG2 and BTG3
3 讨 论
BTG/Tob家族成员可通过正向或负向调节多种信号通路作用于细胞周期循环,实现抗增殖特性[24];多种信号通路也可以靶向BTG/Tob因子,影响其表达、稳定性、活性甚至蛋白定位[1]。目前,对BTG/Tob家族的了解主要集中在癌症发生。视黄酸处理乳腺癌细胞后,引起BTG2表达活性升高,进而通过降低CDK4活性导致癌细胞周期异常[25];肾癌组织中由于DNA甲基化引起BTG3启动子失活,导致其表达量降低[26]。MSTN基因是TGF-β超家族成员,骨骼肌生长的负调控因子。在MSTN的作用下,成肌细胞的细胞周期聚集在G0/G1阶段从而停止生长[27-28]。迄今为止,已经发现绵羊、牛、狗、小鼠及德国一名儿童携带有遗传突变而功能失活的MSTN基因,产生肌肉急剧增加的双肌表型[29-33]。近年来多项研究表明,BTG2/3与肌肉发育有关,但其间关系及表达调控机制仍知之甚少。本研究检测BTG2/3基因在绵羊胚胎发育中后期骨骼肌表达模式,并分析其在特克赛尔及乌珠穆沁羊两品种的差异表达;为了揭示BTG2/3在胚胎骨骼肌作用,利用慢病毒感染方法探讨BTG2/3与MSTN之间的关系。
特克赛尔羊为引入肉用品种羊,具有典型的双肌表型;乌珠穆沁羊原产内蒙古,属肉脂兼用型本地品种羊[34]。胚胎期各日龄阶段特克赛尔羊背最长肌重量显著大于乌珠穆沁羊,且120日龄显著高于100日龄[35]。MSTN表达水平结果显示,特克赛尔羊随胎儿日龄增加而持续下降(图1A),乌珠穆沁羊随胎儿日龄呈先下降后上升趋势,100 d表达量最低,且与特克赛尔羊差异显著(P<0.05),提示两品种绵羊胎儿背最长肌重量可能受到MSTN调控,胎儿100 日龄可能是绵羊胎儿发育的重要时期。特克赛尔羊的BTG2表达丰度在胎儿100 d出现峰值,随后表达下调,而乌珠穆沁羊100 d出现低谷,与特克赛尔羊差异极显著(P<0.01),其他日龄阶段差异不显著(图1B)。特克赛尔羊的BTG3表达丰度同样在胎儿100 d出现高峰,有趣的是乌珠穆沁羊相同阶段表达量也出现高峰,且5倍于特克赛尔羊表达水平,表现出与BTG2完全相反的趋势。特克赛尔羊胚胎期各日龄阶段肌纤维密度连续下降,乌珠穆沁羊呈先上升后下降趋势,100日龄具有折点[36],提示两品种绵羊纤维密度的变化可能与BTG2/3的共同调节密切相关。X.L.Ren等研究结果显示,BTG3可能与癌细胞的入侵和迁移相关,下调BTG3促进细胞增殖[37]。提示BTG3可能与乌珠穆沁羊胚胎中后期生长特性有关。
背最长肌不同发育阶段BTG2和BTG3表达检测可知,绵羊胎儿100 d两基因表达差异极显著(P<0.01),可选择此阶段不同骨骼肌组织表达型进行检测。结果显示,BTG2基因在特克赛尔羊背最长肌和半腱肌表达水平极显著高于乌珠穆沁羊(P<0.01),而BTG3基因在乌珠穆沁羊背最长肌和半膜肌表达量极显著高于特克赛尔羊(P<0.01),两基因在股四头肌表达差异不显著。研究发现,BTG2/3抑制细胞循环G1/S期[38],胚胎发育期的结果表明不同骨骼肌发育机制有差异,BTG2和BTG3在两品种骨骼肌中发挥不同功能,BTG2对特克赛尔羊肌细胞的生长有较大作用;BTG3可能在乌珠穆沁羊胚胎骨骼肌发育中具有更重要作用。
作为一种重要的研究工具,慢病毒载体免疫原性低,能感染分裂相和非分裂相细胞,将自身携带片段整合入宿主细胞基因组,起到稳定表达效果[39-40]。pFU-GW-RNAi 慢病毒载体具有绿色荧光标记,对绵羊成肌细胞感染效率达95%以上。试验组绵羊成肌细胞MSTN基因表达量在增殖和分化阶段极显著降低(P<0.01),说明慢病毒干扰载体pFU-GW-myostatin对MSTN具有明显沉默效果;且增殖阶段MSTN表达量高于分化阶段(图3),这与MSTN抑制成肌细胞分化有关[27]。MSTN表达下调后,增殖和分化细胞BTG2和BTG3基因表达量较对照组极显著降低(P<0.01),表明两基因不但在成肌细胞增殖阶段,也在分化阶段与细胞发育密切相关。与MSTN相同,两基因在增殖阶段表达量极显著高于分化阶段(P<0.01),说明BTG2和BTG3可能主要作用于成肌细胞增殖阶段,抑制细胞分化。MSTN可能通过BTG/Tob家族成员抑制绵羊成肌分化。
100 d胚胎期,乌珠穆沁羊BTG2表达趋势与MSTN相同(图1A,B),成肌细胞MSTN干扰后BTG2表达量极显著降低,说明两基因具有正向调控机制,共同抑制绵羊胚胎期骨骼肌生长。乌珠穆沁羊背最长肌BTG3表达量极显著高于特克赛尔羊,且表现出与BTG2和MSTN相反的趋势,而成肌细胞MSTN干扰后BTG3表达量也极显著降低,提示BTG3可能受到MSTN正向调控,但机体内可能受到多种因子调控,二者之间相互关系及相互作用机制可通过免疫共沉淀等方法进一步研究。
4 结 论
本研究检测了BTG2/3在特克赛尔和乌珠穆沁绵羊胚胎中后期背最长肌的表达模式、分析了BTG2/3在特克赛尔和乌珠穆沁羊胎儿100 d 4种不同骨骼肌中的表达差异,发现BTG2/3在绵羊胎儿中后期背最长肌不同发育阶段表达量具有显著差异。另外利用基因干扰技术验证了BTG2/3可能参与Myostatin信号通路。本研究对绵羊胚胎发育的分子机制及不同品种绵羊胚胎发育的分子水平差异具有重要意义,且对BTG2/3在绵羊胚胎期发育作用具有重要补充。
[1] MAUXION F,CHEN C Y,SERAPHIN B,et al.Btg/Tob factors impact deadenylases[J].TrendsBiochemSci,2009,34(12):640-647.
[2] MATSUDA S,KAWAMURA-TSUZUKU J,OHSUGI M,et al.Tob,a novel protein that interacts with p185erbb2,is associated with anti-proliferative activity[J].Oncogene,1996,12(4):705-713.
[3] YOSHIDA Y,MATSUDA S,IKEMATSU N,et al.Ana,a novel member of tob/btg1 family,is expressed in the ventricular zone of the developing central nervous system[J].Oncogene,1998,16(20):2687-2693.
[4] IKEMATSU N,YOSHIDA Y,KAWAMURA-TSUZUKU J,et al.Tob2,a novel anti-proliferative Tob/Btg1 family member,associates with a component of the Ccr4 transcriptional regulatory complex capable of binding cyclin-dependent kinases[J].Oncogene,1999,18(52):7432-7441.
[5] BUANNE P,CORRENTE G,MICHELI L,et al.Cloning of Pc3b,a novel member of the Pc3/Btg/Tob family of growth inhibitory genes,highly expressed in the olfactory epithelium[J].Genomics,2000,68(3):253-263.
[6] ROUAULT J P,RIMOKH R,TESSA C,et al.Btg1,a member of a new family of antiproliferative genes[J].EMBOJ,1992,11(4):1663-1670.
[7] ROUAULT J P,PREVOT D,BERTHET C,et al.Interaction of Btg1 and p53-regulated Btg2 gene products with Mcaf1,the murine homolog of a component of the yeast Ccr4 transcriptional regulatory complex[J].JBiolChem,1998,273(35):22563-22569.
[8] LIN W J,GARY J D,YANG M C,et al.The mammalian immediate-early Tis21 protein and the leukemia-associated Btg1 protein interact with a protein-arginine N-methyltransferase[J].JBiolChem,1996,271(25):15034-15044.
[9] PREVOT D,VOELTZEL T,BIROT A M,et al.The leukemia-associated protein Btg1 and the p53-regulated protein Btg2 interact with the homeoprotein Hoxb9 and enhance its transcriptional activation[J].JBiolChem,2000,275(1):147-153.
[10] ROUAULT J P,FALETTE N,GUEHENNEUX F,et al.Identification of Btg2,an antiproliferative p53-dependent component of the dna damage cellular response pathway[J].NatGenet,1996,14(4):482-486.
[11] DURIEZ C,FALETTE N,AUDOYNAUD C,et al.The human Btg2/Tis21/Pc3 gene:genomic structure,transcriptional regulation and evaluation as a candidate tumor suppressor gene[J].Gene,2002,282(1-2):207-214.
[12] ZHU J,JIANG J,ZHOU W,et al.The potential tumor suppressor p73 differentially regulates cellular p53 target genes[J].CancerRes,1998,58(22):5061-5065.
[13] 韩立霞,孙少华,白瑞景,等.B细胞异位基因2(Btg2)多态性与河北小尾寒羊多脊椎性状的关联分析[J].农业生物技术学报,2010,18(1):81-86. HAN L X,SUN S H,BAI R J,et al.Association between polymorphism of B-cell translocation gene 2(Btg2) and multi-vertebrae traits of Hebei small tail han sheep[J].JournalofAgriculturalBiotechnology,2010,18(1):81-86.(in Chinese)
[14] KAMAID A,GIRALDEZ F.Btg1 and Btg2 gene expression during early chick development[J].DevDyn,2008,237(8):2158-2169.
[15] GUARDAVACCARO D,CORRENTE G,COVONE F,et al.Arrest of G(1)-S progression by the p53-inducible gene Pc3 is rb dependent and relies on the inhibition of cyclin D1 transcription[J].MolCellBiol,2000,20(5):1797-1815.
[16] RODIER A,MARCHAL-VICTORION S,ROCHARD P,et al.Btg1:a triiodothyronine target involved in the myogenic influence of the hormone[J].ExpCellRes,1999,249(2):337-348.
[17] YONEDA M,SUZUKI T,NAKAMURA T,et al.Deficiency of antiproliferative family protein ana correlates with development of lung adenocarcinoma[J].CancerSci,2009,100(2):225-232.
[18] MIYAI K,YONEDA M,HASEGAWA U,et al.Ana deficiency enhances bone morphogenetic protein-induced ectopic bone formation via transcriptional events[J].JBiolChem,2009,284(16):10593-10600.
[19] MATSUDA S,ROUAULT J,MAGAUD J,et al.In search of a function for the Tis21/Pc3/Btg1/Tob family[J].FEBSLett,2001,497(2-3):67-72.
[20] FENG Z,TANG Z L,LI K,et al.Molecular characterization of the Btg2 and Btg3 genes in fetal muscle development of pigs[J].Gene,2007,403(1-2):170-177.[21] TANG Z,LI Y,WAN P,et al.Longsage analysis of skeletal muscle at three prenatal stages in tongcheng and landrace pigs[J].GenomeBiol,2007,8(6):R115.
[22] LU J,SUN D,XU L,et al.Selection of an effective small interference RNA to silence myostatin gene expression in sheep fibroblast cells[J].BiochemGenet,2012,50(11-12):838-847.
[23] 魏彩虹,王 皓,杜立新,等.乌珠穆沁羊成肌细胞的诱导分化及相关基因表达[J].农业生物技术学报,2012,20(3):283-288. WEI C H,WANG H,DU L X,et al.Induced differentiation and related gene expression of Ujumqin sheep myoblast cellsinvitro[J].JournalofAgriculturalBiotechnology,2012,20(3):283-288.(in Chinese)
[24] WINKLER G S.The mammalian anti-proliferative Btg/Tob protein family[J].JCellPhysiol,2010,222(1):66-72.
[25] DONATO L J,SUH J H,NOY N.Suppression of mammary carcinoma cell growth by retinoic acid:the cell cycle control gene Btg2 is a direct target for retinoic acid receptor signaling[J].CancerRes,2007,67(2):609-615.
[26] MAJID S,DAR A A,AHMAD A E,et al.Btg3 tumor suppressor gene promoter demethylation,histone modification and cell cycle arrest by genistein in renal cancer[J].Carcinogenesis,2009,30(4):662-670.
[27] LANGLEY B,THOMAS M,BISHOP A,et al.Myostatin inhibits myoblast differentiation by down-regulating MyoD expression[J].JBiolChem,2002,277(51):49831-49840.
[28] JOULIA D,BERNARDI H,GARANDEL V,et al.Mechanisms involved in the inhibition of myoblast proliferation and differentiation by myostatin[J].ExpCellRes,2003,286(2):263-275.
[29] SCHUELKE M,WAGNER K R,STOLZ L E,et al.Myostatin mutation associated with gross muscle hypertrophy in a child[J].NEnglJMed,2004,350(26):2682-2688.
[30] MCPHERRON A C,LAWLER A M,LEE S J.Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member[J].Nature,1997,387(6628):83-90.
[31] JOHNSON P L,MCEWAN J C,DODDS K G,et al.A directed search in the region of GDF8 for quantitative trait loci affecting carcass traits in texel sheep[J].JAnimSci,2005,83(9):1988-2000.
[32] MOSHER D S,QUIGNON P,BUSTAMANTE C D,et al.A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs[J].PLoSGenet,2007,3(5):e79.
[33] GROBET L,MARTIN L J,PONCELET D,et al.A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle[J].NatGenet,1997,17(1):71-74.
[34] 国家畜禽遗传资源委员会.中国畜禽遗传资源志·羊志[M].北京:中国农业出版社,2011. China National Commission of Animal Genetic Resource.Animal Genetic Resources in China· Sheep and Goat[M].Beijing:Chinese Agticultral Press,2011.(in Chinese)
[35] 任航行.Texel与乌珠穆沁绵羊妊娠中、后期胎儿骨骼肌基因表达谱及组织学分析[D].北京:中国农业科学院,2010. REN H X.Profiles of gene expression and histological analysis in fetal skeletal muscle between Texel and Ujumqin sheep(Ovisaries) during the second half of gestation[D].Beijing:Chinese Academy of Agricultural Sciences,2010.(in Chinese)
[36] REN H,LI L,SU H,et al.Histological and transcriptome-wide level characteristics of fetal myofiber hyperplasia during the second half of gestation in Texel and Ujumqin sheep[J].BMCGenomics,2011(12):411.
[37] REN X L,ZHU X H,LI X M,et al.Down-regulation of BTG3 promotes cell proliferation,migration and invasion and predicts survival in gastric cancer[J].JCancerResClinOncol,2015,141(3):397-405.
[38] TIRONE F.The gene Pc3(Tis21/Btg2),prototype member of the Pc3/Btg/Tob family:regulator in control of cell growth,differentiation,and DNA repair[J].JCellPhysiol,2001,187(2):155-165.
[39] STEWART S A,DYKXHOORN D M,PALLISER D,et al.Lentivirus-delivered stable gene silencing by RNAi in primary cells[J].RNA,2003,9(4):493-501.
[40] DELENDA C.Lentiviral vectors:optimization of packaging,transduction and gene expression[J].JGeneMed,2004(6):S125-138.
(编辑 郭云雁)
Expression Trend ofBTG2/3 in the Mid to Late Embryonic Skeletal Muscle of Sheep and the Relationship betweenBTG2/3 andMSTN
LIU Rui-zao1,LIU Zhen1,WU Ming-ming1,2,WANG Hui-hua1,ZHU Cai-ye1,ZHANG Li1,ZHAO Fu-ping1,WEI Cai-hong1*,DU Li-xin1*
(1.InstituteofAnimalScience,ChineseAcademyofAgriculturalSciences,Beijing100193,China;2.CollegeofAnimalScienceandTechnology,ChinaAgriculturalUniversity,Beijing100193,China)
The aim of this study was to analyze the expression of the sheepBTG2/3 genes in the mid to late embryonic skeletal muscle and to investigate the relationship betweenMSTNandBTG2/3.Longissimusdorsiof 85 dpc(day postconception),100 dpc,120 dpc and 135 dpc in Texel and Ujimqin sheep were collected as tested subjects.Real-time PCR was used to detect the expression ofMSTNandBTG2/3 in thelongissimusdorsi.Semimembranosus,longissimusdorsi,semitendinosus and quadriceps femoris of 100 dpc in the Texel and Ujimqin sheep were selected to detect the expression ofBTG2/3.Lentiviral vectors pFU-GW-myostatin were constructed to infect sheep myoblasts at the proliferation and differentiation stage stably and the expression ofBTG2/3 were detected at different stages.The results showed that a downward expression trend was emerged ofMSTNin Texel fetuses,whereas the expression level in Ujimqin increased after decline at 100 dpc.The expression ofBTG2 andBTG3 in Texel had downward trend after rising on 100 dpc,butBTG2 represented decrease-increase-decrease trend andBTG3 represented opposite trend toBTG2 in Ujimqin.Expression levels in different muscle on 100 dpc in the Texel and Ujimqin showed that,BTG2 gene expression was significantly higher(P<0.01) in Texellongissimusdorsiand semitendinosus than that in Ujimqin,andBTG3 gene expression was significantly lower(P<0.01) in Texel semimembranosus andlongissimusdorsithan that in Ujimqin.Transfection and interference efficiency of lentiviral vector were very high.After transfected myoblast at proliferation and differentiation stage,respectively,expression levels ofBTG2 andBTG3 also were decreased significantly(P<0.01) at both stages.The results indicate thatBTG2 andBTG3 play an important role in sheep skeletal muscle growth from the mid to late embryonic stage and may be involved in myostatin regulatory pathway.Our study provide an insight into the molecular regulation mechanism of embryonic development in sheep.
BTG2/3;Texel;Ujimqin;fetus;myostatin interference
10.11843/j.issn.0366-6964.2015.11.002
2014-11-28
优质肉、毛羊新品种(系)选育与关键技术研究及示范(2011BAD28B05-2);肉羊产业体系岗位科学家
刘瑞凿(1988-),男,河北鸡泽人,硕士,主要从事动物遗传育种方面的研究,E-mail:rzliu1988@sina.com
*通信作者:魏彩虹,副研究员,E-mail:weicaihong@caas.cn;杜立新,教授,E-mail:lxdu@263.net
S826;S813.3
A
0366-6964(2015)11-1916-08
